Abstract
Centella asiatica is a commonly consumed green leafy vegetable in many developing countries due its high nutritious value and availability at low cost. The present study was conducted to assess the Cd, Cr and Pb uptake associated health risks of Centella asiatica harvested from organic and non-organic cultivations in a chronic kidney disease of uncertain etiology (CKDue) prevalent area in Sri Lanka. The health risk assessment was conducted in terms of the bioconcentration factor (BCF), soil-to-root and root-to-leaf translocation factors (\({\text{TF}}\,({\text{soil-root}})\) and \({\text{TF}}\,({\text{root-leaf}})\)), Target hazard quotient for each heavy metal (THQ) and hazard index (HI). In addition, the spatial variation of physical and chemical parameters of the root zone soil were assessed using MINITAB 17 statistical software. Results indicated significant spatial variations in conductivity, organic matter content and Cr concentrations among organic and non-organic study sites. The Cr, Cd and Pb concentrations recorded from roots and leaves of Centella asiatica were higher than the safe limits for consumption established by the European Union. The health risk analysis indicated that there is a potential of hyper-accumulating Cd in the roots of Centella asiatica. Further, the THQ and HI of the heavy metals indicated possible adverse non-cancer health risks associated with long-term consumption of leaves of Centella asiatica. Therefore, necessary precautionary actions to prevent the excessive buildup of Cr, Cd and Pb in the edible portions of Centella asiatica are essential in order to ensure consumer safety.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Chronic kidney disease is a significant health problem in rural agricultural communities in most of the developing countries [1, 2]. Majority of patients do not show any identifiable cause for the disease and therefore, it has been named CKD of unknown etiology (CKDue). In Sri Lanka, occurrence of CKDue was first recognized in the 1990s in the North Central Province of the country and now almost after two decades of first recognition, this disease has become a major public health issue of high priority in North Central Province with more than 50,000 estimated patients. The prevalence of this disease is now spreading to the adjoining provinces as well [3,4,5,6,7].
The World Health organization have suggested several hypotheses to explain the causative factors for the CKDue prevalent in the North Central Province of Sri Lanka. These hypotheses focus on several key factors including assessing As and Cd concentrations in agrochemicals, drinking water and commonly consumed vegetables in the disease prevalent areas [3, 4, 7].
However, research conducted to assess the heavy metal concentration in the major drinking and agricultural water sources the North Central Province of Sri Lanka have revealed that the concentrations of As, Pb, Cd and U in reservoirs and well water were lower than the maximum contaminant levels recommended by the USEPA [8,9,10]. Therefore, the possibility of drinking water as a source for CKDue is debatable and WHO has recommended continuing ongoing water quality surveillance, with a focus on As and Cd in the affected areas.
Regarding the concentration of nephrotoxic heavy metals in the vegetables growing in the area and agrochemicals used in the cultivations, the only “vegetable” analyzed in the WHO study was lotus root [10]. Although the results indicated high Cd content in lotus rhizomes harvested from CKDue prevalent areas in the North Central Province [8], no further studies were conducted on the contribution of contaminated vegetables toward the health risk of the community [7, 10,11,12].
Green leafy vegetables are also a very common dietary component of the Sri Lankan community. However, no research has been conducted to assess the uptake of heavy metals by the commonly consumed green leafy vegetables grown in the CKDue prevalent areas in Sri Lanka. Therefore, the present study focused on assessing Pb, Cd and Cr concentrations in the edible portions of a commonly consumed green leafy vegetable species, Centella asiatica harvested from a CKDue prevalent community in the North Central Province of Sri Lanka.
2 Methodology
2.1 Study area
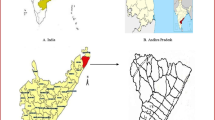
The study area is located in the North Central Province of Sri Lanka. North Central Province (NCP) is situated in the dry zone and it is the largest province in the country. NCP contains about 15% of the total land area of Sri Lanka and most of the land area is used for agricultural activities. NCP consists of two administrative districts, namely Anuradhapura District and Polonnaruwa District. Among these two administrative districts, the highest prevalence of CKDue was reported from the Anuradhapura District and it was recorded that 10% of the adult population in this district was affected from CKDue during the past decade [13]. Among the affected population, the highest number of patients were recorded from the Padaviya Medical Officer of Health (MOH), and therefore, the present study was conducted in Centella asiatica cultivation sites located in Padaviya.
