Abstract
This paper discusses the relationship between the elongation and compression behavior and microstructural changes under redox cycles of porous Ni(O)–YSZ cermets for solid oxide fuel cells (SOFC). Mechanical damage in SOFC and SOEC is one of the most important degradation factors governing the electrical performance of cells. Therefore, it is necessary to know the mechanical properties of each component material, such as elastic and deformation properties, in the operating environment. Particularly, of the Ni(O)–YSZ cermets which currently makes up 90% of the volume of the cell, with present mainstream anode supported SOFC and SOEC. Therefore, understanding the properties of the Ni(O)–YSZ cermets plays an important role in ensuring the performance of the entire SOFC and SOEC. In this study, the microstructural changes of Ni(O)–YSZ cermet by reduction, re-oxidation and re-reduction were observed in detail using microstructural observations and systematically compared with the dimensional change behavior. For the dimensional change behavior, a simple model considering the initial porosity and Ni content is proposed, which successfully predicts the dimensional change due to re-oxidation. Furthermore, Ni(O)–YSZ cermets with high Ni content show large initial dimensional changes, but the dimensional reversibility improves with increase of the number of redox cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One kind of next-generation high-performance energy conversion systems, solid oxide fuel cells (SOFC) and solid oxide electrolysis cells (SOEC) are made exclusively of solid components, including the electrolytes and electrodes. To ensure the long-term reliability and durability of SOFC and SOEC, it is extremely important to know the mechanical properties and deformation behavior of the materials in the SOFC and SOEC. Particularly, of the anode material which currently makes up 90% of the volume of the cell, with present mainstream anode supported SOFC. Therefore, understanding the characteristics of the anode plays an important role in ensuring the overall performance of the SOFC. Ni, with its excellent catalytic function, is commonly used as one of the key components of the anode. However, Ni (16.9 × 10–6 1/°C [1]) has large differences in thermal expansion coefficients (TEC) compared with oxide electrolytes (e.g., YSZ: 10.3 × 10–6 1/°C [1]), and Ni structures cannot be maintained because they aggregate easily in high temperature environments. This is one reason why mixtures of Ni and electrolyte (e.g., YSZ) components (Ni–YSZ cermet) are generally used as the anode material. The presence of YSZ can be suppressed the effect of the different thermal expansion coefficients from YSZ, and at the same time, YSZ, which is stable to environmental changes, forms a solid framework and supports Ni, which prevents Ni aggregation. The Ni–YSZ cermet is a porous body providing gas permeability and electrode reaction area. However, Ni, YSZ (composite backbone), porosity, and their microstructures vary greatly depending on the design concept behind the SOFC, and a systematic evaluation of a range of physical, thermal, and electrical properties is necessary. Therefore, various research institutions report the results of their research, mainly on mechanical properties and elongation properties due to operating environmental conditions. First, the following research has been reported on mechanical properties. Pihlatie et al. [2] evaluated the porosity dependence of the elastic modulus of NiO–YSZ and Ni–YSZ by the Impulse Excitation method. Their results showed that the elastic modulus decreases linearly with the increasing porosity. Radovic et al. [3] also used the Impulse Excitation method, and showed that it can be arranged by the exponential curve. As a result of conducting ring-on-ring method for fracture strength and fracture toughness, it was reported that these properties also follow an exponential law. Pihlatie et al. and Nakajo et al. [4] systematically evaluated the temperature dependence of elastic (Young’s) modulus. For NiO–YSZ, it is an almost constant value from room temperature to 180 °C and then rises till 250 °C. The maximum temperature investigated was 250 °C, and it tended to increase with increasing temperature. As for Ni–YSZ, it has been reported that it gradually decreases as the temperature rises. Watanabe et al. [5] and Huang et al. [6] also systematically examined and discussed the fracture behavior and creep deformation of Ni-based cermet porous bodies during Redox cycles. Wang et al. [7] built a 3D model based on the finite element method (FEM) to investigate the effect of inhomogeneous oxidation of anode on mechanical degradation of SOFC. The stress distributions and critical oxidation strains with considering inhomogeneous oxidation were compared to those of supposed homogeneous oxidation.
