Abstract
This study evaluated the in vitro antiplasmodial efficacy and cytotoxicity of Allanbackia floribunda stem bark extract, leaf extract and oil. It also assessed the phytochemical compositions and antioxidant action of the stem bark fractions as well as the phytochemical fingerprint of the most active fraction (dichloromethane). Trager and Jensen method was used to culture Plasmodium falciparum, Mark III test developed by WHO was used to assess the antiplasmodial activity of the plant’s crude extract and fractions against the ring stage of P. falciparum strain, Pf3D7. Cytotoxicity was determined against Vero cell line using microculture tetrazolium (MTT) test. Gas chromatography with flame ionization detection (GC-FID) was employed to identify phytochemical fingerprint of the most active fraction. The stem bark extract had better antiplasmodial activity (IC50Pf3D7 of 4.3 ± 0.17 μg/mL) compared with the leaf extract (IC50Pf3D7, 8.0 ± 0.28 μg/mL) and oil (IC50Pf3D7 > 100 μg/mL). Both the leaf and stem bark extracts were found to be non-cytotoxic compared with the standard cytotoxic drug, doxorubicin. The selectivity indices (S.I.) of the extracts against the parasite were 20.06 and 8.85 for the stem bark and leaf respectively. Dichloromethane fraction had the highest inhibition against the P. falciparum parasite with IC50Pf3D7 of 1.51 μg/ mL. GC-FID analysis showed high presence antiplasmodial flavonoids and terpenes. This investigation confirmed that A. floribunda stem bark has potent activity against P. falciparum, and it is relatively safe to normal cell.
Article Highlights
-
Allanblackia floribunda methanol stem bark and leaf extracts could inhibit the growth of chloroquine sensitive Plasmodium falciparum (Pf3D7) in vitro.
-
The stem bark infusion of Allanblackia floribunda was found to be nontoxic and safe at moderate doses to normal cell line (Vero cell line).
-
Dichloromethane fraction of the stem bark showed excellent inhibition against chloroquine sensitive malaria parasite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Malaria is a disease that is caused by Plasmodium parasite. The symptoms and clinical manifestations of this protozoan infection is severe when the infection is caused by Plasmodium falciparum in children, pregnant women and individuals from malaria free regions [1]. However, the negative impact of P. falciparum is less complicated when previously exposed to the parasite infection [1]. Though Artemisinin-based combination therapy (ACT) recommended by WHO for the treatment of uncomplicated malaria has been effective in combating this malady. but the scourge of the disease is still fierce in sub-Saharan Africa because of the mortality rate on a yearly basis [2]. For example, there were approximately 216,000,000 cases of malaria infection worldwide resulting in 445, 000 death with 91% of the mortality in Africa [2]. It has been impossible to eliminate malaria from Africa due to the existence of hard-to- reach communities, inaccessibility of antimalarial drugs, exposure to fake antimalarial drugs, drug resistance, lack of information flow on effective antiplasmodial agents and individuals’ low-income status. In addition, recent reports in eastern [3], northern and southern [4, 5] regions of Nigeria suggest possible cases of parasite resistance and recrudescence to the currently used combined antimalarial therapies. The present circumstances necessitate the need for alternative antimalarial drug and adjuvant therapy to curtail the challenges of parasite elimination and curb possible future resistance.
Despite the death toll resulting from parasite plaque in Nigeria, the tribals of southern Nigeria are able to control the ravaging effect to some extent by using plant infusion from Allanblackia floribunda commonly known as elephant rice [6, 7]. A. floribunda is rich in bioactive substances with varied pharmacological properties on living systems [6, 7]. The stem bark, leaf and fruit of A. floribunda infusions are used in African herbal medicine preparation to manage pain and inflammation. In Nigeria, Ghana, Cameroon and Ivory Coast the stem bark extract is used to heal aching tooth and asthma, the pounded bark is used traditionally to stop diarrhea, a decoction of the whole fruit is used to relieve scrotal elephantiasis [6, 7] and the stem back infusion is used to maintain strong erection in aging men [8].
Preliminary phytochemical screening of the stem, leaf and oil showed that stem has more phenolics, flavonoids, terpenoids compared with the leaf extract and oil [Irabor et al., unpublished]. The methanol extract of a close species, Allanblackia parviflora Chev. was reported to have a strong antimicrobial activity against Entaemoeba faecalis [9]. In 2017, high performance liquid chromatography analysis of methanol extract of the seed by Akpanika et al. [10] revealed the presence of important polyphenols (morelloflavone) and glucosides and their antioxidant activities were comparable to vitamin C.