Four Centella asiatica cultivation sites with an area of 100 m2 each were selected as study sites from Padaviya area, Sri Lanka. Sites A (80°47′24.354″E and 8°53′10.341″N) and B (80°48′31.169″E and 8°52′13.805″N) were non-organic cultivation sites which applied chemical fertilizer during the culture cycle of Centella asiatica. Sites C (80°49′13.999″E and 8°51′27.549″N) and D (80°47′15.788″E and 8°50′31.013″N) were organic cultivation sites that did not apply chemical fertilizer. The distance between any two sampling sites were about 8.5 ± 0.2 km.
2.2 Collection of samples
At the end of each culture cycle during dry (April 2019) and rainy seasons (October 2019), twenty plants and their root zone associated soil samples were collected randomly from each sampling site.
The sampled plants were stored in pre-distilled water rinsed polyethylene bags and transported to the laboratory in ice. In the laboratory, the plants were first washed with tap water and then washed with double distilled water to eliminate adsorbed dust and particulate matters. The plant samples were separated into leaves and roots. The roots and leaves were then cut separately and chopped into small pieces using a plastic knife. These chopped pieces were oven dried at 70–80 °C until they attained a constant weight. The dried samples were ground into powder using acid washed commercial mortar and pestle and then sieved through 0.425 mm mesh size sieve. The sieved samples were stored in desiccators until they were acid digested.
At each of the plant sampled locations, root zone soil samples (at 15 cm depth) were collected and were stored in prewashed polyethylene bags and transported to the laboratory in ice. In the laboratory, each soil sample was oven dried at 105 °C for 24 h and then, ground into a fine powder using acid washed commercial mortar and pestle and sieved through 0.425 mm mesh sieve. The sieved soil samples were stored in the polyethylene bags and placed in desiccators until they were subjected to acid digestion.
2.3 Analysis of heavy metals
The powdered sieved plant and soil samples (0.5 g) were acid digested at 270 °C for 3 h in the Kjeldatherm digestion system using concentrated HNO3 and HClO4 in 1:1 (v/v) ratio. The digested solutions were allowed to cool and 5 mL of distilled-deionized water were added to dissolve the precipitate formed on cooling and gently swirled and filtered into a 50 mL volumetric flask through Whatman filter paper no. 42 (125 mm). The clear solution was then diluted up to 50 mL with distilled-deionized water and was stored in acid washed polypropylene bottles until analyzed for heavy metals. The acid digested samples were analyzed for Cd, Cr and Pb using an Atomic absorption spectrophotometer (Analytic jenaModel novAA 400p) on graphite furnace mode following the procedure described in APHA (1998). The minimum detection limits were 0.02 mg/L for all the analyzed metals. Sandy loam certified reference material CRM 023 (Sigma-Aldrich, USA) were used as standard reference material for soil analysis and white cabbage certified reference material BCR 485 (Sigma-Aldrich, USA) were used as standard reference material for plant part analysis. Continuing control verification was done after every 10 samples to check that variability was within 10% [14].
Standard solutions of respective metals were prepared from the commercial standard calibration solutions (Reagecon Diagnostics limited, Ireland) for each metal separately using deionized water. Analytical grade HNO3 and deionized water were used as the blank solutions. Duplicate analysis was performed for each set of samples [14].
2.4 Physical and chemical parameters of soil
Soil pH, electrical conductivity, cation exchange capacity and organic matter content of soil collected from each sampling site at each sampling season were analyzed in the laboratory following the procedures described in APHA 1998 [14].
2.5 Data analysis
2.5.1 The bio-concentration factor (BCF) and translocation factor (TF)
Bio-concentration factor (BCF) is the ratio of the metal concentration in various parts of a plant to that in the soil [15]. If the BCF ≤ 1.00, it indicates the plant can only absorb but not accumulate metal. The plant may have potential to accumulate metal if the BCF > 1.00 [15].
BCF was calculated using the following formula as described by Sulaiman et al. 2016 [16]:
where Cplant is the concentration of metal in plant part and Csoil is the concentration of metal in soil [16].
The translocation capacity from the soil or root to the aboveground part of the plant (leaves), is estimated using the translocation factor (TF) and it was calculated using the following formulas as described by Sulaiman et al. 2016 [16]:
where \({\text{TF}}\,(R - L)\) is the translocation factor from roots to leaves, Cleaves is the concentration of metal in leaves and Croot is the concentration of metal in root. [16]
where \({\text{TF}}\,(S - R)\) is the translocation factor from soil-to-roots Croots is the concentration of metal in roots and Csoil is the concentration of metal in soil [16].