The following research has been reported on volume elongation properties. The model proposed by Klemensø et al. [8] is widely accepted regarding the deformation behavior of the anode. It was reported that the aggregated Ni particles are expanded by reduction, and the YSZ frame is broken to cause irreversible damage. Therefore, it is reported that the cell does not damage if the anode strain is within 0.2% under the Redox cycle. Several research groups are aiming to develop anode materials with oxidation strain within 0.2% even under the Redox cycle. Waldbilling et al. [9] measured the oxidation strains for Ni–YSZ cermets with different Ni content, and found no clear relationship between the Ni content and oxidation strain due to changes in the amount of pores generated during reduction. Pihlatie et al. evaluated the elongation behavior up to three times of re-oxidation treatment when fixing the Ni content and changing the porosity. As a result, it is reported that the elongation can be controlled by the porosity at the sintering stage, and the strain can be suppressed with a porosity of about 35%. In addition, Faes et al. [10, 11] investigated the change behavior of the elongation volume with one re-oxidation using the Ni content, porosity and Ni/YSZ grain size as parameters. The results showed that porosity at sintering may be one of the main factors for the elongation. They also reported that volume elongation can be suppressed when the initial porosity is greater than 35%, similar to Pihlatie et al. Their results show that the initial porosity does indeed represent a general trend in the amount of elongation. In the past few years, FIB-SEM and X-ray computed tomography based anode microstructure evaluation methods have also been developed and are being actively studied. In particular, Jiao et al. [12]succeeded in developing a method for quantitative comparison of anode microstructure changes after long-time operation by increasing the reconstruction size as large as possible, using 3D reconstruction based on FIB-SEM technology. Kim et al. [13] constructed a 3D FEM model and performed thermal stress analysis based on the results observed by FIB-SEM. It was found that the stress exceeding the fracture stress of 8 mol% YSZ appears mainly above 800 °C. The three-phase interface has been designed by quantitatively evaluating the degree of bending, connectivity, and microstructural change of the anode after redox [14,15,16]. In recent years, non-destructive visualization of the microstructural change behavior has been achieved by X-ray computed tomography and other techniques, and the basic research for long-life design has progressed dramatically [17,18,19].
Among them, X-ray absorption near-edge tomography [20, 21] and X-ray holotomography [22, 23] are synchrotron-based techniques that can completely reconstruct the three-dimensional microstructure of anodes with a resolution of less than 50 nm [24]. Holotomography has the advantage of being able to analyze relatively large samples due to the use of high energy X-rays; X-ray ptychography is a scanning technique, one of the coherent diffractive imaging (CDI) techniques, which uses the same coherent micrometer-sized X-ray The image is constructed by combining multiple far-field scattering patterns obtained with overlapping spots using a probe [25]. In addition, ptychography has shown excellent sensitivity to changes in mass density and the highest resolution of any X-ray imaging technique [26]. As mentioned above, research on methods to evaluate anode microstructure has progressed rapidly in the last few years. On the other hand, although the number of studies on the evaluation of the microstructure in the oxidized state has increased in recent years, there are only a few studies on the evaluation of the microstructure and macroscopic deformation in the oxidized and reduced states using the same specimens. In particular, because of the severe redox conditions in high-performance SOFC and SOEC, it is extremely important to develop a simple method to predict the microstructure and deformation state.
In this study, based on the results of these many previous studies, we evaluated the microstructural changes and deformation behaviors caused by redox cycling with Ni content and porosity as parameters. Then, by using multivariate analysis, a prediction equation for re-oxidation elongation considering Ni content and porosity was developed, and we succeeded in relating the microstructural change to macroscopic deformation.
2 Experimental
2.1 Observation of microstructure changes
2.1.1 Specimen preparation
Specimens were prepared from NiO powder (Sumitomo Metal Mining Co. Ltd., Tokyo, Japan; average particle diameter: 0.48 µm), 8YSZ powder (Tosoh Co. Ltd., Tokyo, Japan; average particle diameter: 90 nm), and cross-linked polymethyl methacrylate (PMMA) (Sekisui Plastics Co. Ltd., Osaka, Japan; average particle diameter: 2.5 µm).
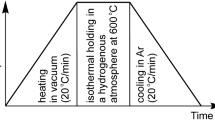
The NiO and 8YSZ powders were weighed so that the volume ratio of Ni:YSZ was 50:50 (vol%), and ground and mixed for 30 min in a planetary mill using water as a solvent. After the mixture was dried, it was crushed in an agate mortar, heated to 600 °C at a heating rate of 200 °C h−1 at atmospheric pressure, and calcined for 1 h. The PMMA was added to the obtained NiO, YSZ mixed powder to control the amount of pores. The volume fractions of Ni, 8YSZ and PMMA were 40:40:20 (vol%), 20:20: 60 (vol%), respectively. The powder was uniaxially compacted at 30 MPa using a carbide die of ϕ10 mm to produce cylindrical green pellets 10 mm in diameter and 5 mm in thickness. The NiO–YSZ green bodies were pressed at 100 MPa by cold isostatic pressing (CIP), and then heated to 1400 °C at 200 °C·h−1 at atmospheric pressure and sintered for 3 h. The produced NiO–YSZ was subjected to a reduction treatment for 12 h in an Ar–1%H2 condition at 800 °C, and a re-oxidation treatment was carried out for 12 h in a 100%O2 gas condition at 800 °C. After that, re-reduction treatment was performed for 12 h in an Ar–1%H2 atmosphere at 800 °C. In addition, specimens were weighted after the redox treatment to confirm the redox status. Schematic image of redox cycle process is shown in Fig. 1.