Despite the relevance of A. floribunda in Africa folk medicine there exist a limited scientific data and documentation on its antimalarial activity, the relative abundance of its pharmacologically active substances, best solvent for its extraction and level of safety of its most active infusions. This investigation therefore examined the in vitro antiplasmodial activity, cytotoxicity, antioxidant action and GC-FID analysis of Allanblackia floribunda extracts.
2 Materials and methods
2.1 Plant collection and identification
The plant parts used in this study were selected based on ethnopharmacological information as antimalarial agents used in southern part of Nigeria. Allanblackia floribunda leaf, stem bark and seed were collected during the period of January and July 2016, from a forest area at Ohogua community, Ovia East Local Government, Benin City, Edo Sate, Nigeria. The plant was authenticated by Dr. Henry A. Akinnibosun at the Department of Plant Biology and Biotechnology, Faculty of Life Sciences, University of Benin, Nigeria, and voucher specimen of the sample (UBHA361) was deposited at the herbarium of the same department. The plant scientific name was checked with http://www.theplantlist.org on 26th October, 2020.
2.2 Preparation of extract
A 100°g of the A. floribunda stem bark and leaf were macerated in 1000 mL of absolute methanol for 72 h with occasional stirring. The extracts were then filtered using double layered muslin cloth and the filtrate concentrated to dryness by using a rotary evaporator at reduced pressure while the oil was obtained from about 200 g of the powdered seed using hot water floatation method. The extracts obtained were stored at 4°C until used.
2.3 Cultivation of parasites
Trager and Jensen procedure was used to culture P. falciparum [11, 12]. The Plasmodium strain (Pf3D7) used was chloroquine sensitive isolate obtained from the Malaria Research and Reference Reagent Resource Center (MR4) at the American Type Culture Collection (ATCC) in Manassas, Virginia, US. The parasites were cultured in O + RBC as host cells and maintained in RPMI 1640 medium supplemented with gentamicin solution 0.01 mg/mL, 25 mM HEPES buffer, 25 mM NaHCO3 and 1% Albumax II maintained in 5% CO2 and incubated at 37 °C. Parasitaemia was determined using light microscopy (Giemsa stain).
2.4 In vitro antiplasmodial activity
The growth inhibition of chloroquine-sensitive Plasmodium falciparum strain (Pf3D7) by the plant extracts was evaluated by means of the Mark III test, as developed by WHO [13]. The stock solutions were filter-sterilised through a 0.2 μm Millipore filters. Two-fold serial dilutions of drug/extracts were performed to generate required concentrations (refer to supplementary file S2) for treatment of parasitized cells in vitro. One hundred microliters (100 μL) of 0.5% P. falciparum culture that had been synchronized (refer to supplementary file S1) was aliquoted into the wells of the pre-treated 96-well microtitre plate to a final hematocrit of 3%. Wells with parasitized red blood cells without plant extract serve as negative controls whereas wells containing cultures with chloroquine diphosphate serve as positive control. Parasitaemia was evaluated after 48 h by Giemsa stain and the average suppression of parasitaemia was calculated by using the formula below:
The antiplasmodial activities of the extracts were expressed using IC50. Each sample was tested in triplicate and the IC50 obtained were pooled and expressed as geometric means and standard deviations. The independent sample t-test was used to compare mean IC50 of antimalarial activity between plant extracts. Finally, the in vitro antiplasmodial activity was rated in accordance with the system of antiplasmodial activity of Rasoanaivo et al. [14]. According to this system, an extract is regarded to be very active if IC50 is less than 5 μg/mL, active if IC50 is greater than 5 μg/mL but less than 50 μg/mL, weakly active if IC50 is greater than 50 but less than 100 μg/mL and inactive if IC50 is greater than 100 μg/mL.
