Abstract
The presented method is focused on simultaneous determination of retinol and two carotenoids (lutein, β-carotene) in selected Malaysian food products. A Dionex Ultimate 3000 Series High-Performance Liquid Chromatography system equipped with two Dionex Acclaim Polar Advantage II C18 columns was utilized in this study. Simultaneous elution of retinol, lutein and β-carotene was achieved within 45 min with gradient profile of methanol and ultra-pure water (95:5–100:0–95:5) programmed at 0.8 mL/min flow rate and 30 °C. Retinol was acquired at 325 nm while lutein and β-carotene were acquired at 440 nm. Limits of detection and quantification of retinol and lutein were at 0.10, 0.34 µg/mL, and 0.08, 0.26 µg/mL; respectively while higher levels were observed for β-carotene at 1.06 and 3.54 µg/mL. Linearity test indicated R2 of 0.996–0.999, along with high accuracy (71.92–116.29%) and excellent repeatability for all analytes. Extraction efficiency was determined using standard addition into representative food product with recovery obtained in the range of 61.86–116.90% for all three analytes. The developed in-house HPLC method incorporates an enclosed solid-phase extraction system that is believed to further improve sample purification and analytes detection. On the whole, the developed on-line SPE-HPLC technique and efficient sample pre-treatment is deemed a reliable approach for simultaneous determination of retinol, lutein and β-carotene, as analyzed among the selected Malaysian food products. Additionally, the described procedure could also benefit routine analysis of these analytes in other food matrices or for determination of other analytes with similar chemistry and faster elution.
Article highlights
-
A Dionex HPLC system integrated with on-line solid phase extraction within an enclosed system for improved detection of retinol, lutein and β-carotene in several food matrices.
-
Sample processing verification via standard addition technique into selected food products.
-
Superiority of the proposed method in comparison to other available techniques and recommendation for future applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vitamins are micronutrients that are essential for human life, and are divided into two categories of “fat” and “water” soluble compounds. Retinol, lutein and β-carotene belongs to the fat-soluble vitamins (FSVs); where each is known to possess various roles in vital biological functions. Vitamin A is involved in cell regulation, tissue growth as well as differentiation and can only be obtained from food source [1, 2]. Meanwhile, carotenoids are classified as a group of pigmented compounds that gives rise to various colors such as the red, yellow and orange hues of plant leaves, fruits and flowers, and even several birds, fish or insects [3]. Carotenoids are well-known to be effective antioxidants, although it is very much dependent on its structure as well as the oxidizing species [4]. The isolation and determination of FSVs is quite complex considering that they can co-exist on the lipid fraction of foods with other lipid constituents such as triglycerides, sterols and phospholipids [5]. The matrix complexity as well as their stability further complicates the development of rapid and simple extraction and analytical methods [6].
To date, various extraction techniques have been reported; and these include liquid–liquid, solid-phase or supercritical fluid extraction [7,8,9,10]. Extractions are performed upon alkaline saponification, a process that is well-known to result in analyte degradation [11]. Despite the unstable nature of all these vitamins, addition of antioxidants assists to prevent oxidation during the saponification process. Ascorbic acid, pyrogallol, sodium ascorbate, ethoxyquin and butylated hydroxytoluene (BHT) are some of the commonly used antioxidants [12]. Varying range of solvents or solvent mixtures has been utilized for extracting FSVs. The commonly recommended solvents include ethyl acetate, mixtures of methanol and tetrahydrofuran (THF), hexane, acetone, and other organic solvents [13,14,15].
High Performance Liquid Chromatography (HPLC) is the most common technique reported for analytical determination of vitamins that can be coupled to a range of detectors such as ultraviolet–visible (UV–Vis), diode array detection (DAD), fluorescence or mass spectrometry (MS) [5]. Among all, MS portrays significantly high selectivity and sensitivity. This was evident through studies presenting improved quantification of vitamins with utilization of HPLC-tandem MS [16, 17]. In addition, the ionization technique in MS also plays an important role where the atmospheric pressure chemical ionization (APCI) generates less matrix interferences comparatively to electrospray ionization (ESI) [18]. The application of MS technique has been tremendously applied for vitamin analysis in a wide range of matrices such as infant formula, vegetables, milk and plant foods [2, 19,20,21].
Simultaneous detection of multiple vitamins from complex matrices could be achieved by normal phase, ion-exchange or reverse phase chromatography under both isocratic and gradient elution. Among these, reverse phase chromatography is the most widely used technique for all FSVs except for vitamin E that portrays higher retention in normal phase chromatography [12, 22, 23]. Recent technological advancements also indicated availability of a latest technique for FSV determination, known as ultra-performance convergence chromatography (UPC2) that is environmental-friendly and also cost-effective along with rapid analysis time. This technique integrates the superiority of both supercritical fluid chromatography and ultra-performance liquid chromatography (UPLC) in a single viable technique [24].
A recent study by Katsa and colleagues [5] reported on the determination of FSVs in rice cereal baby foods with the application of HPLC–DAD and UHPLC-APCI-MS/MS techniques. A total of three methods were performed and compared in this study. The basic principle of all three methods was enzymatic hydrolysis, hot saponification and liquid–liquid extraction. Among the three methods, one method was preferred since it could detect all three vitamins (A, D3, E) simultaneously and is deemed faster, eco-friendly as well as cheaper for routine analysis. However, the presented method reported higher detection limits compared to previous studies; that was presumed to be in relation to the matrix effect [5].
In another study, the carotenoid pattern of pineapple infructescence was investigated via application of HPLC–DAD–APCI–MS technique [25]. The finding showed presence of diverse esters of violaxanthin with caprylic, capric, lauric and myristic acid as the responsible color imparting pigments of the flesh. In addition, the pineapple peel indicated an interesting profile with presence of chloroplast-specific pigment profile consisting of (all-E)-violaxanthin, (all-E)-neoxanthin, (all-E)-lutein and (all-E)-β-carotene. Apart from carotenoid profiling, the carotenoids extracted from the pineapple pulps were also quantified by HPLC–DAD. The presented research highlights the importance of HPLC–DAD technique for accurate quantification of the extracted vitamins [25].
