Abstract
Mine tailings (MT) waste valorization in construction materials can be one possible solution because they may allow an alternative for some applications as an important contribution for a more circular economy. The aim of this work was to study the feasibility of using a sulfidic mine tailing in the production of building materials such as ceramic roof tiles. The introduction of 5, 10 and 20% MT in ceramic roof tiles promoted an improvement on the final properties of these materials. The use of 20%MT has decreased the firing temperature from 1150º to 1050 ºC, hence promoting energy savings and lower costs. Properties as density and water absorption were improved. Firing shrinkage, many times responsible for cracking, also decrease with the use of MT and, in this way, improve the production rate. The 20% MT ceramic formulation achieved the highest value of strength with lowest firing temperature. For the effects of sulphates' emission (SO2 and SO3 gases) upon firing, a solution was proposed involving their reaction with water and, through condensation, providing afterwards sulphuric acid as a process by-product. The use of high sulphide MT in ceramic roof tiles processing could be viewed as a potential safe waste management solution for these particular mine tailings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The world economy has been built based on a linear business model, which is now under threat due to the limited availability of natural resources. Circular economy is a concept that looks at the waste as an alternative raw material for natural ones. In summary, it is possible to reduce, reuse, recover, and recycle waste to reduce the exploitation of natural resources as well as production costs and increase job creation in order to reduce environmental problems.
Mine tailings (MT), resulting from mining extraction operations, are typically a mud-like material. Mine tailing storage is an important environmental issue, and these wastes need to be placed in environmentally controlled landfills, which occupies a lot of space [1, 2]. These wastes composition depends on the ore mineralogical composition as well as on the chemical and physical processes used for extracting the final product. Therefore, different industries who extract the same material, can produce wastes with different mineralogical compositions and, consequently, different physical and chemical characteristics [3]. Nevertheless, the great difficulty of using mine tailings is the presence of heavy metals and sulfates in their composition, although, the contents of these materials change with the mine and ore extraction process.
Several authors have studied the production mine tailing incorporation in different kind of building materials [4,5,6,7,8,9,10]. Concerning bricks production with MT, there were also experiments made by firing cement-based bricks or by alkali-activated (geopolymer) bricks [11]. Ahmari e Zhang [2] investigated bricks production through geopolymerization of copper mine tailings and concluded that geopolymer bricks could also immobilize heavy metals in structure. Onuaguluchi and Eren [4] applied dry copper mine tailing as an additive in mortars but the water absorption increased. Blessen et al. [12] have concluded that copper MT waste could be used for partial replacement of natural aggregates up to 60% replacement. Yliniemi et al. [13] used mine tailing to produce lightweight aggregates by granulation and after that used it successfully as aggregates in mortar and concrete compositions.
According to Argane et al. [14], the mortar alkaline pH values versus the MT acid pH value make cement mortars able to prevent heavy metal leaching. Previous studies [15,16,17] that Argane et al. [15] has considered, showed that cement matrices promote chemical and physical bonding for heavy metal retention.
Another solution was the use of MT in ceramic construction materials. Liu et al. [18] studied the feasibility of recycling tungsten mine tailings to produce a ceramic substrate. Mine tailing was the major component to prepare these ceramic substrates sintered at different temperatures (from 1000 to 1250 ºC). After sintering the best performance temperature were 1050 ºC according to the final properties’ values obtained. These authors indicated that tungsten mine tailing ceramic substrates can be a good application to reduce landfill disposal. Cetin et al. [19] used mine tailings (boron and basalt-rich rock) to produce ceramic substrate tiles. However, the ceramic substrate showed high water absorption and it was necessary to apply a glaze layer made also with a recycled borosilicate glass and soda lime glass. X-ray diffraction (XRD) analysis showed that samples are mainly constituted of silicon oxide, potassium aluminium silicate, calcium carbonate and calcium hydroxide for compositions without MT, while the composition with MT also present a small amount of iron sulphide (up to 4%).