2.5 Cytotoxicity test (cell viability assay)
Vero cells were provided by the National Veterinary Research Institute, Vom Jos, Plateau State, Nigeria to assess the cytotoxicity of the most active plant extracts. Vero cells were maintained in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), glutamine (2 mM), penicillin (100 units/mL) and streptomycin (100 µg/mL). The cells were seeded in 96 well plates at 10,000 cells per well in 100 μL culture medium. The cells were incubated at 37°C in a humidified 5% CO2 atmosphere. After 48 h, the medium was discarded by inversion of the microtitre plate and thereafter replaced with 100 μL of fresh culture medium followed by 100 μL of crude plant extract at a concentration of 2000 µg/mL in row H of the 96 well plate and serially diluted twofold to give concentrations ranging from 1000 to 1.95 µg/mL. Row A of the 96 well plate serve as control wells. Doxorubicin was used as standard cytotoxic drug and concentration range tested for doxorubicin was 5- 0.01ug/ mL. The cells were maintained at 37°C in a CO2 incubator before determining their viability by using MTT assay as described by Mosmann [15]. The absorbance for each well was measured between 490 nm in a micro-titre plate reader and the percentage cell viability (CV) was calculated using the formula below. A dose–response curve was plotted to calculate the concentration that resulted in 50% death of viable cells (CC50).
2.6 Fractionation of A. floribunda stem bark extract
The stem bark extract was most active against Plasmodium falciparum (Pf3D7) compared with the leaf extract and oil of A. floribunda hence it was fractionated. Fractionation of the stem bark extract was done using solvent of increasing polarity as described by Hassan et al. [16]. The methanol stem bark extract of A. floribunda was partitioned into fractions of n-hexane, dichloromethane, ethyl acetate and hydromethanol. The fractions obtained were concentrated using a rotary evaporator (RE 300, Bibby Scientific, UK) with reduced pressure at 45˚C.
2.7 Qualitative phytochemical screening and antioxidant activity of A. floribunda stem bark fractions
The qualitative test for phytochemicals present in fractions were carried out using standard procedures [17, 18]. The free radical scavenging capacity of the fractions against 1, 1–diphenyl–2–picrylhydrazyl (DPPH) radical was determined by the method of Brand-Williams et al. [19] and phosphomolybdate reduction capacity was estimated using the method described by Prieto et al. [20].
2.8 Gas chromatography (GC) analysis of most active fraction (dichloromethane fraction)
The phytochemical fingerprint for flavonoids, terpenes, alkaloids, terpenoids, and volatile organic constituents of the most active fraction was determined using gas chromatography with flame ionization detector. Gas chromatography analysis was carried out on a HP 6890 Powered with HP ChemStation Rev. A 09 01[1206] Software. Active constituents were identified based on comparison of the retention times of the peaks with those of corresponding pure standard mixtures used. The flavonoids extraction was carried out by the method described by Millogo-Kone et al. [21]. The extraction of terpenes was carried out by method described by Ortan et al. [22]. The alkaloids extraction was carried out by the method described by Ngounou et al. [23]. The composition of volatile organic compounds was carried out on a HP 6890 powered with HP ChemStation Rev. Bioactive metabolites were identified based on comparison of the retention times of the peaks with those of the corresponding reference standards (Supelco Inc.) mixtures used (refer to supplementary file for the detailed methods of extraction and GC-FID conditions).
2.9 Statistical analysis
The various results obtained from this study were expressed as mean ± SEM. One way analysis of variance (ANOVA) followed by Tukey's HSD (honest significant difference) test was used to determine significance differences between the groups. Statistical significance was declared when P value was less than 0.05. The statistical analysis was performed using the statistical package for social science (SPSS) for windows, version 16.0.
3 Results
3.1 In vitro antiplasmodial activity of A. floribunda stem bark extract, leaf extract and oil
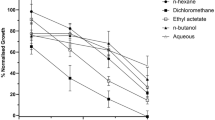
The inhibition of parasite growth by the plant extracts was dose dependent from 0.78 to 25 μg/mL. At about 5 μg/mL and above, both leaf and stem bark extracts had over 45% parasite inhibition. However, A. floribunda oil extract recorded less than 40% of P. falciparum inhibition even at the highest dose (see Fig. 1a). The IC50 values of the tested medicinal plant against P. falciparum are listed in Table 1. The stem bark extract of A. floribunda showed excellent antiplasmodial activity (4.3 ± 0.17 μg/ mL) followed by the leaf extract (8 ± 0.28 μg/mL). However, the oil extract showed IC50 value that was over 100 μg/mL.