Over the years, there have been numerous research reports presenting on various techniques or modifications that could be utilized for multiple applications. The growing interest in this research scope added with the tremendous benefits of the vitamin compounds led us to further look into improvisation efforts as an alternative approach. Although the MS technique has gained much popularity for its superiority in terms of high sensitivity and selectivity; however, it is relatively higher in cost and this is deemed unsuitable for applications that require routine analysis. Hence, HPLC coupled to UV–Vis or DAD are mostly preferred for common and routine analysis for its versatility, robustness, selectivity, sensitivity and cost-effectiveness. Furthermore, it is also important to understand that regardless of the selected analytical method superiority, the suitability of any technique is still dependent on the extraction efficiency. Hence, much attention should also be placed on development of good extraction technique that is mostly based on principle of enzymatic hydrolysis, alkaline hydrolysis, hot saponification and liquid–liquid extraction.
In the present work, we intend to establish an efficient extraction technique and development of a robust in-house HPLC technique for concurrent detection of retinol, lutein and β-carotene in selected Malaysian food products. The key factor of the current study relies on the combination of HPLC system integrated with on-line solid phase extraction (SPE). This approach instead of the conventional off-line SPE technique is both time- and cost-effective as it does not require additional solvents or cartridges. In addition, it is easily auto-programmed via HPLC analytical method that involves sample purification within an enclosed system. This further highlights its importance that is especially beneficial for compounds that are usually present in trace amounts, leading to an improved detection capacity via purification and impurities elimination. To date, there have been no reports on simultaneous detection of retinol, lutein and β-carotene incorporating the on-line SPE-HPLC system.
2 Materials and methods
2.1 Chemicals and reagents
Vitamin A (retinol ≥ 95%) and β-carotene (≥ 93%) standards were purchased from Sigma Aldrich (St Louis, USA), whereas lutein (≥ 95%) was purchased from Extrasynthese (Lyon, France). All standards were stored at -20 °C. Standard Reference Material®3280 (SRM 3280) of multivitamin tablets was purchased from National Institute of Standards and Technology (NIST, USA). Potassium hydroxide (KOH) pellets (≥ 85%) was purchased from Merck (Darmstadt, Germany). BHT (≥ 99.0%) was purchased from Sigma Aldrich (St Louis, USA). HPLC grade methanol (purity ≥ 99.9%) was purchased from Merck (Darmstadt, Germany). The other organic solvents include ethanol, methanol and 1-chlorobutane (purity ≥ 99.9%), all of analytical grade also purchased from Merck (Darmstadt, Germany). The ultra-pure water (UPW) used for mobile phase mixture was purified through a Sartorius Arium® lab water system (Göttingen, Germany).
2.2 Raw materials
One kilogram each of all analyzed products were purchased from local supermarkets in Klang Valley region. Three different matrices representing raw and processed food products were selected for this study. The selected products (minimum n = 3) ranged from several varieties of cordial (mango, soursop, sarsi), nut (pistachio, gingko, macadamia, flour coated groundnut) and flour (chickpea, soya) products. The products were confirmed to represent brands/varieties consumed and available nationwide. The food products of similar brand were homogenized finely using food grinder and stored in chiller at 4 °C till further analysis.
2.3 Standard preparation and calibration
Standard stock solutions of retinol, β-carotene and lutein (200 µg/mL each) were prepared in ethyl acetate. Calibration mix standards were prepared via serial dilution with analytical grade methanol at varying concentration levels in the range of 0.2–20.0 µg/mL. A linear correlation between peak areas and concentrations were determined within the defined range for each standard.
2.4 Sample preparation and extraction
5.0 g of each food product and 2.0 g of SRM 3280 (in duplicate) was weighed individually into 100 mL amber volumetric flask. 10 mL of UPW was added to the sample and well-mixed. This was then followed by addition of 10 mL of 0.3% (w/v) BHT and 30 mL of 7.5% (w/v) KOH, both in ethanol. This mixture was well-shaken and subjected to saponification in water bath at 80 °C for 45 min. Upon saponification, samples were allowed to cool for about 15 min prior to addition of 20 mL UPW and 30 mL of 1-chlorobutane pre-added with 0.3% BHT (w/v). The solvents were well-mixed few times and left to stand allowing for formation of separate layers. 20 mL of the resulting upper supernatant was transferred into 50 mL centrifuge tube and added with equivalent volume of UPW. This solution was vortexed and subjected to centrifugation at 3076×g for 10 min. 1.5 mL of the upper supernatant was transferred into micro-centrifuge tubes and evaporated using rotary evaporator for 90 min. The evaporated sample was re-constituted in 0.75 mL of methanol, vortexed, and filtered using 0.2 µm membrane filters. The filtered samples were finally subjected to HPLC analysis.
2.5 Apparatus and separation conditions
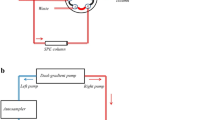
The analyses of retinol and the selected carotenoids (lutein and β-carotene) were performed using HPLC Dionex Ultimate 3000 Series system (Thermo Fisher Scientific, Waltham, MA USA) equipped with Photodiode Array Detector (PDA). Separation was achieved using two Dionex Acclaim Polar Advantage II Reversed-phase C18 column; where one served as the on-line SPE column (3.0 × 75 mm, 3 µm) connected to left pump and the other as an analytical column (4.6 × 250 mm, 5 µm) connected to right pump with gradient elution of methanol in combination with UPW. The graphical representation of the on-line SPE system is as depicted in Fig. 1 [26]. The gradient profiles for both pumps are identical where elution was initiated using methanol: water; 95:5 (v/v) which was then modified to 100:0 (v/v) at 15 min. This composition was maintained up to 42 min; and was altered back to initial composition of 95:5 (v/v) at 44 min till the end of analysis at 45 min. Flow rate for both pumps and column oven temperature was kept constant at 0.8 mL/min and 30 °C throughout the analyses, respectively. Injection volume for each analysis was fixed at 20 μL. Column switching was programmed to initiate at position 1_2 that was altered to 6_1 position from 1 to 15 min; prior to re-switching to 1_2 valve. Retinol was acquired at 325 nm whereas β-carotene and lutein were acquired at 440 nm with a total analysis time of 45 min. The resulting peaks were identified by their retention time in comparison to their respective standards. Data sets were processed and integrated using Chromeleon 7 software (Thermo Fisher Scientific, Waltham, MA USA).