Literature presents many other studies on mine tailing recycling, however, in most of these studies the sulphate content is very low or unreported. This work aims to demonstrate that it is possible to use high-sulfidic mine tailings in a safe and sustainable way, although it presents a very difficult challenge.
2 Materials and methods
2.1 Materials and formulations
Mine tailings coming from a copper mine exploration in southern Portugal, included in the Iberian pyrite belt (Fig. 1), were characterized in this work.
This particular mine tailings present about 30% of humidity and, because of that, they need to be dried before use in different applications. They also present a powder bulk density (according to EN 1097–3 standard) of 1.28 g/cm3.
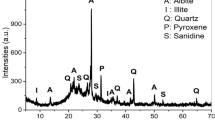
Figure 2 presents the mine tailing mineral composition obtained by X-ray diffraction (XRD). No relevant amount of amorphous material is found, which might indicate a low geopolymer reactivity potential and, on the other hand, the fact that alumina content is also low in this particular mine tailing results on a low potential to be alkali-activated, meaning to act as a geopolymer precursor, but rather to be used as an aggregate or filler in the quoted building materials. This fact is corroborated by the results of X-ray fluorescence (XRF) in Tables 1 and 2.
Indeed, from XRF this waste shows a low content of Al2O3 which is not favorable to geopolymerization (or alkali activation) process. SO3 and some heavy metals such as Pb, Cr, Zn, and Co were found, so this mine tailing needs to be carefully addressed in potentially safe applications as an alternative raw material. However, the XRD analysis showed that sulphur is mainly present in the form of pyrite (FeS2). FeS2 is not soluble in water but, even at room temperature, the oxidation of pyrite (FeS2), in the presence of air, promotes the oxidation of sulfur producing SO2. SO2 in contact with water, gives rise to an important common environmental problem in coal mining regions, the generation of acid mine drainage. H2SO4 (sulfuric acid) is formed, and iron is released in the form of Fe+2 and Fe+3. In the simultaneous presence of water and air, the classic equation of pyrite oxidation is [20]:
Figures 3 and 4 show the mineral composition obtained by X-ray diffraction (XRD) for the red and yellow clays used in the ceramic roof tile formulation. The main components are quartz and clay minerals (ilite, muscovite and kaolinite) that are phyllosilicates (normally hydrated aluminosilicates). Structurally, ilite is very similar to muscovite, but richer in silicon, magnesium and iron. The high iron content is responsible for the clay red color.
MT thermal analysis was also performed to determine the appropriate sulphate decomposition temperature in these mine tailings. Differential thermal analysis (DTA) measures the temperature difference between a thermally inert material (calcined alumina—Al2O3) and the sample material under study, when both materials are subjected to a temperature rise at a set heating rate (here 5 ºC/ min up to 1000 ºC). DTA curves reveal endothermic or exothermic transformations that occur in a material during heating or cooling. Energy changes related to these transformations can be related to phase transitions, solid state decompositions or reactions with an active gas, such as oxygen. Gravimetric thermal analysis (GTA) is an analytical method that allows the evaluation of mass variations that occur during the heating of the sample. Weight losses in the sample under study more commonly occur at different temperatures due to decomposition of compounds, loss of structural water or moisture.
The simultaneous analysis of DTA and GTA allows to classify the reactions related to decomposition or oxidation processes.
Regarding particle size distribution, MT exhibits a particle size distribution ranging from 2 μm to 200 μm. In a first analysis, using dry sieving, it was verified that 26% of the MT particles have dimensions higher than 63 μm up to 200 μm. The red and yellow clays presented 0.63% and 11.98% of particles higher than 63 μm, respectively. Clay minerals are usually a lot smaller than 63 microns, so the particles larger than 63 microns are mainly due to quartz presence in the clays. This fraction bigger than 63 microns can be found between 63 microns and 1 mm. Figure 5 shows the sand particle size distribution also used in the ceramic paste along with clays.