2.5.2 Target hazard quotient (THQ)
The potential health risk for heavy metal consumption through consumption of leaves of Centella asiatica was assessed by calculating the target hazard quotient (for adults and children) using the following formula as described by the United States Environmental Protection Agency [17, 18].
where \(E_{F}\) is the exposure frequency (156 days/year considering C. asiatica is included in the diet 3 days per week); \(E_{D}\) is the exposure duration (77 years, equivalent to the average lifetime of the Sri Lankan population); \(F_{IR}\) is the food ingestion rate (US EPA recommended average leafy vegetable consumption rates for adults and children is 2.2 g/person/day, respectively [19]); C is the metal concentration in the edible parts of vegetables (mg/kg(wet weight)); \(R_{FD}\) is the oral reference dose (Pb, Cd, Cr and Ni values were 0.0035, 0.001, 1.5 and 0.02 mg/kg/day, respectively [20, 21]); \(W_{AB}\) is the average body weight (70 kg for adults and 30 kg for children [22]) and \(T_{A}\) is the average exposure time for non-carcinogens (\(E_{D}\) × 365 days/year). A THQ value greater than 1, indicates that the exposure is likely to cause obvious adverse effects to human health [18].
2.5.3 Hazard index (HI)
The hazard index in the study sites were calculated as the sum of individual THQs for each metal [23].
2.6 Statistical analysis of data
Data were tested for normality using Anderson Darling test and the non-normalized data were log transformed. One-way analysis of variance followed by Tukey’s pairwise comparison was used to analyze the variation of physical and chemical parameters of root zone soil and the heavy metal concentrations of roots, leaves and root zone soil collected from the non-organic and organic cultivation sites.
Principal component analysis (PCA) was used to determine the soil quality parameters that characterizes the study sites during dry and rainy seasons. The data were analyzed using MINITAB 17 software. The statistical significance level was defined at p < 0.05. All the concentrations are expressed as mg metal /kg dry weight of the soil, root or leaves.
3 Results and discussion
3.1 Physicochemical parameters and heavy metal concentrations of root zone soil of Centella asiatica
Variation of physicochemical parameters and heavy metal concentrations in root zone soil of Centella asiatica is presented in Table 1. Mean soil pH of the study sites ranged from 6.49 to 7.15. Soil pH did not show significant variations among study sites during both seasons. Mean soil conductivity ranged from 72.20 to 174.60 µS/cm. Mean soil conductivity in the organic study sites were significantly higher than those of the non-organic sites during both seasons (ANOVA, Tukey’s pairwise comparison p < 0.05, Table 1). However, during the rainy season, there was a significant reduction in soil conductivity in all the sites (Table 1).
Mean percentage organic matter content of soil ranged from 2.66 – 6.94%. The organic matter content of the organic cultivation sites was significantly higher than that in the non-organic sites during both seasons (ANOVA, Tukey’s pairwise comparison p < 0.05, Table 1). The mean cation exchange capacity of soil ranged from 8.50 to 14.5 meq/100 g. There was no significant variation of cation exchange capacity of soil among the study sites during both seasons (ANOVA, p > 0.05, Table 1).
Mean Cd, Cr and Pb concentrations in soil ranged from 0.01 to 0.40, 10.56 to 28.70 and 2.75 to 6.12 mg/kg, respectively (Table 1). Mean Pb concentrations in soil did not show significant variations among study sites during both sampling events (ANOVA, p > 0.05, Table 1). However, mean Cr concentration in soil of non-organic sites were significantly higher than that of the organic sites during both sampling events (ANOVA, Tukey’s pairwise comparison p < 0.05, Table 1). Cd concentration in soil in the non-organic sites were significantly higher than that of the organic sites during the dry season (ANOVA, Tukey’s pairwise comparison p < 0.05, Table 1). However, during the rainy season, Cd concentration did not show significant variations among study sites (ANOVA, p > 0.05, Table 1). The pattern of heavy metal concentrations of soil varied as, Cr > Pb > Cd in both sampling events (Table 1). The Cd, Cr and Pb concentrations in soil during the rainy season were higher than those in the dry season (Table 1). The Cr, Cd and Pb can be added to the natural environment due to various reasons. Mainly, these are components of the agricultural chemicals and long-term application of agricultural chemicals can result is accumulation of these heavy metals in soil [24]. In addition, application of biosolids such as animal manure, compost can also lead to accumulation of some heavy metals such as Pb in soil [25]. The high concentrations of heavy metals in soil during the rainy season may have caused by accumulation of non-point source surface runoff. However, the soil Pb, Cd, Cr concentrations in all the study sites were below the levels recommended by European Union (EU) safe limits (300 mg/kg for Pb; 180 mg/kg for Cr and 6.4 mg/kg for Cd) (Table 1).