2.6 Analytical method validation
Limit of detection (LOD), limit of quantification (LOQ), linearity, accuracy and repeatability were the selected main parameters for method validation as per the International Conference on Harmonization (ICH) tripartite guidelines Q2 [27]. The detailed protocol for each parameter were performed as explained below.
2.6.1 LOD and LOQ
There are several approaches that can be applied in the determination of LOD and LOQ. Signal to noise (S/N) approach was selected in our study, where this is done by comparing the measured signals from samples with known low concentrations against those of blank samples in establishing the minimum concentration at which the analyte can be reliably detected. Three concentrations of each standard were prepared and injected three times, and was compared against three injections of blank samples. LOD and LOQ were determined as a S/N ratio of 3 and 10, respectively with reference to background noise from a blank sample.
2.6.2 Linearity and range
The linearity range for each analyte was determined through a calibration curve with a minimum of five standard concentrations. The determinant coefficient (R2) obtained was used to assess linearity.
2.6.3 Accuracy and repeatability
The accuracy of the method was assessed by evaluating the recovery percentage of known concentration levels against five standards with known concentrations. Repeatability was determined via three injections at five different concentration levels in the range of 1.0–20.0 µg/mL.
2.6.4 Recovery
The suitability of sample extraction was determined via standard addition technique. Samples were spiked with known amount of each standard into a selected product of each group prior to commencement of the extraction protocol. The recovery was presented as the percentage against the spiked amount of each analyte, R (%) = (AS – AU/SA) × 100%, where AS is the analyte content in spiked product, AU is analyte content in the unspiked product, while SA represents the standard amount added to the product. Similarly, this approach was also applied using standard reference material (SRM®3280) for further confirmation on the extraction efficiency against the known reference value.
2.7 Statistical analysis
Data were analyzed using SPSS software (version 26.0). All results were expressed as mean, standard deviation, standard error of mean and range. Normality test was performed and determined by Shapiro Wilk, where data is normally distributed at probability of p > 0.05. The means and median difference between groups were compared using t-test (normally distributed data) and Kruskal–Wallis along with Mann–Whitney test (not normally distributed data). Significance was accepted at the probability of p < 0.05.
3 Results and discussion
3.1 Validation of HPLC method and extraction technique
An in-house analytical method for detection of retinol and carotenoids were developed and optimized. The method validation results of our study were compared to reported literatures, to further justify its suitability and potential applicability. Based on the comparative evaluations, it was concluded that the developed in-house method is suitable for routine analysis application and is discussed below.
As presented in Table 1, LOD and LOQ of retinol were 0.10 and 0.34 µg/mL, while lutein recorded LOD and LOQ of 0.08 and 0.26 µg/mL, respectively. In contrast, β-carotene resulted in higher LOD and LOQ at 1.06 and 3.54 µg/mL. The determination of linearity range showed linear responses ranging from 0.5 to 20.0 µg/mL for retinol, 0.3–20.0 µg/mL for lutein, whereas β-carotene falls within 4.0–20.0 µg/mL. The correlation coefficient (R2) was used as the reference point for the linearity analysis, where this ranged from 0.996 to 0.999, indicating a good fit of the calibration function (Table 1).
In terms of LOD and LOQ parameters, a study by Katsa and colleagues [5] showed that comparison of two instruments resulted in 0.98 and 2.9 mg/L with HPLC–DAD and 0.43 and 1.3 mg/L with HPLC–MS/MS, respectively. In reference to this finding, we observe that the LOD and LOQ obtained with our application is better with retinol at 0.10 and 0.34 µg/mL (Table 1). However, for the other two analytes (lutein and β-carotene), a much lower LOD and LOQ was reported by Gebregziabher et al. [28] using HPLC–UV–Vis with C30 column. The LOD and LOQ presented was at 0.0096 and 0.0290 µg/mL for lutein; while 0.0175 and 0.0529 µg/mL was observed for β-carotene.
In addition, Hrvolova and co-workers [29] developed an advanced HPLC–MS/MS method for concurrent determination of carotenoids and FSV in human plasma. Analytical evaluation of the developed method proposed for detection of all analytes reported much lower LOD and LOQ, compared to the commonly applied HPLC–DAD technique. Comparatively, the LOD for retinol, lutein and β-carotene was reported at 0.002, 0.008 and 0.041 µg/mL whereas the LOQ was recorded at 0.005, 0.028 and 0.138 µg/mL; respectively. This confirms the conclusion by Hrvolova et al. [29] in their study that the limits obtained were almost 100-fold lower compared to HPLC–DAD technique; as observed in our current research. Based on the different findings, it could be observed that it varies according to different instrumentation parameters and sensitivity. Despite of improved sensitivities with MS techniques that could generate a much lower detection and quantification limit, however we suggest the application of a more robust HPLC technique as presented in our study, especially for routine analysis in relevance to easy maintenance and also, being more cost-effective.
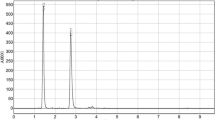
Repeatability testing at five concentration levels within the range of 1.0–20.0 µg/mL indicated comparable results for each injection. The results obtained for retinol, lutein and β-carotene at representative levels of 2.0, 4.0 and 10.0 µg/mL with overlay of three injections are presented in Fig. 2. All three compounds successfully eluted within 45 min in the order of retinol (8.8 min), followed by lutein (17.2 min) and β-carotene (41.0 min). Our analysis resulted in significantly longer duration; however, this is in correlation with the column length. Although the usage of shorter column could minimize the analytical duration, the preference for longer column is mainly due to its ability to achieve better separation and higher resolution. The finding of our study was comparably similar to another study conducted by Steingass and team [25] which presented on method for carotenoid profiling and quantification in pineapple peel and pulp using HPLC–DAD–APCI–MS. In the study, it was shown that retention time of lutein and β-carotene was almost similar to our finding despite application of MS techniques where it was also achieved around 18 and 45 min, respectively. In addition, the total run time analyzed in the study was mentioned to be conducted for a duration of 80 min. As such, we justify that the proposed method is reliable in view that efficient elution of these carotenoid compounds is practically acceptable for HPLC–DAD applications and should be correlated to the column used.