The treated specimens were immersed in UV-curable epoxy resins to fix the Ni(O)–YSZ structure. During the impregnation process, a vacuum treatment was performed for 30 min so that the pores were sufficiently impregnated with the resin. Next, the resin was irradiated with UV light cured for 1 h. The resin impregnated specimens were cut by a low-speed cutter to a size of approximately 5 × 3 × 3 mm. After mechanical polishing, the surface was finished with water-resistant polishing paper No. 2000.
2.1.2 FIB-SEM observation and 3D image reconstruction
The microstructure was observed using a focused ion beam scanning electron microscope (FIB-SEM: NVision 40, Carl Zeiss-SIINT). The angle between the electron beam and the ion beam is 54°. The specimen is adjusted so that it is normal to the FIB light source, and the FIB mills the resin to the observation surface while moving it in the Z-axis direction. When the polished surface appeared, a carbon coating with a thickness of 0.1 mm was applied to prevent the sample from being charged. After that, continuous SEM images of the xy plane were obtained by shaving about 10 mm in the z-axis direction using the FIB. The obtained cross-sectional SEM images were constructed in three dimensions using AVIZO 6 (Maxnet), an image processing software [16]. We aligned the images obtained by using markers processed on the specimen in advance. After that, a ternary process was performed to discriminate the three phases of Ni(O)–YSZ, and porosity based on the EDX spectra and the luminance values of the SEM images. The anode microstructure was reconstructed in three dimensions on the basis of the data obtained after the ternary processing as described above. Based on the reconstructed anode microstructure, the volume ratio of each phase, the surface area of each phase relative to the total volume of each phase, the three-phase interfacial length per specimen volume, the degree of connectivity, and the particle size distribution were calculated. The interfacial length was calculated using the domain extension method, which is based on the line lengths of the overlapping regions when the three phases (Ni(O)–YSZ, and porosity) are independently extended outward. As shown in Fig. 2, a line segment is extended in six directions from a point in the phase, and the distance between the line segment and the other phase boundaries is measured [14]. The average of the distances in the six directions obtained was calculated, and twice the distance was determined as the average particle size of the phase. Histograms of the local average grain size and the overall average grain size were calculated accordingly. If a line was extended in six directions and went out of the observation area, only the distance in that direction was ignored, and the average of the remaining directions was taken to determine the average particle size. The connectivity is defined as the ratio of the volume of the connected phase to the total volume, where the phase contacting one of the six faces of the rectangle is the connected phase and the phase not contacting any of the six faces is the isolated phase.
2.2 Elongation and compression testing
2.2.1 Specimen preparation
The powders used in this study had three different compositions with volume fractions of 15:85 (vol%), 30:70 (vol%), and 50:50 (vol%) of NiO:YSZ, prepared by the same method as in 2.1.1. The powder was uniaxially compacted at 30 MPa using a carbide die of ϕ20 mm and 3 mm thick to produce cylindrical green pellets 20 mm in diameter and 3 mm in thickness. The NiO–YSZ green bodies were pressed at 100 MPa by cold isostatic pressing (CIP) and then heated to 1400 °C at 200 °C·h−1 at atmospheric pressure and sintered for 3 h. The specimens were then cut by a low-speed cutter and polished to obtain NiO–YSZ specimens for the further experiments. The final size of the specimens is 2.3 × 0.5 × 14 mm.
2.2.2 Elongation and compression measurements
The measurements of the elongation coefficient was carried out using a volume dilatometer (BRUKER TD5000S), which is capable of controlling temperature (RT to 1000 °C) and atmospheric pressure. The temperature of the specimen is monitored by thermocouples located near the specimen and controlled by a heater to maintain the specified temperature. The gas atmosphere was maintained by using a mass flow controller to keep the gas in the chamber in which the specimen was set at a constant flow rate. In this study, 5% H2–95%N2 and 100%O2 were used as the specimen reduction and oxidation gases, respectively. When switching between the reducing gas and the oxidizing gas, 0.1% O2–99.9%N2 gas was used as the replacement gas, and the gas was switched after confirming that the gas in the chamber was sufficiently replaced. Cylindrical Al2O3 was used as a reference material for calibration. The displacement of the specimen was measured by securely fixing one end of the specimen in the longitudinal direction, contacting the probe to the other end, and measuring the displacement by linear variable differential transformer (LVDT).