The determination of the particle size distribution of MT, for the fraction with dimensions smaller than 63 microns, was made using a Coulter LS230 equipment, which uses laser diffraction as an analysis methodology (Fig. 6). It is possible to consider that MT and red clay are very similar from their particle size distributions.
Normally, in the ceramic roof tiles production, the paste is extruded into slabs and then pressed (plastic pressing) to obtain the final shape. A ceramic roof tiles typical formulation consisting of 60% yellow clay, 30% red clay, 10% sand and 25% water was used as the reference composition (C_0%MT). Introduction of 0, 5, 10 and 20% of MT was made replacing part of the yellow clay and the obtained different compositions were fired at temperatures of 850º, 950º, 1050º, 1150º and 1200 ºC, in order to assess their properties variation with MT and firing temperature. Roof tile formulations prepared in this work to evaluate the role of MT are presented in Table 3.
2.2 Characterization methods
Cylindrical ceramic samples having about 15 cm long were prepared by plastic extrusion. Twelve samples of each composition were fired at a heating rate of 10 ºC/min and remained for one hour at the maximum sintering temperature. Ceramic roof tiles samples with and without MT were characterized in terms of flexural strength (bending test), according to standard NP EN 538 using an universal testing machine (FORM + TEST type Beta 2 3000D). These 12 samples of each formulation were used in each test. Firing shrinkage and firing weight loss were also measured. Water absorption and bulk density were measured on these samples, according to standard NP EN ISO 10,545–3.
3 Results analysis
MT thermal analysis was performed to determine the appropriate sulphate decomposition temperature in these mine tailings, and it was found that this reaction occurs around 700 ºC (Fig. 7). During thermal treatment, the following reactions may occur. At lower temperature one can have:
Then, around 480 ºC the following reactions occur:
And above 650 ºC, it results on:
Figure 7 shows the results from DTA/GTA thermal analysis where it is possible to observe an endothermic reaction after 600 ºC. Some authors [21] indicate this temperature as the thermal decomposition of pyrite. Thermogravimetry (GTA) analysis performed simultaneously indicated a weight loss at temperatures between 600 and 800 ºC.
Furthermore, in terms of characterization, XRD analysis of MT was also performed. Figure 8 shows the one relative to the MT-containing composition after calcining at 700 ºC. Above 700 ºC there are no sulphates in the mine tailing composition. Usually, SO2 and SO3 gases in contact with water can give rise to acid solutions (sulfuric acid) which can be environmentally dangerous.
Regarding the produced ceramic roof tiles, Table 4 shows the evolution of flexural mechanical strength with the increase of firing temperature for compositions with different MT contents. From Fig. 9 an increase in flexural strength is observed up to a certain temperature and then there is a decrease.
The temperature corresponding to the maximum flexural strength is the appropriate firing temperature for each composition. Iron oxide (such as hematite Fe2O3) has a lower softening and melting temperature, acting as a flux agent and, consequently, contributes to decrease the ceramic firing temperature with the increasing of MT content.
Looking to the 20%MT composition results, the higher flexural strength achieved was obtained at the firing temperature of 1050 ºC, while the reference formulation (0%MTP) and the 10%MTP formulation presents its higher flexural strength only at 1150 ºC.
Moreover, this 20% MT formulation shows the higher flexural strength of them all. These results are due to the higher iron content. The decrease in the mixture softening temperature promotes glassy phase production which, on cooling, aggregates the different materials acting as a binder. Flexural strength increases with the increase in MT content. The 20% MT ceramic formulation presents the highest value of flexural strength with at the lowest firing temperature (1050 ºC).
Regarding the 5% MT formulation, it was decided not to continue its evaluation because it did not show any improvement in properties when compared to the reference sample (0%MT).