The accuracy parameter was determined against five standard concentrations in the range of 1.0–10.0 µg/mL (Table 2). Based on the obtained results, it was found that high accuracy levels were attained for all three analytes, with retinol (81.80–107.33%), lutein (73.88–104.66%) and β-carotene (71.92–116.29%). We evaluated our results against several reported studies where an almost similar observation was obtained. The application of HPLC–DAD by Katsa and team [5] presented recovery in the range of 85.00 -107.00% for retinol. On the other hand, other studies utilizing HPLC–MS/MS application demonstrated recoveries in the range of 93.7–111.5% for retinol, 84.7–106.58% and 83.12–103.3% for lutein and β-carotene; respectively [5, 28, 29]. Therefore, we recommend that our proposed method is equally competitive in all three analytes determination.
Standard addition technique was applied for evaluation of sample processing efficiency where recovery for retinol and lutein was achieved in the range of 74.30–116.90% and 85.43–101.16%, respectively. On the other hand, recovery for β-carotene ranged within 61.86–89.81% (Table 3). In addition to standard addition technique, the efficiency of recovery analysis was also verified using SRM®3280 whereby recovery of retinol and β-carotene was achieved at 89.58 and 85.84%, while lutein resulted in recovery of 108.82% (Table 4). Upon comparison, the proposed method was deemed suitable in view that the SRM recovery also correlates within the similar range for recovery results obtained with the various sample matrices.
Furthermore, the importance of SPE technique as an additional step for sample purification has been highlighted in study findings by Katsa et al. [5]. Comparative evaluation of the tested methods showed that method with SPE technique integration assisted in purification and sample pre-concentration that resulted in an improved analysis. Based on the similar concept, our work here combines the proposed HPLC technique with an on-line SPE system to purify the sample and eliminate unnecessary impurities towards enhanced detection, all performed within an enclosed system. In line with the comparative evaluation, we propose that our developed in-house HPLC method is robust, sensitive, selective and highly suitable for detection of all three analytes; especially for routine diagnostic applications.
3.2 Retinol and carotenoids analysis in selected Malaysian food products using the developed in-house HPLC method
Food products that are subjected to various processing steps prior to extraction have been reported to possess higher risk of technical and nutritional alterations. Food processing technique assists to enhance nutritional quality, shelf-life, increase variation as well as a preservation method [30, 31]. Thermal, mechanical, chemical and irradiation are some of the common food processing methods widely applied [30]. Since these nutrients are highly prone to degradation, we hypothesized that processing conditions could possibly impact the naturally available nutrient levels.
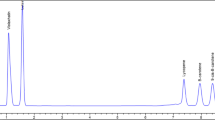
In this study, we attempted to investigate the content of three analytes (retinol, lutein, β-carotene) in raw food product (nuts) and processed food products (flour and cordials). The results of the analyzed food products are presented as mean value of all brands in Table 5. In addition, chromatograms of SRM®3280 and selected food products of respective analytes is presented as representation of real sample analysis (Figs. 3, 4, 5). Shapiro–Wilk analysis indicated both non-normally distributed data, thus further statistical analysis was conducted using non-parametric tests as mentioned in Sect. 2.7. The results were analyzed respective to each analyte (retinol, lutein, β-carotene) and product group (cordials, nuts, flours). In order to validate the findings of our study, the results were compared to available data of respective food product reported in United States Food Composition Database (USDA).
Daily consumption of fruits has been recommended to promote and sustain healthy lifestyle. Fruits are known for their remarkable therapeutic and preventive effects accounted by their nutrient rich composition comprised of minerals, vitamins, fibers, carbohydrates and phytochemicals. Among the essential functions of their nutritional attributes include proper body function, blood pressure and digestive system regulation, along with lower risk of coronary heart disease, obesity, certain cancer as well as vision loss [32,33,34]. The perishable nature of fruits only allows for its fresh consumption or processing within stipulated period; hence they are usually processed into other products such as dried fruits, canned fruit, frozen fruit, jam, fruit juice concentrates or alcoholic beverages that allows them for off-season consumption [35, 36].
Among these, fruit juice concentrates are one of the fruit products that are widely produced to supply nutritionally rich juices throughout the year. The process of producing fruit juice concentrates mainly involve water evaporation step, where the juices are usually boiled under vacuum. Apart from this technique, these concentrates are also prepared by freezing water as ice from the juices [37, 38]. Production of juice concentrates are highly beneficial as lower water content leads to longer shelf-life, reduces the packaging requirement, storage and transport-related costs with simplified handling process of the final product. In current practice, enormous methods for production of these fruit concentrates are applied. However, it was also highly recommended to ensure proper selection in order to minimize possible detrimental effects especially in terms of sensory and nutritional profile [36].
Based on results presented in Table 5, it was observed that only β-carotene and retinol were present in cordial samples. Soursop cordial indicated presence of retinol in brand 3 (1.031 mg/100 g) while β-carotene was present in brand 2 of mango cordial (3.911 mg/100 g). However, statistical evaluation indicated no significant difference for both retinol and β-carotene, as per Kruskal–Wallis analysis (p > 0.05). The reference data for fruit concentrate available in USDA was mainly reported on calculation basis, whereby mango concentrate signified presence of 714 IU of vitamin A that is equivalent to 0.214 mg/100 g retinol and 0.428 mg/100 g β-carotene, respectively [39]. Similarly, soursop fruit concentrate yields 160 IU of vitamin A (0.048 mg/100 g retinol and 0.096 mg/100 g β-carotene) [40]. In comparison with our findings, we noticed higher contents of both analytes respective to the tested cordial products.
A study by Gouado and team [41] presented strong evidence on the processing conditions effects indicated by presence of varying carotenoid composition levels with different forms. The study was conducted using mango and papaya fruits, where similar trend was noticed with higher concentration of carotenoids composition in the form of juice followed by fresh fruits and dried slices. The reported β-carotene content in mango juice was higher at 15.8128 µg/100 g, whereas a lower content of 3. 1743 µg/100 g and 0.1320 µg/100 g was found with fresh mango fruits and dried mango slices [41]. This finding proves the benefits of fruits preservation via concentrated liquid for longer term storage. However, high sugar content in these concentrates should also be taken into consideration where frequent consumption of these instead of fresh fruits may not be a healthier alternative, and are commonly associated with emergence of several non-communicable disease (NCD); namely obesity, type 2 diabetes and cardiovascular disease [42].