In this study, elongation and compression tests were conducted in a gas atmosphere at 800 °C. 0.1% O2–99.9%N2 gas was supplied for the heating up process. The redox cycle test was started after the specimen temperature reached 800 °C and stabilized. As a first step, the NiO–YSZ is reduced with a reducing gas. The complete reduction of the specimens was proved by supplying the reducing gas, 5% H2–95%N2, into the chamber and confirming that the specimen dimensions were constant. The gas supply time is not specified because it depends on the density and dimensions of the specimen. For the subsequent re-oxidation process, 0.1%O2–99.9%N2 gas is supplied for 50 min after it is confirmed that the dimensions have been maintained, as described above. In this study, the gas was completely displaced, and 100%O2 was then supplied for the re-oxidation process. As with the reduction treatment, we confirmed that the specimens did not change in size, and then 0.1% O2–99.9%N2 gas was applied for 50 min. This cycle was repeated a maximum of six times to evaluate the dimensional changes. In addition, microstructural changes and dimensional changes were systematically evaluated by measuring the dimensions of the test pieces observed by FIB-SEM at room temperature.
3 Results and discussion
3.1 Microstructural observations
3.1.1 3D image reconstruction of the Ni(O)–YSZ microstructures
The porosity of NiO–YSZ after sintering was measured and the porosity was shown to be 11% and 32%, respectively. Following paragraph, the microstructural changes in NiO–YSZ with these two types of initial porosity will be discussed next. Reduction and re-oxidation conditions are shown in Fig. 1. FIB-SEM observations were performed on each specimen. Typical cross-sectional SEM images are shown in Fig. 3 (initial porosity 11%) and Fig. 4 (initial porosity 32%), respectively. Cross-sectional SEM images can be classified into four types of colors. From the elemental analysis, light gray, gray, and black or white indicate the Ni(O)–YSZ, and pores, respectively. The difference in color of the pores is due to the level of penetration of the resin. Black indicates the area completely impregnated with resin, and white indicates the area not fully impregnated with resin. The observed area size, volume fraction, particle size, specific surface area, contact area and connectivity are shown in Table 1 (initial porosity 11%) and Table 2 (initial porosity 32%), respectively. In calculating these parameters, the voids in the NiO were considered to be NiO.
All the specimens evaluated in this study were synthesized to have a Ni:YSZ volume ratio of 50:50 (vol%). On the other hand, the volume ratios obtained by the 3D reconstructions sometimes differed from the ideal volume ratios by up to 4% in some cases. The uneven distribution of the material when mixing the raw powder and the narrow observation area may be the cause. When more accurate 3D information is required, we need to increase the number of observations or expand the observation area.
In the present research, the connectivity of the Ni phase, YSZ phase, and gas phase was close to 100% in all specimens regardless of the initial porosity. In addition, almost no change in the connectivity of the re-reduced Ni–YSZ, and the electronic conduction path of Ni does not decrease in a single redox cycle. However, for the specimens with an initial porosity of 11%, micro cracks were observed between the YSZ particles during re-oxidation and reduction, suggesting that the re-oxidation of NiO caused damage to the YSZ frame. This suggests that the redox cycle has affected the mechanical properties, especially the elastic modulus and fracture strength, in the case of low porosity, and it is necessary to evaluate the mechanical properties after redox cycling in future.
3.1.2 Microstructure changes in Ni(O)
Based on the size distribution of grains obtained from the 3D reconstruction, microstructural changes in Ni(O) are discussed. Figure 5 (initial porosity 11%) and Fig. 6 (initial porosity 32%) show the grain size distribution obtained from the 3D reconstruction. As shown in Figs. 5a and 6a, the peak of the graph shifts to the left side of the mountain-shaped graph. In other words, this means that the size of the grains becomes smaller by reduction. The upper part of Table 3 (initial porosity 11%) and Table 4 (initial porosity 32%) shows the size change of Ni(O) grains obtained in the redox cycle test, where the size of the NiO/Ni grains shrank from 1.51 to 1.25 µm at the initial porosity of 11%. The initial porosity of 32% shrank from 1.36 to 1.19 µm as well. This was found to be in agreement with the observations carried out in a previous study by De Angelis et al. [17]. Therefore, in order to clarify the mechanism of grain size contraction as NiO grains become Ni particles during the first reduction, we first consider a simple spherical elongation/compression model.
Assuming that the NiO in the NiO–YSZ is a sphere, a comparative study was carried out to quantitatively evaluate whether the change in molar volume of NiO and Ni coincides with the actual change in grain size.
The volume of NiO in grain size DNiO is
where VNiO is the volume of NiO and DNiO is the grain size of NiO.
When NiO is completely reduced, the volume of reduced Ni is
where VNi is the volume of Ni, VmNiO is the molar volume of NiO, and VmNi is the molar volume of Ni, expressed as 10.7 (cm3 mol−1) and 6.6 (cm3 mol−1), respectively.