3.2 Cytotoxic activity of A. floribunda crude stem and leaf extract
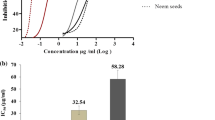
Results of the cytotoxicity of A. floribunda stem bark extract, leaf extract and doxorubicin against Vero cell line are shown in Fig. 1b and 1c. The stem bark extract at 1.95 to 1000 μg/mL had cell viability that was majorly over 50%. This same trend was observed for the leaf extract at 1.95 to 250 μg/mL. However, we observed less than 50 percent cell viability at concentration greater than 250 μg/mL for the leaf extract. The methanol extracts of A. floribunda exhibited non significant activity against the Vero cell line with CC50 values of 88.26 ± 0.15 μg/mL (stem bark) and 70.81 ± 0.06 μg/mL (leaf) as shown in Table 1. The standard cytotoxic drug, doxorubicin was found to be highly cytotoxic having CC50 of 0.05 ± 0.00 µg/mL; the selectivity index for A. florinbunda leaf was lower (8.85) when compared with the stem bark extract (20.06) Table 1.
3.3 Phytochemical composition, in vitro antiplasmodial and antioxidant activity of the partitioned fractions of A. floribunda methanol stem bark extract
Table 2 depicts the phytochemical screening results of the partition fractions of A. floribunda most active infusion, stem bark extract. The result revealed the presence of quinones, flavonoids, cardiac glycosides, alkaloids, terpenoids, tannins and phenols in all the fractions. Majority of the phytochemicals were highly detected in the dichloromethane, ethylacetate and hydromethanol fractions. However, some of these bioactive compounds were less detected in the hexane fraction. Quinones were undetected in the hydromethanol fraction. Figure 2a represents the total antioxidant capacity obtained for the fractions of the stem bark extract; these results have similar pattern to the data obtained for the phytochemical screening of A. floribunda stem bark fractions. The reduction of molybdenum (VI) to molybdenum (V) by the fractions was highest in the hydromethanol (205 μg/mL) followed by ethylacetate fraction (101.4 μg/mL) and then the dichloromethane (93.2 μg/mL). The hexane fraction had the lowest antioxidant activity (82.4 μg/mL). DPPH radical scavenging activity of the stem bark fractions of A. floribunda (Fig. 2b) was over 70% at a concentration range of 20 to 100 μg/ mL. An exception to this was the activity recorded for hexane fraction in which less than 60% DPPH radicals were inhibited at 100 μg/ mL. Table 3 shows the IC50DPPH of the various fractions as follow: ethylacetate (IC50DPPH = 14.62 ± 0.41), hydromethanol (IC50DPPH = 5.26 ± 0.55 μg/ mL) and dichloromethane (IC50DPPH = 18.42 ± 0.65). Ethylacetate, hydromethanol and dichloromethane fractions had better DPPH inhibiting ability compared to hexane fraction (IC50 DPPH = 37.55 ± 0.79 μg/ mL) which was less active against free radical generated by DPPH.
Antioxidants capacity and free radical inhibiting activity of A. floribunda stem bark fractions a Total antioxidant capacity of A. floribunda stem bark fractions; b DPPH radical scavenging ability of A. floribunda stem bark fractions. Data are expressed as mean ± SEM of results from three independent experiments. Bars with different lowercase letters represent significant difference between mean at p ˂ 0.05
The activity of the various fractions of A. floribunda stem bark with respect to inhibition of parasite growth is shown in Table 3 while Fig. 3 shows the dose response curve. Among the fractions, dichloromethane (DCM) had the best inhibitory potential against the P. falciparum parasite (IC50 Pf3D7 1.51 μg/ mL). DCM was closely followed by the hydromethanol (HMet) fraction (IC50 Pf3D7 5.26 ± 0.55) while that of ethylacetate (EAct) and hexane (Hxn) fractions were almost similar (IC50 Pf3D7 = 6 and 6.25 μg/ mL, respectively). In general, the in vitro antiplasmodial activity of the fractions followed the order: DCM > HMet > EAct > Hxn.