Flours happen to be the most prominent processed products from wheat that includes all-purpose, self-raising, whole wheat and others [43]. In addition, several crop varieties are also processed into flour products such as corn, soy and others. Soybeans (Glycine max) are rich source of phytochemicals (isoflavones, saponins, phytic acids, phytosterols, trypsin inhibitors, bioactive peptides) that are usually processed to produce soybean flour [44]. Previous studies reported that the rich nature of soybean extracts with isoflavones and other polyphenols have been associated with numerous biological activities in relevance to their antioxidative nature [45]. Traditionally, extractions are usually performed with usage of organic solvents, that require elevated temperatures and longer incubation period. Despite of continuous improvement of the extraction techniques for a more sustainable approach, however, it should be noted that certain level of processing is still required that could affect or alter nutritional content to an extent.
The resulting data of flour products showed that only lutein was detected for both soya and chickpea flour (Table 5). Interestingly, lutein was present in all six brands of chickpea flour (0.02–0.119 mg/100 g) and five brands of soya flour (0.024–0.035 mg/100 g). Statistical evaluation showed significant difference among the two flours, as analyzed with Kruskal–Wallis followed by Mann–Whitney pairwise comparison (p < 0.05). According to reference data in USDA, almost similar results with slight variations were noticed with both flours. Our results only signified presence of lutein, however this deviated with USDA that reported 0 μg for combined measure of lutein and zeaxanthin. In addition, it was noted that USDA reported detection of vitamin A with 0.002 mg/100 g RAE along with 0.025 mg/100 g and 0.024 mg/100 g β-carotene for chickpea and soy flour, respectively [46, 47].
A recent study by Gebregziabher and colleagues [28] presented an interesting finding on simultaneous detection of carotenoids and chlorophylls in soybean seeds using HPLC–UV–Vis. The study presented the development of a HPLC method that is suitable to separate carotenoids and chlorophylls with good linearity, accuracy and precision. The authors claimed that despite the complexities of carotenoids analysis, utilization of reversed-phase C30 column managed to successfully separate multiple carotenoids and its isomers via simple processing. It was noticed that the separation of lutein and zeaxanthin were more evident with C30 column, whereas it merged together with C18 column [28]. Based on our finding, only a single peak was detected using C18 and verified as lutein in relevance to the analytical standard peak. However, the possibility of co-emergence of zeaxanthin within the detected lutein peak could not be confirmed since our investigation was not inclusive of zeaxanthin analysis. An additional justification for usage of C18 is in relevance to simultaneous detection of retinol together with carotenoids, that could also be further expanded for other analytes, instead of focusing on carotenoids separation alone.
Chickpea or scientifically called Cicer arietinum L., is a member of Fabaceae family and is grown widely in tropical, sub-tropical and temperate regions. They are also commonly known as Bengal grams or Garbanzo beans [48]. It is highly nutritious and serves as vital source of vegetarian-based diets [49]. Chickpea seeds, are well-recognized as suitable source of dietary proteins in relevance to their good balance of amino acid, high protein bioavailability and relatively low antinutrient levels. It is consumed in several forms, where its unripe form (raw green and tender stage) are commonly consumed in Egypt. In addition, its mature dry seeds have gain recognition as a popular snack [50, 51]. Chickpea seeds are also commonly processed into chickpea flour where it is first dehusked into ‘dhal form’ followed by milling [48]. In a study by Arab and colleagues, the nutritional properties of chickpea flour subjected to various processing conditions were presented. It was shown that the protein, total nitrogen, non-protein nitrogen content, in-vitro protein digestibility as well as mineral content, functional properties, amino acid composition and amino acid scores were affected with different processing (traditional, microwave, fried) compared to the raw flour [50]. Hence, it could be presumed that the processing conditions; right from dehusking to its final ready for consumption stage are extremely essential and is highly accountable for the discrepancies observed in nutrient profiling.
Nuts are energy-dense food source, accounted mainly by its high fat content. In general, nuts are high in unsaturated and low in saturated fatty acids along with considerable levels of fiber, folate, minerals and antioxidants [52,53,54]. Dry fruits with one seed in which the ovary wall becomes hard at maturity are known as tree nuts. Almonds (Prunus amigdalis), walnuts (Juglans regia), pistachios (Pistachia vera), cashews (Anacardium occidentale), and macadamias (Macadamia integrifolia) are some of the commonly known tree nuts. On the other hand, although peanuts (Arachis hypogea) are classified as groundnuts or legumes; it also shows similar nutrient profile and hence are also included as tree nuts [55]. Nutritional properties of nuts were highly associated with various health benefits including decreased risk of cardiovascular disease, as well as risk factors for cholesterol and diabetes [56,57,58,59,60]. In addition, regular consumption of nuts has been associated with weight management that led them to become an important part of human diet, around the world [61,62,63,64].
As presented in Table 5, β-carotene was not detected in any of the tested nut products. In contrast, lutein was present in all six brands analyzed among pistachio and gingko nuts in the range of 0.063–0.188 mg/100 g and 0.029–0.088 mg/100 g, respectively. This scenario was of total opposite with retinol; where it was not detected in any brands of the similar nut product. Retinol was only shown to be present in selected brands of macadamia nut and flour-coated groundnut. Brand 2 and 3 of flour-coated groundnut indicated presence of retinol at 0.050 and 0.063 mg/100 g, while 0.117 mg/100 g of retinol was reported for macadamia nut (Brand 4). Based on Kruskal–Wallis analysis, no significant difference was obtained among the nut products for retinol (p > 0.05). In contrast, lutein showed significant differences (p < 0.05) among all four tested nut products. Further analysis to determine significant difference between specific nut products were evaluated using Mann–Whitney pairwise comparison. The test revealed significant difference among each nut products, with exception to pairwise comparison of macadamia nut and flour-coated groundnut (Table 5).