Table 5 presents the firing shrinkage with the increase in firing temperature. The clay minerals are mainly responsible for the shrinkage during the firing of ceramic materials. Clay minerals are lamellar systems. They have water that is part of their structure between the layers. At temperatures above 500 ºC this water is removed, the layers start to get closer, promoting the shrinkage of the system. The partial substitution of yellow clay for mine tailing decreases the amount of clay minerals and, this way, decreases the shrinkage of the system. Indeed, the increase in iron content increases the amount of vitreous phase created acting as a flux and a binder. This increases the density and consequently decreases the water absorption. Thus, when clay is replaced by MT, it is possible to observe a decrease in shrinkage. The reference composition, without MT, presents the higher total shrinkage and 20%MT composition the lower one following the flexural strength variation. One should consider shrinkage since it can be responsible, if excessive, for material cracking during production, decreasing the company's productivity level.
Weight loss variation with temperature was also measured and presented in Table 6. Weight loss in the reference composition without MT is mainly due to the structural water from clay minerals. The increase of the weight loss with the increase in the MT content, besides the clay minerals water, is also due to the sulfate’s decomposition reaction since sulfate ions are released as gas around 600º–700 ºC.
Regarding the ceramics water absorption and porosity, they also followed the trend of the previous results in terms of showing that the firing temperature decreases, with the incorporation of MT, down to 1050 ºC with the 20%MTP composition (Tables 7 and 8), shown there by the lowest water absorption achieved. Bulk density shows a correspondent normal behavior with firing temperature for each composition. Since 20% MTP composition has the lowest water absorption at 1050 ºC (directly related with open porosity), it also presents the highest bulk density, defining so the best sintering or firing temperature.
Ceramic roof tiles can be used with or without the application of a ceramic glaze. Specifically, in this case, it is advisable to use ceramic glaze to guarantee the roof tiles total waterproofing. In the very low possibility that FeS2 still exists in the ceramic body, the ceramic glaze prevents the penetration of air (O2) and water into the material, avoiding its contact with the reminiscent FeS2.
This solution of waste valorization of mine tailings in ceramic roof tiles could be interesting but still presents the problem of sulphates’ emission content (SO2 and SO3 gases) during the firing process, that could damage the exhaust system besides their eventual emission to the atmosphere.
However, if the gas emissions, instead of going directly to the chimney, were redirected, from the oven to a condensation chamber, with a controlled temperature (T) and relative humidity (HR) (20 ± 5 °C and 95 ± 5% HR) atmosphere, this problem could be solved, providing at the same time, trough condensation, an acid solution as a by-product. During condensation following reactions occur:
The remaining effluent would go up to the chimney, that would have filters suitable for the remaining sulphates, if they were not fully condensed. The condensation chamber should be in borosilicate glass due to its chemical resistance and very low thermal expansion coefficient and it should also be designed according to the flow temperature and velocity.
4 Discussion
Looking at the overall waste management solution implications, the following aspects should be considered. This waste management solution is only an alternative but complementary option to other ones considered for mine tailings so far to minimize them, since they have a great impact not only in the environment but are a strong limiting factor on the lifetime of the mine itself and any complementary solution to reduce them is very important, if viable. Other solutions besides ceramic processing exists, for instance, incorporation in mortars [14]. However, this study has used a low sulphate mine tailing while the presence of high sulfidic mine tailings, such as the ones used here, can lead to severe sulphate-related problems in mortars that could be prevented if a high temperature processing (such as the ceramic one) is used. That was one main reason for attempting here this waste management solution instead of incorporating these MT in mortars.
Besides, this solution assures the same ceramic body properties at a lower firing temperature (around 10% less) which is important from the energy saving and economic point of view. On the other hand, a medium size ceramic plant of bricks or roof tiles produce, in average, 100 000 tons of product per year and the 20% replacement content, found to be technically viable for this ceramic formulation, becomes quite relevant. This is so, not only because of the 20 000 tons of mine tailings that would be used and avoiding its landfill deposition, but also for the 20 000 tons of natural raw materials of the ceramic formulation that would be saved to be explored. Moreover, the mine tailing landfill management involves working costs of at least 10 euros/ton which would mean, at this rate, around 200 000 € per year savings for the mine, which could be used on the alternative disposal of the mine tailings, including eventual transportation costs. To minimize this solution related transportation costs, a ceramic plant installed nearby the mine, or to be installed, could be a solution to minimize transportation and other costs.