The grain size of Ni when NiO is reduced in DNiO is
where DNi is the grain size of Ni.
The grain sizes calculated using the above equations are shown in the lower part of Table 3 (initial porosity 11%) and 4 (initial porosity 32%). The results showed good agreement with the experimental and calculated data in both cases of 11% and 32% initial porosity. From these results, it was found that a simple spherical elongation/compression model could predict the Ni grain size during the first reduction. In other words, the first reduction does not cause Ni aggregation and can be discussed only in terms of molar volume change. The model is applicable to a wide range of initial porosity conditions because no Ni aggregation is observed at 11% initial porosity, where the possibility of Ni aggregation is high under certain conditions.
Next, we discuss the mechanism of grain size change by re-oxidation. The results shown in the upper part of Tables 3 (initial porosity 11%) and IV (initial porosity 32%) show that the grain size did not return to the initial NiO grain size, but increased by more than ten percent after re-oxidation. If a simple molar volume change model can be applied to the re-oxidation, the initial NiO grain size should return to that of the re-oxidized NiO grain size. There are two possible causes for this non-return. The first mechanism is that the oxidation of Ni is an outward diffusion process, which leads to the formation of internal vacancies in NiO during oxidation, leading to irreversible elongation. The second mechanism is that NiO expanded during oxidation contacts nearby NiO and aggregates [27]. Therefore, the simple model described above was used for verification. First, we would like to consider the first mechanism. The size of the NiO grains were calculated and compared with the experimental data, assuming that all of the Ni in the area was replaced by voids due to outward diffusion of Ni during re-oxidation. However, as shown in the SEM image of Figs. 3c and 4c, the void in the NiO is quite small, and it is not possible to replace all the original Ni with a void, so the calculated value calculated by this model is an upper limit, which should be larger than the experimental value in reality it is. If the experimental values are larger than the calculated values, it is necessary to consider other mechanisms.
So, the Ni volume of the grain size DNi is
when Ni is completely oxidized, the volume of NiO is
therefore, the grain size of NiO when Ni is oxidized in grain size DNi is
The grain sizes of NiO calculated using the above equations are shown in the lower part of Table 3 (initial porosity 11%) and Table 4 (initial porosity 32%). As a result, the initial porosity of 11% had the same size variation as the experimental values, and the experimental values for 32% porosity were lower than the calculated values. As mentioned above, the calculated value is the upper limit of the outward diffusion of Ni, and ideally, the experimental value should be smaller than the calculated value. On the other hand, for the 11% porosity sample, the calculated and experimental values are in agreement and cannot be explained by the mechanism of outward diffusion only. As mentioned above, the second mechanism, aggregation, may be responsible for this. Nakagawa et al. [27] observed the microstructural changes of Ni(O)–YSZ in a redox cycle condition in situ using an environment controlled SEM (E-SEM). On re-oxidation, aggregation with nearby NiO was observed due to the elongation of Ni due to oxidation. This result is supported by the SEM observation that 11% porosity is closer to the distance between NiO than to the 32% porosity. Therefore, it is reasonable to explain the 11% porosity by a mechanism that includes all changes in molar volume due to oxidation, outward diffusion, and changes in particle size due to aggregation. On the other hand, the molar volume change and outward diffusion were found to be the main factors in the 32% porosity size change mechanism.
Last, we discuss the mechanism of re-reduction, where 11% initial porosity was found to be smaller than that of the initial reduction. The reason for this is thought to be that the aggregated NiO is fragmented into several Ni at the YSZ frame when it is reduced and contracted during re-oxidation. On the other hand, 32% porosity is higher than in the initial reduction. It is reasonable to assume that this is due to the outward diffusion during re-oxidation, which leads to the irreversible deformation, and it is expected that these phenomena will be repeated in the microscopic region by the redox cycle. D. Sarantaridis et al. [28] had reported similar results, and the present results support them.
3.1.3 Microstructural changes in YSZ and pores
Next, the microstructural changes in the YSZ and pores are discussed. Similarly, Fig. 5b and c (initial porosity 11%) and Fig. 6b and c (initial porosity 32%) show the size distribution of the grains obtained from the 3-D reconstruction. However, 11% porosity re-oxidation resulted in smaller (about half) grain size. As mentioned above, the elongation of the NiO aggregation was observed during the re-oxidation of Ni, and it is assumed that the elongation caused large tensile stress or bending stress in the YSZ frames. This is supported by the fact that there was no change in grain size at 32%. Next, we would like to consider the pore size. The pore size tends to be reduced through re-oxidation and re-reduction. This is a result that supports the previous mechanism. Particularly in materials with low porosity, there is a concern that when the Ni particles are oxidized to NiO, they may become coarsened, which may lead to damage to the YSZ frame because the buffer of the pores is not sufficient to withstand it.