Comparison with data available for nut products also indicated certain similarities and disparities with our obtained results. Pistachio products yielded lutein presence, that was also noticed in reference to USDA. Nevertheless, the reference data was higher at 2.903 mg/100 g which is actually a combined measure of lutein and zeaxanthin, whereas our analysis only reports on the presence of lutein. However, β-carotene was reported to be present at 0.305 mg/100 g that contradicted our observations [65]. Furthermore, gingko nut products showed absence of retinol, while presence of other carotenoids were not mentioned [66]. USDA reference data for macadamia nut [67] was noted to be similar to our results, apart from detection of retinol in one of the tested brands. Although retinol was absent in all the available data, however presence of vitamin A was reported with respect to gingko (0.028 mg/100 g RAE) and pistachio nuts (0.026 mg/100 g RAE), respectively [65, 66].
Pistachios have been reported as the nut product that is comprised of significant amount of lutein and zeaxanthin, contributing to its color [68]. In addition, pistachios were well-known to be rich in several FSVs inclusive of vitamin A, K and γ-tocopherol. Similar observation was reported with pistachio nuts in another study conducted by Stuetz and colleagues in 2017. The study evaluated on five types of nuts (almonds, walnuts, pistachios, macadamia and hazelnuts). Out of these, pistachios were found to be the richest source of lutein, zeaxanthin and β-carotene [69]. In common practice, most nuts are consumed upon roasting, however it was deduced that roasting process might affect certain properties of the compounds, in comparison to its raw form. Thus, Stuetz et al. [69] attempted to evaluate the probable difference observed in terms of lutein/zeaxanthin, β-carotene, α- and γ-tocopherol in several raw and roasted nut products. This investigation showed that lutein/zeaxanthin level was lower in roasted almonds and walnuts, whereas a decreased level of β-carotene was detected in roasted almonds, hazelnuts and pistachios. Based on the findings, it was suggested that the concentration levels of certain vitamins upon roasting are subject to different sensitivity as well as the nut variety [69].
Through our study, we intend to demonstrate applicability of the analytical method and extraction technique for detection of retinol and carotenoids in several food matrices. As shown in the results, all three analytes could be detected respective to the different product groups that indicates suitability of the proposed technique. Despite of the discrepancies in comparison with the reference database, we would like to highlight that these could be probably due to various food processing conditions. Nevertheless, we observe an almost similar pattern of vitamin detection among our results and USDA. In addition, it is undeniable that these food products are highly nutritious regardless of the possible compositional alterations upon certain processing conditions. Moreover, the association of these food products with lower risk of chronic diseases, further strengthens their importance. At the same time, recent advances in development of new technologies could be beneficial in improvisation of the processing effects, along with fortification approach that could also play its role to counter-act nutrient loss.
4 Conclusion
A reliable and robust HPLC method along with efficient sample preparation for the simultaneous analysis of retinol, lutein and β-carotene was developed and validated. Good quality and reasonable values of LOD, LOQ, linearity, accuracy, repeatability and recoveries were attained by the proposed method. The present work highlights on the integration of HPLC technique with an on-line SPE configuration within an enclosed system that proves to be both time- and cost-effective along with its superiority of auto-programming via the HPLC instrumentation method. In this on-line SPE approach, there is no necessity for additional accessories such as the cartridges or even solvents to perform extra sample pre-treatment steps that are common with the conventional SPE technique. Sample purification could be easily programmed and performed within an enclosed system with the intention to eliminate the targeted impurities and further purify the samples that is vital especially for detection of analytes that are usually present in trace amounts.
Although there are continuous reports on HPLC method improvement and optimization, however it is difficult to conclude and establish a general standardized model for all analysis since it is highly reliable on various factors such as matrix effect, choice of solvent, flow rate, temperature and others. This is the first report on proposed method for concurrent analysis of three analytes (retinol, lutein and β-carotene) in several selected Malaysian food products within an enclosed SPE-HPLC system. Although the current study is only focused on several food matrices such as flour, nuts and cordial products; however, it is also believed to be beneficial for other type of food matrices. At the same time, the proposed method could also be integrated for other analytes with faster elution and similar chemistry in consideration of its wide applicability. Despite of the highlighted advantages, the present work also encounters certain limitations where it results in longer analytical duration that was mainly desired to achieve better peak with higher retention that is also inter-related with the selection of column used. On the whole, we believe that the developed on-line SPE-HPLC method and efficient sample pre-treatment gave a reliable and satisfactory results, that is equally competitive to other published methods and could be applied for various food-based routine analysis in the future.
Availability of data and materials
All data generated during this study is analyzed and included in this article.
Abbreviations
- APCI:
-
Atmospheric pressure chemical ionization
- BHT:
-
Butylated hydroxytoluene
- DAD:
-
Diode array detector
- ESI:
-
Electrospray ionization
- FSV:
-
Fat-soluble vitamin
- HPLC:
-
High performance liquid chromatography
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- MS:
-
Mass spectrometry
- NCD:
-
Non-communicable diseases
- R 2 :
-
Determinant coefficient
- RAE:
-