3.4 Gas chromatography with flame ionization detection (GC-FID) analysis on dichloromethane fraction (most active fraction)
Flavonoids profile of dichloromethane fraction showed the presence of higher level of kaempferol; other flavonoids found in high amount are catechin, naringenin, luteolin, epigallocatechin, quercetin and myricetin (Table 4). Table 5 shows the relatively abundance terpenes, they are azulene, α-pinene, pinene-2- ol, γ-terpinene, camphor, 1, 8-cineole, borneol, neryl acetate, β-caryophyllene and humulene. Humulene was significantly higher in the terpenes profile compared to others. Terpenoids profile showed the presence of low α-amyrin, and β-amyrin in the DCM fraction but their concentrations were still much higher than others like taraxerol, lupeol, bauerenol acetate (Table 6). The analyzed A. floribunda dichloromethane fraction was generally low in alkaloids and volatile organic constituents (Tables 7 and 8) compared with the flavonoids fingerprint. However, alkaloids such as coniine, coniceine, cassine, spectaline and volatile organic constituents like butanoic acid, 2-methyl butenoic acid were detected in relatively high amount compared to other alkaloids and volatile organic compounds in DCM fraction. See the chromatograms for all the phytochemical fingerprint analyzed in supplementary Fig. S1-S6.
(a) Dose response curve showing percentage parasite growth inhibition after 48 h exposure to different doses of A. floribunda leaf extract, stem bark extract and oil; (b) Dose response curve showing percentage cell viability after 48 h exposure to different doses of A. floribunda leaf and stem bark extracts in Vero cells; (c) Dose response curve showing percentage cell viability after 48 h exposure to different doses of doxorubicin in Vero cells. Data are expressed as mean ± SEM of results from three independent experiments.
4 Discussion
Allanblackia floribunda stem bark extract, leaf extract and oil were examined for their in vitro antiplasmodial activity, the stem bark and leaf infusions were thereafter assessed for their cytotoxic activity; fractionation of the most active extract (stem bark extract) was done with solvents of increasing polarity and phytochemical screening, in vitro antioxidant and antiplasmodial assays were further investigated on the various fractions, finally, phytochemical fingerprint analysis of the most active fraction (dichloromethane) was carried out using gas chromatography with flame ionization detector.
The result of this investigation showed that the inhibition of parasite growth by the plant extracts in vitro was dose dependent, the stem bark and leaf extracts had better antiplasmodial activities compared with the oil which had low activity against the strain of Plasmodium parasite used (Pf3D7). The stem bark extract of A. floribunda showed excellent antiplasmodial activity followed by the leaf extract. However, the oil extract had weak activity against Pf3D7 because the IC50 Pf3D7 was over 100 μg/ mL, hence inactive against the parasite in vitro. IC50 is the inhibitory concentrations of the drug/extracts that can cause 50% reduction in parasitaemia level. According to the classification of Rasoanaivo et al. [14], the stem extract is considered very active while the leaf extract, is active. This investigation confirms the report of Ayoola et al. [6] and Azebaze et al. [7] that A. floribunda has antiplasmodial activity [24, 25]. From this investigation, we could establish that the most active part of this plant against chloroquine sensitive P. falciparum is the stem bark extract.
The result of the cytotoxicity study showed that the stem and leaf extracts of A. floribunda were non-toxic compared with the standard cytotoxic drug, doxorubicin (an anthracycline antibiotic), which was found to be highly cytotoxic. The selectivity indices, a ratio of CC50 to IC50 Pf3D7 revealed that the two extracts were selectively active against the parasite at the concentrations used but the stem bark infusion was more selectively toxic to the parasite.
Among the four different fractions obtained from the stem bark extract, hydromethanol, ethylacetate and dichoromethane fractions showed the presence of more bioactive compounds including phenolics, terpenoids, tannins, flavonoids and quinones cardiac glycosides compared with hexane fraction which had the list phytochemicals. In vitro antiplasmodial activity showed that the dichloromethane fraction was highly active against the parasite compared with the ethylacetate, hydromethanol and hexane fractions. Literature report on the antimalaria activity of Allanblackia gabonensis, and Allanblackia monticola had parasite inhibition close to the data obtained for dichloromethane fraction against chloroquine sensitive Plasmodium falciparum [7]. Dichloromethane fraction significantly suppressed the growth of P. falciparum compared with ethylacetate, hydromethanol and hexane fractions. The better antimalarial activity of the dichloromethane fraction may be credited to its ability to extract highly active antiplasmodial compounds of flavonoids and terpenes’s origin that are intrinsic to A. floribunda stem bark compared with other solvents used for extraction.