3.1.4 Elongation behavior due to a re-oxidation
The strain of Ni–YSZ due to a re-oxidation is shown in Table 4. The re-oxidation strain was calculated by the following equation.
where εre-oxi is the strain of NiO–YSZ due to re-oxidation, Loxi is the initial length of the specimen at 800 °C, and Lre-oxi is the length of the specimen after re-oxidation at 800 °C. Based on these results, the re-oxidation strain versus the porosity is plotted in Fig. 7. Figure 8 shows the re-oxidation strain with increasing porosity, at all Ni contents. In addition, the deformation measurement results using the test specimens used in Chapter 3.1 are also plotted. The re-oxidation strain increased with increasing Ni content when comparing the Ni content for the same level of porosity. However, as the porosity increases, the effect of Ni content on the amount of strain decreases: it is possible that the porosity may have a greater effect on the dimensional change than the Ni content. Pihlatie et al. and Faes et al. also showed that porosity is the main factor determining the elongation properties. However, looking at the results of this present research, the influence of Ni content on the elongation behavior is not negligible, so it is necessary to know the elongation behavior considering the effect of Ni content. Therefore, the present study was formulated using a multiple regression analysis with two variables, porosity and Ni content. Since the relationship between porosity and re-oxidation strain has been approximated experimentally by Faes et al. based on that equation, the following equation was used as a basic form to study the relationship.
where Vp is the porosity and a is assumed to be a material constant that depends on the Ni content. Multiple regression analysis of the relationship between Ni content and elongation was performed using linear, exponential, and exponential analysis, and it was found that the Ni content could be expressed as a power, since the correlation coefficient of the power was high. The value of b is a constant dependent on porosity from multiple regression analysis. Based on this, we can rewrite Eq. (3.8) to obtain Eq. (3.9).
substituting the experimental data from Table 3 into Eq. (3.9) and analyzing it, we finally obtained Eq. (3.10).
Figure 9 shows the linear relationship between the experimental and calculated values. Therefore, it is possible to predict the re-oxidation strain from the initial microstructure and Ni content from Eq. (3.9).
3.1.5 Elongation behavior due to multiple redox cycles
Eight specimens, VNi30–Vp7, VNi30–Vp35, VNi30–Vp38, VNi30–Vp39, VNi50–Vp29, VNi50–Vp34, VNi50–Vp36-1, and VNi50–Vp36-2, were subjected to 3–5 redox cycle tests to observe the elongation behavior. The results are summarized in terms of strains after reduction and oxidation as shown in Fig. 10. Similar to the single redox cycle test, specimens that had been subjected to multiple redox cycle tests tended to show an increase in strain as the porosity of the material decreased. In order to measure quantitatively how much the volume of the redox strain is restored by the reduction, the degree of strain restoration is shown in Fig. 11. Figure 12 shows the relationship between the number of reductions and the degree of recovery of strain by the reduction [degree of redox reversibility (DRR)].
The DRRN for the Nth reduction was calculated from the following Eq. (3.11).
where εNred and εN−1oxi are the strains during the Nth reduction and (N − 1)-oxi are the strains during the (N − 1)-oxidation (Fig. 12). The closer the DRR is to 1, the higher the reversibility and the less affected by the elongation during oxidation, even if the number of redox cycles is increased. In overall, the DRR was found to be higher in the 50 vol% range where the Ni content was relatively high. In addition, there was a tendency for DRR to improve compared to the initial stage. It is necessary to clarify this mechanism in future.
4 Conclusions
In this study, the microstructure and deformation behavior of Ni(O)–YSZ were evaluated to determine reductions in the mechanical degradation of SOFCs due to redox cycling with the ultimate goal of improving the reliability and durability of SOFC anodes. The microstructure of Ni(O)–YSZ was observed by FIB-SEM and the mechanism of the microstructural changes in Ni(O)–YSZ during reduction, re-oxidation, and re-reduction was investigated by using the initial porosity as a parameter. In the first reduction, only a simple sphere elongation/compression model, that is, a molar volume change, can be considered for a wide range of initial porosity. The initial porosity of 11% was found to be due to a combined mechanism of molar volume change due to oxidation, outward diffusion, and particle size change due to aggregation. The molar volume change and outward diffusion can be explained by the 32%. The 11% of the Ni aggregates that were found to aggregate in the re-reduction process were fragmented into several Ni at the time of compression. On the other hand, 32% of the Ni content was not returned to the original size due to outward diffusion during re-oxidation. The redox cycle may be repeated in the micro region. This is in general agreement with the results observed in some previous studies. In addition, the model proposed by A. Faes et al. was modified to accurately predict the dimensional change due to re-oxidation, taking into account the initial porosity and Ni content. Although the initial size change was larger at higher Ni contents, the size reversibility increased with the increase in the number of redox cycles. In future, research will be conducted to develop a model for predicting multiple redox cycle changes, and research will be carried out to design a system that takes other mechanical and electrical properties into account.