Retinol activity equivalents
- RDA:
-
Recommended dietary allowance
- SPE:
-
Solid phase extraction
- SRM:
-
Standard Reference Material
- THF:
-
Tetrahydrofuran
- UPC2 :
-
Ultra-performance convergence chromatography
- UPLC:
-
Ultra-performance liquid chromatography
- UPW:
-
Ultra-pure water
- USDA:
-
United States Food Composition Database
- UV:
-
Ultraviolet
References
Coultate T, Food: the chemistry of its components. Fourth, (eds) (2002) Cambridge. Royal Society of Chemistry, UK
Plozza T, Craige Trenerry V, Caridi D (2012) The simultaneous determination of vitamins A, E and β-carotene in bovine milk by high performance liquid chromatography–ion trap mass spectrometry (HPLC–MSn). Food Chem 134(1):559–563
Stahl W, Sies H (2003) Antioxidant activity of carotenoids. Mol Aspects Med 24(6):345–351
Young AJ, Lowe GM (2001) Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys 385(1):20–27
Katsa M, Papalouka N, Mavrogianni T, Papagiannopoulou I, Kostakis M, Proestos C, Thomaidis NS (2021) Comparative Study for the Determination of Fat-Soluble Vitamins in Rice Cereal Baby Foods Using HPLC-DAD and UHPLC-APCI-MS/MS. Foods 10(3):648
Melfi MT, Nardiello D, Cicco N, Candido V, Centonze D (2018) Simultaneous determination of water- and fat-soluble vitamins, lycopene and beta-carotene in tomato samples and pharmaceutical formulations: Double injection single run by reverse-phase liquid chromatography with UV detection. J Food Comp Anal 70:9–17
Gómez-Prieto MS, Caja MM, Herraiz M, Santa-María G (2003) Supercritical fluid extraction of all-trans-lycopene from tomato. J Agric Food Chem 51(1):3–7
Kozukue N, Friedman M (2003) Tomatine, chlorophyll, β-carotene and lycopene content in tomatoes during growth and maturation. J Sci Food Agric 83(3):195–200
Rozzi N, Singh R, Vierling R, Watkins B (2002) Supercritical fluid extraction of lycopene from tomato processing byproducts. J Agric Food Chem 50(9):2638–2643
Tzouganaki ZD, Atta-Politou J, Koupparis MA (2002) Development and validation of liquid chromatographic method for the determination of lycopene in plasma. Anal Chim Acta 467(1):115–123
Scalia S, Renda A, Ruberto G, Bonina F, Menegatti E (1995) Assay of vitamin A palmitate and vitamin E acetate in cosmetic creams and lotions by supercritical fluid extraction and HPLC. J Pharm Biomed Anal 13(3):273–277
Jedlička A, Klimeš J (2005) Determination of water-and fat-soluble vitamins in different matrices using high-performance liquid chromatography. Chem Pap 59(3):202
Wieliński S, Olszanowski A (2001) Development and validation of HPLC method for simultaneous determination of fat-soluble vitamins in capsules. J Liq Chromatogr Relat Technol 24(2):201–213
Lin CH, Chen BH (2003) Determination of carotenoids in tomato juice by liquid chromatography. J Chromatogr A 1012(1):103–109
Rodriguez-Amaya DB, Kimura M (2004) HarvestPlus handbook for carotenoid analysis. HarvestPlus Technical Monograph Series 2. vol 2. IFPRI, Washington, DC, USA, and CIAT: Cali, Colombia
Fanali C, D’Orazio G, Fanali S, Gentili A (2017) Advanced analytical techniques for fat-soluble vitamin analysis. TrAC Trends Analyt Chem 87:82–97
Wang X, Li K, Yao L, Wang C, Van Schepdael A (2018) Recent advances in vitamins analysis by capillary electrophoresis. J Pharm Biomed Anal 147:278–287
Souverain S, Rudaz S, Veuthey JL (2004) Matrix effect in LC-ESI-MS and LC-APCI-MS with off-line and on-line extraction procedures. J Chromatogr A 1058(1–2):61–66
Heudi O, Trisconi MJ, Blake CJ (2004) Simultaneous quantification of vitamins A, D3 and E in fortified infant formulae by liquid chromatography-mass spectrometry. J Chromatogr A 1022(1–2):115–123
Huck CW, Popp M, Scherz H, Bonn GK (2000) Development and evaluation of a new method for the determination of the carotenoid content in selected vegetables by HPLC and HPLC–MS–MS. J Chromatogr Sci 38(10):441–449
Gentili A, Caretti F (2011) Evaluation of a method based on liquid chromatography-diode array detector-tandem mass spectrometry for a rapid and comprehensive characterization of the fat-soluble vitamin and carotenoid profile of selected plant foods. J Chromatogr A 1218(5):684–697
Kamal-Eldin A, Görgen S, Pettersson J, Lampi A-M (2000) Normal-phase high-performance liquid chromatography of tocopherols and tocotrienols: Comparison of different chromatographic columns. J Chromatogr A 881(1–2):217–227
Salo-Väänänen P, Ollilainen V, Mattila P, Lehikoinen K, Salmela-Mölsä E, Piironen V (2000) Simultaneous HPLC analysis of fat-soluble vitamins in selected animal products after small-scale extraction. Food Chem 71(4):535–543
Rathi D-N, Liew CY, Fairulnizal MNM, Isameyah D, Barknowitz G (2017) Fat-soluble vitamin and carotenoid analysis in cooking oils by ultra-performance convergence chromatography. Food Anal Methods 10(4):1087–1096
Steingass CB, Vollmer K, Lux PE, Dell C, Carle R, Schweiggert RM (2020) HPLC-DAD-APCI-MSn analysis of the genuine carotenoid pattern of pineapple (Ananas comosus [L.] Merr.) infructescence. Food Res Int 127:108709
Rathi D-N, Md Noh MF, Abd Rashed A, Dasuki I (2019) Simultaneous analysis of vitamin D and K in processed food products via ultra high- performance liquid chromatography (UHPLC). J Food Meas Charact 13(3):1947–1957
ICH, I.C.o.H., ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology; ICH Q2. 2005. ICH: Geneva, Switzerland, 4–12
Gebregziabher BS, Zhang S, Qi J, Azam M, Ghosh S, Feng Y, Huai Y, Li J, Li B, Sun J (2021) Simultaneous determination of carotenoids and chlorophylls by the HPLC–UV–VIS method in soybean seeds. Agronomy 11(4):758
Hrvolová B, Martínez-Huélamo M, Colmán-Martínez M, Hurtado-Barroso S, Lamuela-Raventós RM, Kalina J (2016) Development of an advanced HPLC–MS/MS method for the determination of carotenoids and fat-soluble vitamins in human plasma. Int J Mol Sci 17(10):1719
Vanga SK, Raghavan V (2017) Processing effects on tree nut allergens: a review. Crit Rev Food Sci Nutr 57(17):3794–3806
Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50(10):3010–3014
Cho E, Seddon JM, Rosner B, Willett WC, Hankinson SE (2004) Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch Ophthalmol 122(6):883–892
Rafieian-Kopaei M, Baradaran A, Rafieian M (2013) Plants antioxidants: From laboratory to clinic. J Nephropathol 2(2):152–153
Slavin JL (2005) Dietary fiber and body weight. Nutrition 21(3):411–418