GC-FID analysis of A. floribunda dichloromethane fractions showed significant levels of flavonoids (kaempferol, catechin, naringenin, luteolin, epigallocatechin, quercetin and myricetin) and terpenes (α-pinene, pinene-2- ol, γ-terpinene, camphor, 1,8-cineole, borneol, neryl acetate, β-Caryophyllene, and humulene). However, there appeared to be low levels of terpenoids, alkaloids and volatile organic compounds in the dichloromethane fraction. Some of the phytochemicals present in the dichloromethane fraction have been reported to be potent against P. falciparum [26,27,28]. The better antimalarial activity observed in dichloromethane fraction of A. floribunda agrees with the result obtained by Lekana-Douki et al. [29] who used different solvents to extract Tetrapleura tetraptera and Copaifera religiosa. These authors reported that the dichloromethane extract of the aforementioned plants had significant inhibition against parasite growth compared with other solvent used for extraction.
Literature has earlier reported that some of these compounds detected in dichloromethane fraction have strong inhibitory activity against chloroquine sensitive and chloroquine resistant strains of P. falciparum [30, 31]. Although the mechanism by which A. floribunda inhibited Pf3D7 growth has not been identified, however some malaria researchers have suggested possible ways by which the pharmacologically active compounds (flavonoids and terpenes) which were detected in A. floribunda can exhibit schizonticidal activity against Plasmodium parasite. Herbert et al. [32] reported that terpenes are capable of inhibiting Plasmodium parasite growth because of the structural similarity it has to the substrate needed for isoprenoids biosynthesis in Plasmodium parasite. Isoprenoids’ biosynthesis depends on the DOXP/2-C-methyl-d-erythritol-4-phosphate (MEP) pathway in Plasmodium falciparum [33] while in humans, isoprenoids are synthesized via the mevalonate pathway [34]. This made isoprenoid biosynthesis a potential target for P. falciparum especially in the presence of phytochemicals such as terpenes. Terpenes inhibit dolichol biosynthesis in the trophozoite and schizont stages and it may be acting through inhibition of the isoprenyl diphosphate synthases [32]. This family of enzymes catalyzes consecutive 1′-4 condensations of isopentenyl-pyrophosphate with the allylic substrate to form the linear backbone for all isoprenoid compounds, including prenylated proteins, prenylated quinones, and dolichol [35]. Due to the structural similarity terpenes have with the substrates required for dolichol synthesis, it might interfere with the parasite's biosynthesis of polyisoprenoids by competing with original substrates in enzyme–substrate reactions or by interfering with the mechanisms of elongation of isoprenic chains [32]. This metabolic interference may have accelerated parasite death when chloroquine-sensitive P. falciparum culture at ring stage were treated with A. floribunda stem bark infusion. Other possible mechanisms of parasite inhibition by the plant extracts could be through parasite enzyme inhibition (1, 5-lipoxygenase, carbonic anhydrase, and aspartic protease) by phenolics [31], prevention of merozoites invasion of erythrocytes by flavonoids, inhibition of haemozoin biocrystallization by alkaloids, inhibition of P. falciparum fatty acid biosynthesis and protein synthesis disruption by triterpenoids [36,37,38,39].
5 Conclusions
Findings from this study show that A. floribunda methanol stem bark and leaf extracts have some levels of activity against Plasmodium falciparum parasite and are relatively non-toxic to normal cells. Dichloromethane fraction was the most active fraction from the stem bark extract. Based on the GC-FID results, we are suggesting that flavonoids and terpenes may have been responsible for the excellent antimalarial activity of A. floribunda stem bark extract. This therefore supports the use of A. floribunda in the treatment of malaria in ethno-medicinal practices. The next phase of this study is to conduct activity guided isolation on the dichloromethane fraction to obtain the active antimalarial compound in A. floribunda stem bark.