References
Mori M, Yamamoto T, Itoh H, Inaba H, Tagawa H (1998) Thermal expansion of nickel-zirconia anodes in solid oxide fuel cells during fabrication and operation. J Electrochem Soc 145:1374–1381. https://doi.org/10.1149/1.1838468
Pihlatie M, Kaiser A, Mogensena M (2009) Mechanical properties of Ni(O)-YSZ composites depending on temperature, porosity and redox cycling. J Eur Ceram Soc 29:1657–1664. https://doi.org/10.1016/j.jeurceramsoc.2008.10.017
Radovic M, Lara-Curzio E (2004) Mechanical properties of tape cast nickel-based anode materials for solid oxide fuel cells before and after reduction in hydrogen. Acta Mater 52:5747–5756. https://doi.org/10.1016/j.actamat.2004.08.023
Nakajo A et al (2012) Compilation of mechanical properties for the structural analysis of solid oxide fuel cell stacks. Constitutive materials of anode-supported cells. Ceram Int 38:3907–3927. https://doi.org/10.1016/j.ceramint.2012.01.043
Watanabe S, Sukino S, Miyasaka T, Sato K, Yashiro K, Kawada T, Hashida T (2020) Influences of Ni content and porosity on mechanical properties of Ni–YSZ composites under solid oxide fuel cell operating conditions. J Mater Sci 55:8679–8693. https://doi.org/10.1007/s10853-020-04624-4
Huang Y, Sato K, Kumada K, Hashida T (2019) Evaluation of creep properties in anode materials for solid oxide fuel cells by using small punch testing method. ECS Trans 91:761–769. https://doi.org/10.1149/09101.0761ecst
Wang Y et al (2020) Effect of inhomogeneous oxidation on the mechanical degradation of anode supported solid oxide fuel cell. J Power Sources 450:227663. https://doi.org/10.1016/j.jpowsour.2019.227663
Klemensø T, Chung C, Larsen PH, Mogensen M (2005) The mechanism behind redox instability of anodes in high-temperature SOFCs. J Electrochem Soc 152:A2186–A2192. https://doi.org/10.1149/1.2048228
Waldbillig D, Wood A, Ivey DG (2005) Thermal analysis of the cyclic reduction and oxidation behaviour of SOFC anodes. Solid State Ionics 176:847–859. https://doi.org/10.1016/j.ssi.2004.12.002
Faes A, Fuerbringer J-M, Mohamedi D, Wyser AH, Caboche G, J.V. herle, (2011) Design of experiment approach applied to reducing and oxidizing tolerance of anode supported solid oxide fuel cell. Part I: Microstructure optimization. J Power Soources 196:7058–7069. https://doi.org/10.1016/j.jpowsour.2010.07.092
Faes A, Nakajo A, Wyser AH, Dubois D, Brisse A, Modena S, Herle JV (2009) RedOx study of anode-supported solid oxide fuel cell. J Power Sources 193:55–64. https://doi.org/10.1016/j.jpowsour.2008.12.118
Jiao Z, Shikazono N (2016) 3D reconstruction size effect on the quantification of solid oxide fuel cell nickel–yttria-stabilized-zirconia anode microstructural information using scanning electron microscopy-focused ion beam technique. Sci Bull 16:1317–1323. https://doi.org/10.1007/s11434-016-1044-8
Kim JW et al (2018) Three-dimensional thermal stress analysis of the re-oxidized Ni–YSZ anode functional layer in solid oxide fuel cells. J Alloys Compd 752:148–154. https://doi.org/10.1016/j.jallcom.2018.04.176
Iwai H et al (2010) Quantification of SOFC anode microstructure based on dual beam FIB-SEM technique. J Power Sources 195:955–961. https://doi.org/10.1016/j.jpowsour.2009.09.005
Kubota M, Okanishi T, Muroyama H, Matsui T, Eguchi K (2015) Microstructural evolution of Ni–YSZ cermet anode under thermal cycles with redox treatments. J Electrochem Soc 162:F380–F386. https://doi.org/10.1149/2.0311504jes
Sumi H, Kishida R, Kim J-Y, Muroyama H, Matsui T, Eguchi K (2010) Correlation between microstructural and electrochemical characteristics during redox cycles for Ni–YSZ anode of SOFCs. J Electrochem Soc 157:B1747–B1752. https://doi.org/10.1149/1.3491345
De Angelis S, Jørgensen PS, Esposito V, Tsai EHR, Holler M, Kreka K, Abdellahi E, Bowen JR (2017) Ex-situ tracking solid oxide cell electrode microstructural evolution in a redox cycle by high resolution ptychographic nanotomography. J Power Sources 360:520–527. https://doi.org/10.1016/j.jpowsour.2017.06.035