Principal component analysis showed categorization of study sites according to the soil quality parameters during dry and rainy seasons. Two principal components displaying a cumulative variance of 78.6% were obtained after applying PCA on soil quality parameters. PCA score plot for variation of soil quality parameters among the study during dry and rainy seasons is given in Fig. 1. The eigenvalues of the first two principal components, eigenvectors of the soil quality variables and the principal component scores for the study sites are given in Table 2. According to the results of the PCA on soil quality parameters, the non-organic cultivation sites during the dry season (Site A-Dry and Site B-Dry) were grouped together and were characterized by high Cr concentration in soil. Similarly, these two study sites in the rainy season were grouped together and were characterized by high Pb and Cd concentrations in soil. The organic cultivation sites (Site C and Site D) of the rainy season and site C in the dry season were categorized together and were characterized by high conductivity. Site C in the rainy season were separated by other sites and was characterized by high cation exchange capacity in soil (Fig. 1, Table 2).
3.2 Heavy metal concentrations in the roots of Centella asiatica
Mean concentrations of Cr, Cd and Pb in the roots of Centella asiatica collected from non-organic and organic cultivation sites are given in Table 3. The mean concentration of Cd, Cr and Pb recorded from roots ranged from 1.23 to 2.19, 3.00 to 15.98 and 0.49 to 2.88 mg/kg respectively (Table 3). There was no significant variation of the Cr, Cd and Pb concentrations in roots among the study sites during both sampling seasons. The Pb and Cd concentrations in the roots of Centella asiatica collected from all the study sites during the rainy season were significantly higher, than those of the dry season. The Cr concentration in the roots of Centella asiatica collected from the non-organic study sites were significantly lower during the rainy season compared to that of the dry season. However, Cr concentration in the roots of Centella asiatica collected from the organic study sites were significantly higher during the rainy season compared to that of the dry season. The mean Pb, Cd, Cr concentrations in roots of Centella asiatica in all the study sites were higher than the levels for consumption recommended by European Union (EU) (0.3 mg/kg for Pb; 2.3 mg/kg for Cr and 0.2 mg/kg for Cd) (Table 3).
3.3 Heavy metal concentrations in the leaves of Centella asiatica
Mean concentrations of Cr, Cd and Pb in the leaves of Centella asiatica collected from non-organic and organic cultivation sites are given in Table 4. The mean concentration of Cd, Cr and Pb recorded from roots ranged from 0.80 to 1.65, 3.09 to 6.17 and 0.83 to 3.93 mg/kg, respectively (Table 4). There was no significant variation of the Cd and Pb concentrations in leaves among the study sites during both sampling seasons. However, the Cr concentration in the leaves of Centella asiatica collected from the non-organic study sites were significantly higher than that of the organic study sites in both sampling seasons (Table 4). The mean Pb, Cd, Cr concentrations in leaves of Centella asiatica in all the study sites were also higher than the levels recommended by European Union (EU) safe limits for consumption (0.3 mg/kg.for Pb; 2.3 mg/kg for Cr and 0.2 mg/kg for Cd) (Table 1).
The Cr, Cd and Pb concentrations in the leaves of Centella asiatica were less than those in the roots in all sites at all the sampling events. Similar results were recorded by Tang et al. 2009, Ong et al. 2011 and 2015 [26,27,28]. The roots are the major route of taking up water and nutrients into the plants. Therefore, the roots are in direct contact with the soil solution which contains a mixture of nutrients, microorganisms and heavy metals, organic compounds and their derivatives. In addition, the presence of root hairs increases the absorption and adsorption abilities of roots and further enhance the contact with heavy metal cations in the soil solution. The metal cations that enter into the roots can be immobilized within the root or they can be subjected to temporary storage within roots until they are transported to the aerial parts of the plant. This may result in high concentrations of heavy metal ions in the roots compared to other parts of the plant [27].