References
Trampuz A, Jereb M, Muzlovic I, Prabu RM (2003) Clinical review: severe malaria. Crit Care 7(4):315–323. https://doi.org/10.1186/cc2183
World Health Orgnization (2018) World malaria report. Geneva: https://apps.who.int/iris/bitstream/handle/10665/275867/97892 41565653-eng.pdf?ua=1
Ajayi NA, Ukwaya KN (2013) Possible ACT resistant malaria in Nigeria – a report of 3 cases. Rev Soc Bras Med Trop 46(4):525–527
Wundermann GS, Osiki AA (2017) Currently observed trend in the resistance of malaria to artemisinin based combination therapy in Nigeria – a report of 5 cases. Int j trop dis health 21(2):1–5
.Ebohon O, Irabor F, Ebohon LO, Omoregie ES, (2019) Therapeutic failure after regimen with artemether-lumefantrine combination therapy: a report of three cases in Benin City Nigeria. Rev Soc Bras Med Trop 52:e20190163
Ayoola GA, Coker HA, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC (2008) Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res 7:1019–1024
Azebaze AGB, Teinkela JEM, Nguemfo EL, Valentin A, Dongmo AB, Vardamides JC (2015) Antiplasmodial activity of some phenolic compounds from Cameroonians Allanblackia. Afri Health Sci 15(3):835–840
.Boudjeko T, Ngomoyogoli KEJ, Woguia LA, Yanou NN, (2013) Partial characterization, antioxidative properties and hypolipidemic effects of oilseed cake of Allanblackia floribunda and Jatropha curcas. BMC Complem Altern M 13:352. https://doi.org/10.1186/1472-6882-13-352
Adu JK, Amoah E, Ayensu I, Osei-Djarbeng S, Peter J (2016) Phytochemical screening and antimicrobial.activities of the stem bark of Allanblackia parviflora Chev (Clusiaceae). J of phytopharmacol 5 (6) 215–219
Akpanika GA, Winters A, Wilson T, Ayoola GA, AA, Bello A, Hauck B, (2017) Polyphenols from Allanblackia floribunda seeds: identification, quantification and antioxidant activity. Food Chem 222:35–42. https://doi.org/10.1016/j.foodchem.2016.12.002
Trager W, Jensen JB (1976) Human malaria in continuous culture. Science 193:673–675
Olasehinde GI, Olusola O, Adegboyeg OA, Obasola EF, Neena V, Isaac OA, Adesola AA, Louis OE (2014) In vitro studies on the sensitivity pattern of Plasmodium falciparum to anti-malarial drugs and local herbal extracts. Mala J 13:63
WHO (2001) In vitro micro-test (Mark III) for the assessment of P. falciparum to, mefloquine, quinine, amodiaquine, sulfadoxine/pyrimethamineand artemisinin. Geneva, Switzerland: CTD/MAL/9720 Rev 2
Rasoanaivo A, Ravi P, Petitjean S, Ratsimamanga-Urverg A, Rakoto R (1992) Medicinal plants used to treat malaria in Madagascar. J Ethnopharmacol 37:117–127
Mosmann T (1983) Rapid colorimeter assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Hassan ES, Verma S, Srivastava SK, Dwarn S (2013) Activity guided isolation and characterization of antiplasmodial agents of some local medicinal plants. Nig J Basic Appl Sci. https://doi.org/10.4314/njbas.v21i3.2
Trease GE, Evans WC (1989) Pharmacognosy. 11thedition London Bailliere Tindall Ltd Pp. 60 -75.
Harborne JB (1973) Phytochemical Methods, London, Chapman and Hall, Ltd. Pp. 49–188
Brand-Williams W, Cuvelier ME, Berset C (1979) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28:25–30
Prieto P, Pineda M, Anguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of Vit E. Analytical Biochem 269:337–341
Millogo-Kone H, Lompo M, Kini F, Asimi S, Guissou IP, Nacoulma O (2009) Evaluation of flavonoids and total phenolic contents of stem bark and leaves of Parkia biglobosa (Jacq.) Benth. (Mimosaceae) – free radical scavenging and antimicrobial activities. Res J Med Sci 3(2):70–74
Ortan A, Popescu M, Gaita A, Dinu-Pirvu C, Campeanu G (2009) Contribution to the pharmacological study on Anethum graveolens, Dill (Apiaceae). Romanian Biotechnol Lett 14(2):4342–4348
Ngounou FN, Manfouo RN, Tapondjou LA, Lontsi D, Kuete V (2005) Antmicrobial diterpenoids alkaloids from Erythrophleum Suaveoleus. Bull Chem Soc Ethiop 19(2):221–226
Burkill HM (1985) The useful plants of West Tropical Africa. Royal Botanical Garden Kew 4:385–386