Shearing PR, Bradley RS, Gelb J, Tariq F, Withers PJ, Brandon NP (2012) Exploring microstructural changes associated with oxidation in Ni–YSZ SOFC electrodes using high resolution X-ray computed tomography. Solid State Ionics 216:69–72. https://doi.org/10.1016/j.ssi.2011.10.0
De Angelis S, Jørgensen PS, Tsai EHR, Holler M, Fevola G, Bowen JR (2020) Tracking nickel oxide reduction in solid oxide cells via ex-situ ptychographic nano-tomography. Mater Charact. https://doi.org/10.1016/j.matchar.2020.110183
Kennouche D, Chen-Wiegart YK, Cronin JS, Wang J, Barnett SA (2013) Three dimensional microstructural evolution of Ni-–ttria-Stabilized Zirconia solid oxide fuel cell anodes at elevated temperatures. J Electrochem Soc 160:F1293–F1304. https://doi.org/10.1149/2.084311jes
Chen-Wiegart YK, Cronin JS, Kennouche D, Yakal-Kremski KJ, Barnett SA, Wang J (2013) Morphological evolution of solid oxide fuel cell in operational environment with X-ray nano-tomography. In: Meeting abstract, the electrochemical society. pp 765–765. https://ecs.confex.com/ecs/224/webprogram/Abstract/Paper24364/B6-0765.pdf
Villanova J, Laurencin J, Cloetens P, Bleuet P, Delette G, Suhonen H, Usseglio Viretta F (2013) 3D phase mapping of solid oxide fuel cell YSZ/Ni cermet at the nanoscale by holographic X-ray nanotomography. J Power Sources 243:841–849. https://doi.org/10.1016/j.jpowsour.2013.06.069
Laurencin J, Quey R, Delette G, Suhonen H, Cloetens P, Bleuet P (2012) Characterisation of solid oxide fuel cell Ni–8YSZ substrate by synchrotron X-ray nano-tomography: from 3D reconstruction to microstructure quantification. J Power Sources 198:182–189. https://doi.org/10.1016/j.jpowsour.2011.09.105
Cronin JS, Chen-Wiegart YK, Wang J, Barnett SA (2013) Three-dimensional reconstruction and analysis of an entire solid oxide fuel cell by full-field transmission X-ray microscopy. J Power Sources 233:174–179. https://doi.org/10.1016/j.jpowsour.2013.01.060
Dierolf M, Menzel A, Thibault P, Schneider P, Kewish CM, Wepf R, Bunk O, Pfeiffer F (2010) Ptychographic X-ray computed tomography at the nanoscale. Nature 467:436–439. https://doi.org/10.1038/nature09419
Holler M, Diaz A, Guizar-Sicairos M, Karvinen P, Färm E, Härkönen E, Ritala M, Menzel A, Raabe J, Bunk O (2014) X-ray ptychographic computed tomography at 16 nm isotropic 3D resolution. Sci Rep. https://doi.org/10.1038/srep03857
Nakagawa Y, Yashiro K, Sato K, Kawada T, Mizusaki J (2007) Microstructural changes of Ni/YSZ cermet under repeated redox reaction in environmental scanning electron microscope (ESEM). ECS Trans 7:1373–1380. https://doi.org/10.1149/1.2729241
Sarantaridis D, Chater RJ, Atkinson A (2008) Changes in physical and mechanical properties of SOFC Ni–YSZ composites caused by redox cycling. J Electrochem Soc 155(5):B467–B472. https://doi.org/10.1149/1.2883731
Acknowledgements
This research was conducted as part of the New Energy and Industrial Technology Development Organization (NEDO) (JPNP20003) on Fundamental study for a rapid evaluation method of SOFC durability in ‘‘Technology development for promoting SOFC Commercialization.’’ and, MEXT under Grants-in-Aid for Scientific Research (B) (No.16K14052, No. 16H04229).
Funding
This study was funded by New Energy and Industrial Technology Development Organization (NEDO) (JPNP20003) and, MEXT under Grants-in-Aid for Scientific Research (B) (No.16K14052), (No. 16H04229). The sponsors had no control over the design, interpretation, writing or publication of this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sato, K., Watanabe, S., Huang, Y. et al. Relationship between microstructure and deformation of porous Ni-based cermets under redox cycling. SN Appl. Sci. 3, 810 (2021). https://doi.org/10.1007/s42452-021-04789-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04789-w