Berk Z (2016) Chapter 9 - Production of Citrus Juice Concentrates. Citrus fruit processing. Academic press, San Diego, pp 187–217
Adnan A, Mushtaq M, Islam Tu (2018) Chapter 12 - fruit juice concentrates, in fruit juices. In: Rajauria G, Tiwari BK (eds) Academic Press, San Diego, pp 217–240
Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC, Ahn DU, Lee SC (2004) Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J Agric Food Chem 52(11):3389–3393
Jiao B, Cassano A, Drioli E (2004) Recent advances on membrane processes for the concentration of fruit juices: a review. J Food Eng 63(3):303–324
USDA 454102, B. Mango. 19th January 2021]. Available from: https://fdc.nal.usda.gov/fdc-app.html#/food-details/454102/nutrients
USDA 495428, B. Soursop. 19th January 2021]; Available from: https://fdc.nal.usda.gov/fdc-app.html#/food-details/495428/nutrients
Gouado I, Schweigert FJ, Ejoh RA, Tchouanguep MF, Camp JV (2007) Systemic levels of carotenoids from mangoes and papaya consumed in three forms (juice, fresh and dry slice). Eur J Clin Nutr 61(10):1180–1188
Te Morenga L, Mallard S, Mann J (2013) Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ Brit Med J 346:e7492
Kumar P, Yadava R, Gollen B, Kumar S, Verma RK, Yadav S (2011) Nutritional contents and medicinal properties of wheat: a review. Life Sci Med Res 22(1):1–10
Isanga J, Zhang G-N (2008) Soybean bioactive components and their implications to health—A review. Food Rev Int 24(2):252–276
Nikoo M, Regenstein JM, Ahmadi Gavlighi H (2018) Antioxidant and antimicrobial activities of (−)-Epigallocatechin-3-gallate (EGCG) and its potential to preserve the quality and safety of foods. Compr Rev Food Sci Food Saf 17(3):732–753
USDA 172444, S.L. Soy flour, Low-fat. 19th January 2021]; Available from: https://fdc.nal.usda.gov/fdc-app.html#/food-details/172444/nutrients
USDA 174288, S.L. Chickpea flour. 21st September 2021]; Available from: https://fdc.nal.usda.gov/fdc-app.html#/food-details/174288/nutrients
Chang Y-W (2006) Isolation and characterization of proteins from chickpea (Cicer arietinum L.) seeds
Sotelo, A. and R. Adsule, Chickpea (Cicer arietinum L.). , in Food and Feed from Legumes and Oilseeds, E. Nwokolo and J. Smartt, Editors. 1996, Chapman & Hall: London, UK. p. 82–89.
Arab EA, Helmy I, Bareh G (2010) Nutritional evaluation and functional properties of chickpea (Cicer arietinum L.) flour and the improvement of spaghetti produced from its. J Am Sci 6(10):1055–1072
Alajaji SA, El-Adawy TA (2006) Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J Food Comp Anal 19(8):806–812
Venkatachalam M, Sathe SK (2006) Chemical composition of selected edible nut seeds. J Agric Food Chem 54(13):4705–4714
Kornsteiner M, Wagner KH, Elmadfa I (2006) Tocopherols and total phenolics in 10 different nut types. Food Chem 98(2):381–387
Ros E, Tapsell LC, Sabaté J (2010) Nuts and berries for heart health. Curr Atheroscler Rep 12(6):397–406
Ros E (2010) Health benefits of nut consumption. Nutrients 2(7):652–682
Mozaffarian D (2016) Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 133(2):187–225
Ros E (2015) Nuts and CVD. Br J Nutr 113(Suppl 2):S111–S120
Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D (2015) Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am J Clin Nutr 102(6):1347–1356
Asghari G, Ghorbani Z, Mirmiran P, Azizi F (2017) Nut consumption is associated with lower incidence of type 2 diabetes: The Tehran Lipid and Glucose Study. Diabetes Metab 43(1):18–24
Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, Fiol M, Gómez-Gracia E, López-Sabater MC, Vinyoles E, Arós F, Conde M, Lahoz C, Lapetra J, Sáez G, Ros E (2006) Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 145(1):1–11
Bes-Rastrollo M, Wedick NM, Martinez-Gonzalez MA, Li TY, Sampson L, Hu FB (2009) Prospective study of nut consumption, long-term weight change, and obesity risk in women. Am J Clin Nutr 89(6):1913–1919
Jackson CL, Hu FB (2014) Long-term associations of nut consumption with body weight and obesity. Am J Clin Nutr 100 Suppl 1(1):408S-S411
O’Neil CE, Keast DR, Fulgoni VL 3rd, Nicklas TA (2010) Tree nut consumption improves nutrient intake and diet quality in US adults: an analysis of National Health and Nutrition Examination Survey (NHANES) 1999–2004. Asia Pac J Clin Nutr 19(1):142–150
Alasalvar C, Shahidi F (2008) Tree nuts: composition, phytochemicals, and health effects. In: Alasalvar C, Shahidi F (eds) Nutraceutical Science and Technology. CRC Press, Boca Raton
USDA 170184, S.L. Nuts, Pistachio nuts, Raw. 19th January 2021]; Available from: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170184/nutrients
USDA 170584, S.L. Nuts, Gingko nuts, Raw. 19th January 2021]; Available from: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170584/nutrients
USDA 170178, S.L. Nuts, Macadamia nuts, Raw. 19th January 2021]; Available from: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170178/nutrients
Bulló M, Juanola-Falgarona M, Hernández-Alonso P, Salas-Salvadó J (2015) Nutrition attributes and health effects of pistachio nuts. Br J Nutr 113(Suppl 2):S79-93
Stuetz W, Schlörmann W, Glei M (2017) B-vitamins, carotenoids and α-/γ-tocopherol in raw and roasted nuts. Food Chem 221:222–227
Acknowledgements
The authors thank the Director General of Health Malaysia and the Director of Institute for Medical Research (IMR), Malaysia, for giving the permission to publish this article. We also thank the staff of Nutrition, Metabolism and Cardiovascular Research Centre, Institute for Medical Research, National Institute of Health for their continuous support.
Funding
This research was funded by a grant from the National Institute of Health, Ministry of Health Research Grant.
Author information
Authors and Affiliations
Contributions
Conceptualization: D-NGR, AA.R, M.F.M.N; Investigation and Methodology: D-NGR; Writing—Original draft: D-NGR; Writing—Review and Editing: D-NGR, AAR, MFMN; Revisions and Final editing: D-NGR. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no competing interest, and approves it for publication.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rathi, DN.G., Rashed, A.A. & Noh, M.F.M. Determination of retinol and carotenoids in selected Malaysian food products using high-performance liquid chromatography (HPLC). SN Appl. Sci. 4, 93 (2022). https://doi.org/10.1007/s42452-022-04955-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-04955-8