Odugbemi TO (2006) Outlines and Pictures of Medicinal Plants from Nigeria. University of Lagos Press, Lagos, Nigeria, p 137
Salehi B, Upadhyay S, Orhan IE, Jugran AK, Jayaweera SLD, Dias DA, Sharopov F, Taheri Y, Martins N, Baghalpour N, Cho WC, Sharifi-Rad J (2019) Therapeutic Potential of α- and β-Pinene. A Miracle Gift of Nature Biomol 9(11):738. https://doi.org/10.3390/biom9110738
Soré H, Sanon S, Hilou A (2018) Antiplasmodial properties of plants isolated flavonoids and their derivatives. Int. J Herb Med 6(5). 43–56. wwwflorajournal.com
Kamaraj C, Balasubramani G, Siva C, Raja M, BalasubramanianV., Raja RK, Tamilselvan S, Benelli G, Perumal P, (2017) Ag nanoparticles synthesized using b-caryophyllene isolated from murraya koenigii: antimalarial (plasmodium falciparum 3D7) and anticancer activity (A549 and HeLa cell lines). J Clust Sci 28:1667–1684
Lekana-Douki JB, Oyegue Liabagui SL, Zatra BJB, R, Lebibi J, Toure-Ndouo, FS, (2011) In vitro antiplasmodial activity of crude extracts of Tetrapleura tetraptera and Copaifera religiosa. BMC Res Notes 4:506. https://doi.org/10.1186/1756-0500-4-506
Zofou D, Kowa TK, Wabo HK, Ngemenya MN, Tane P, Titanji VPK (2011) Hypericum lanceolatum (Hypericaceae) as a potential source of new anti-malarial agents: a bioassay-guided fractionation of the stem bark. Malar J 10:167
Pham AT, Nguyen C, Malterud KE, Diallo D, Wangensteen H (2013) Bioactive favone-C-glycosides of the African medicinal plant Biophytumum braculum. Molecules 18:10312–10319
Herbert RG, Emília AK, Valnice JP, Alicia SC, Fulgencio AAD, Alejandro MK (2004) Terpenes Arrest Parasite Development and Inhibit Biosynthesis of Isoprenoids in Plasmodium falciparum. Antimicrob Agents Chemother 48(7):2502–2509
Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E (1999) Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573–1576
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343:425–430
Wang KC, Ohnuma S (2000) Isoprenyl diphosphate synthases. Biochem Biophys Acta 1529:33–48
Ravikumar S, Inbaneson SJ, Suganthi P (2012) In vitro antiplasmodial activity of ethanolic extracts of South Indian medicinal plants against Plasmodium falciparum. Asian Pac J Trop Biomed 1–9 Available at: www.elsevier.com/locate/apjtb
Kirby GC, O’Neil MJ, Philipson JD, Warhurst DC (1989) In vivo studies on the mode of action of quassinoids with activity against chloroquine resistant Plasmodium falciparum. Biochem Pharmacol 38:4367–4374
Dubar F, Egan TJ, Pradines B, Kuter D, Ncokazi KK, Forge D (2011) The antimalarial ferroquine: role of the metal and intramolecular hydrogen bond in activity and resistance. ACS Chem Biol 6(3):275–287
Wright CW (2009) Antiprotozoal Natural Products. In: Evans EC (ed) Trease and Evans Pharmacognosy, 16th edn. Edinburgh, Saunders, pp 428–434
Acknowledgements
The authors would like to acknowledge the Department of Biochemistry, university of Benin; Department of Basic Sciences, Benson Idahosa University; Department of Cell Biology, University of Lagos; Department of Biochemistry, National Institute of Medical Research and Metro Research and Biotechnology Africa Limited for their technical supports and research facilities.
Funding
This work received financial support from Obariase Foundation. Equally, the in vitro testing was materially supported by Dr. O.A. Adebesin, Department of Cell Biology and Genetics, University of Lagos.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FI, OE, NE and OT. O. The first draft of the manuscript was written by FI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval
The study was approved by the Institutional Ethics Review Committee, University of Benin (LS19114).
Data Availability
All data generated and analyzed are included in this research article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Irabor, F., Ebohon, O., Erhunse, N. et al. In vitro antiplasmodial activity, cytotoxicity, antioxidant action and GC-FID analysis of Allanblackia floribunda Oliv. SN Appl. Sci. 3, 820 (2021). https://doi.org/10.1007/s42452-021-04812-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04812-0