5 Conclusions
This study has evaluated the possibility of a waste management solution using sulfidic mine tailings as raw material in ceramics roof tiles. The use of mine tailings in ceramic roof tiles promoted a flexural strength increase with the incorporation of MT content. This effect of MT also shows in other related final properties such as bulk density and water absorption. The 20% MT ceramic formulation presents the highest value of flexural strength with lowest firing temperature. MT inclusion decreases the energy costs because it considerably lowers the needed firing temperature of the roof tiles formulations.
Sulphate decomposition reaction (around 600º–700 ºC) and consequently the release of the sulfate gas is higher for the composition with 20% MT. However, if the kiln emissions are redirected to a condensation chamber it is possible to produce an acid solution as a process by-product, preventing it to go up to the chimney into the atmosphere.
From the point of view of the environmental impact prevention, one could say that this solution is only an alternative one to deal with the huge amount of mine tailings generated in mines throughout the world and it should specifically be applied at places that gather the specific conditions to make it a viable solution. If a ceramic plant is installed or could be installed at a 50 km radius from the mine site, then, this solution would be attractive not only from the energy savings point of view but also because of ceramic natural raw materials savings and because it would contribute to reduce the mine tailings landfill deposition, which is not only and environmental problem but also a limiting factor on the mine lifetime.
References
Adiansyah JS, Rosano M, Vink S, Keir G (2015) A framework for a sustainable approach to mine tailings management: disposal strategies. J Clean Prod 108:1050–1062. https://doi.org/10.1016/j.jclepro.2015.07.139
Ahmari S, Zhang L (2013) Durability and leaching behavior of mine tailings-based geopolymer bricks. Constr Build Mater 44:743–750. https://doi.org/10.1016/j.conbuildmat.2013.03.075
Ritcey GM (2005) Tailings management in gold plants. Hydrometallurgy 78:3–20. https://doi.org/10.1016/j.hydromet.2005.01.001
Onuaguluchi O, Eren Ö (2012) Recycling of copper tailings as an additive in cement mortars. Constr Build Mater 37:723–727. https://doi.org/10.1016/j.conbuildmat.2012.08.009
Moukannaa S, Loutou M, Benzaazoua M, Vitola L, Alami J, Hakkou R (2018) Recycling of phosphate mine tailings for the production of geopolymers. J Clean Prod 185:891–903. https://doi.org/10.1016/j.jnoncrysol.2018.12.031
Moukannaaa S, Nazaric A, Bagheric A, Loutou M, Sanjayanc JG, Hakkou R (2019) Alkaline fused phosphate mine tailings for geopolymer mortar synthesis: Thermal stability, mechanical and microstructural properties. J Non-Crystalline Solids 551:76–85. https://doi.org/10.1016/j.jnoncrysol.2018.12.031
Yildirim Ozen M, Moroydor Derun E (2019) A comparative study: Effects of different nanoparticles on the properties of gold mine tailings containing cement mortars. Constr Buil Maters 202:396–405. https://doi.org/10.1016/j.conbuildmat.2019.01.042
Kim Y, Lee Y, Kim M, Park H (2019) Preparation of high porosity bricks by utilizing red mud and mine tailing. J Clean Prod 207:490–497. https://doi.org/10.1016/j.jclepro.2018.10.044
Pyo S, Tafesse M, Kim BJ, Kim HK (2018) Effects of quartz-based mine tailings on characteristics and leaching behavior of ultra-high-performance concrete. Constr Build Mater 166:110–117. https://doi.org/10.1016/j.conbuildmat.2018.01.087