3.4 Bioconcentration factor
The bioconcentration factor (BCF) of Cr, Cd and Pb in Centella asiatica during rainy and dry seasons is given in Fig. 2. The BCF of Cr, Cd and Pb in Centella asiatica during the dry season was higher than those in the rainy season. The BCF of Cd was higher than 1 in all the sites during both seasons. However, the BCF of Cr was lower than 1 in all the sites during both seasons. The BCF of Pb showed slight variations among study sites in both seasons (Fig. 2). BCF is an indicator used in environmental toxicology and risk assessment to determine the degree of intake and storage of toxic substances in animals and plants [29, 30]. A value higher than 1 for BCF indicates that particular species is a hyper-accumulator for heavy metals [29, 31, 32]. Therefore, the results of the present study also indicate that Centella asiatica is a hyper-accumulator species for Cd in both organic and non-organic cultivations.
3.5 Translocation factor
The soil-to-root translocation factor (TFsoil–root) of Cr, Cd and Pb in Centella asiatica sites during rainy and dry seasons is given in Table 5. In the dry season, the TFsoil–root of Pb and Cd did not show significant variation among non-organic and organic cultivation sites. However, the TFsoil–root of Cr in the dry season was significantly higher in the non-organic cultivation sites compared to that of the organic cultivation sites. Variation of the TFsoil–root of heavy metals showed a different pattern compared to the dry season. In the rainy season, the TFsoil–root of Pb, Cr and Cd were significantly higher in the organic cultivation sites compared to non-organic cultivation sites (Table 5, Student t test, p < 0.05).
The root-to-leaf translocation factor (TFroot–leaf) of Cr, Cd and Pb in Centella asiatica sites during rainy and dry seasons is given in Table 6. The TFroot–leaf of Cd and Pb did not show significant variations among the non-organic and organic cultivation sites during rainy and dry seasons. However, the TFroot–leaf of Cr in the organic cultivations were significantly higher than that of the non-organic sites during the dry season and was significantly lower in the rainy season (Table 6, Student t test, p < 0.05).
The TF is another factor that can be used to measure the hyper-accumulator potential of plants. Further, TF is used to comprehend the transport mechanisms of heavy metals in different plant components such as roots, stems and leaves [33, 34]. Similar to the BCF, the TF > 1 indicates that the species is a hyper-accumulator for the corresponding heavy metal [35]. In the present study, TFsoil–root for Cd was higher than 1 in both organic and non-organic cultivations during both seasons indicating hyper-accumulation of Cd in the roots of Centella asiatica. However, the TFroot–leaf of Cd were less than 1 indicating decreased translocation of Cd from roots to the leaves of Centella asiatica. Both TFsoil–root and TFroot–leaf of Cr and Pb were less than 1 indicating that these metals are not hyper-accumulated in Centella asiatica.
3.6 Target hazard quotient (THQ) and hazard index (HI)
The target hazard quotient and hazard index of the heavy metals for adults and children are given in Table 7. The values of THQ of Cr and Pb were less than 1 for both adult and child populations and these values were similar for both non-organic and organic cultivations. A THQ less than one indicates that there is no or negligible health hazard associated with long time consumption of Centella asiatica [17]. However, the THQ for Cd was slightly higher than 1 for both populations in organic and non-organic cultivations indicating possibility of causing significant health effects due to long-term consumption of Centella asiatica (Table 7). HI derived using target hazard quotients in both organic and non-organic cultivations was higher than 1.0 for both adult and child populations, and it indicates that there is a possibility of causing adverse non-cancer health effects over a lifetime of exposure [17, 23].
4 Conclusion
The cultivation type had no significant effect on heavy metal uptake or translocation within Centella asiatica. The mean Pb, Cd, Cr concentrations in the leaves and roots of Centella asiatica in all the study sites were higher than the safe concentrations for consumption recommended by European Union. The bioconcentration factor indicated that Centella asiatica is a hyper-accumulator of Cd. However, the soil-to-root translocation factor and root-to-leaf translocation factor indicated that there is a less potential of translocating Cd from roots to the leafy parts of Centella asiatica. Further, the target hazard quotient and hazard index indicated that there is a possibility of causing adverse non-cancer health risks associated with long-term consumption of leaves of Centella asiatica. However, as the roots of Centella asiatica has a potential to hyper-accumulate Cd, the consumers need to be advised to properly remove the roots from the plant before consumption. Further, it is advisable to regulate the use of Centella asiatica roots in ayurvedic medicines in order to prevent unforeseen ill health conditions in patients due to Cd.
Data availability
Data are available on request from the authors.