Kiventera J, Lancellotti I, Catauro M, Poggetto F, Leonelli C, Illikainen M (2018) Alkali activation as new option for gold mine tailings inertization. J Clean Prod 187:76–78. https://doi.org/10.1016/j.jclepro.2018.03.182
Zhang L (2013) Production of bricks from waste materials—a review. Constr Biuld Mater 47:643–655. https://doi.org/10.1016/j.conbuildmat.2013.05.043
Thomas BS, Damare A, Gupta RC (2013) Strength and durability characteristics of copper tailing concrete. Constr Build Mater 48:894–900. https://doi.org/10.1016/j.conbuildmat.2013.07.075
Yliniemi J, Paiva H, Ferreira VM, Tiainen J, Illikainen M (2017) Development and incorporation of lightweight waste-based geopolymer aggregates in mortar and concrete. Constr Build Mater 131:784–792. https://doi.org/10.1016/j.conbuildmat.2016.11.017
Argane R, Benzaazoua M, Hakkou R, Bouamrane A (2016) A comparative study on the practical use of low sulfide base-metal tailings as aggregates for rendering and masonry mortars. J Clean Prod 112:914–925. https://doi.org/10.1016/j.jclepro.2015.06.004
Choi W-H, Lee S-R, Park J-Y (2009) Cement based solidification/stabilization of arsenic-contaminated mine tailings. Waste Manag 29:1766–1771. https://doi.org/10.1016/j.wasman.2008.11.008
Choi YW, Kim YJ, Choi O, Lee KM, Lachemi M (2009) Utilization of tailings from tungsten mine waste as a substitution material for cement. Constr Build Mater 23:2481–2486. https://doi.org/10.1016/j.conbuildmat.2009.02.006
Cartiedge FK, Butler LG, Chalasani D, Eaton HC, Frey FP, Herrera E, Tittlebaum ME, Yang SL (1990) Immobilization Mechanisms in Solidification/Stabilization of Cd and Pb Salts Using Portland Cement Fixing Agents. Environ Sci Technol 24:864–873. https://doi.org/10.1021/es00076a012
Liu W, Wu T, Li Z, Hao XJ, Lu A (2015) Preparation and characterization of ceramic substrate from tungsten mine tailings. Constr Build Mater 77:139–144. https://doi.org/10.1016/j.conbuildmat.2014.12.094
Cetin S, Marangoni M, Bernardo E (2015) Lightweight glass-ceramic from the sintering of mine tailings. Ceram Int 41:5294–5300. https://doi.org/10.1016/j.ceramint.2014.12.049
Ayora C, Caraballo MA, Macias F, Rötting TS, Carrera J, Nieto J-M (2013) Acid mine drainage in the Iberian Pyrite Belt: 2. Lessons learned from recent passive remediation experiences. Environ Sci Pollut Res 20:7837–7853. https://doi.org/10.1007/s11356-013-1479-2
Yan J, Long Xu, Yang J (2008) A study on the thermal decomposition of coal-derived pyrite. J Anal Appl Pyrolysis 82:229–234. https://doi.org/10.1016/j.jaap.2008.03.013
Acknowledgements
This experimental work was carried out under the auspices of the GEOSULF ERA-MIN project, supported by the Finnish Funding Agency for Technology and Innovation (Tekes), Portuguese National Funding Agency for Science, Research and Technology (FCT) and the National Centre for Research and Development of Poland (NCBR), whom the authors wish to acknowledge. The authors wish also to acknowledge SOMINCOR for the supply of the mine tailings. The work was also supported by the Foundation for Science and Technology (FCT)—Aveiro Research Centre for Risks and Sustainability in Construction (RISCO), Universidade de Aveiro, Portugal [FCT/UIDB/ECI/04450/2020].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paiva, H., Simões, F., Maljaee, H. et al. Production of ceramic construction materials as an environmental management solution for sulfidic mine tailings. SN Appl. Sci. 3, 751 (2021). https://doi.org/10.1007/s42452-021-04735-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04735-w