References
Ghosh R, Siddarth M, Singh N, Tyagi V, Kare PK, Banerjee BD, Kalra OP, Tripathi AK (2017) Organochlorine pesticide level in patients with chronic kidney disease of unknown etiology and its association with renal function. Environ Health Prev Med. https://doi.org/10.1186/s12199-017-0660-5
Kafle K, Balasubramanya S, Horbulyk T (2019) Prevalence of chronic kidney disease in Sri Lanka: a profile of affected districts reliant on groundwater. Sci Total Environ 694:133767. https://doi.org/10.1016/j.scitotenv.2019.133767
Abeysekera DTDJ, Kaiyoom SAA, Dissanayake SU (1996) Place of peritoneal dialysis in the management of renal failure patients admitted to General Hospital Kandy. In: Kandy society of medicine 18th annual academic conference.
Jayasumana C, Paranagama P, Agampodi S, Wijewardane C, Gunatilake S, Siribaddana S (2015) Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura. Sri Lanka Environ Health 14(6):1–10
Johnson S, Misra SS, Sahu R, Saxena P (2012) Environmental contamination and its association with chronic kidney disease of unknown etiology in North Central Region of Sri Lanka. Report from Centre for Science and Environment, New Delhi, India
Jayasumana MACS, Paranagama P, Amarasinghe M, Wijekon DVK, Fonseka SI (2011) Presence of Arsenic in pesticides used in Sri Lanka, Symposium of Water professionals’ Day, University of Peradeniya, October 01, Peradeniya, Sri Lanka
Kaur P, Gunawardena N, Kumaresan J (2020) A review of chronic kidney disease of unknown etiology in Sri Lanka, 2001–2015. Indian J Nephrol 30:245–252
Bandara JM, Senevirathna DM, Dasanayake DM, Bandara JM, Abeysekara DT, Rajapaksha KH (2008) Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (Tilapia). Environ Geochem Health 30(5):465–478
Chandrajith R, Nanayakkara S, Itai K, Aturaliya TN, Dissanayake CB, Abeysekera T, Harada K, Watanabe T, Koizumi A (2010) Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: geographic distribution and environmental implications. Environ Geochem Health 33(3):267–278. https://doi.org/10.1007/s10653-010-9339-1
Jayatilake N, Mendis S, Maheepala P, Mehta FR (2013) Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. https://doi.org/10.1186/1471-2369-14-180
Jayasekara J, Dissanayake D, Adhikari S, Bandara P (2013) Geographical distribution of chronic kidney disease of unknown origin in north central region of Sri Lanka. Ceylon Med J 58(1):6–10. https://doi.org/10.4038/cmj.v58i1.5356
Herath C, Jayasumana C, De Silva PMCS, De Silva PHC, Siribaddana S, De Broe ME (2018) Kidney diseases in agricultural communities: a case against heat-stress nephropathy. Kidney Int 3(2):271–280
Paranagama DGA, Bhuiyan MA, Jayasuriya N (2018) Factors associated with Chronic Kidney Disease of unknown aetiology (CKDu) in North Central Province of Sri Lanka: a comparative analysis of drinking water samples. Appl Water Sci. https://doi.org/10.1007/s13201-018-0792-9
APHA (1998) Standard methods for the examination of water and wastewater, American Public Health Association, American Water works Association, Water Environment Federation Publication, Washington, DC, USA, 20th edition
Liu WX, Liu JW, Wu MZ, Li Y, Zhao Y, Li SR (2009) Accumulation and translocation of toxic heavy metals in winter wheat (Triticum aestivum L.) growing in agricultural soil of Zhengzhou, China. Bull Environ Contam Toxicol 82(3):343–347
Sulaiman FR, Mustaffa NFS, Mohd-Khazaai SN (2016) Preliminary assessment of selected metals in agricultural soils in Jengka, Pahang, Malaysia. Environ Earth Sci 75(3):223
Antoine JMR, Fung LAH, Grant CN (2017) Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol Rep 4:181–187. https://doi.org/10.1016/j.toxrep.2017.03.006
Zhou H, Yang W, Zhou X, Liu L, Gu J, Wang W, Zou J, Tian T, Peng P, Liao B (2016) Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int J Environ Res Public Health 13(3):289. https://doi.org/10.3390/ijerph13030289
Ametepey ST, Cobbina SJ, Akpabey FJ, Duwiejuah AB, Abuntori ZN (2018) Health risk assessment and heavy metal contamination levels in vegetables from tamale metropolis, Ghana. Int J Food Contam. https://doi.org/10.1186/s40550-018-0067-0
US EPA (2002) A review of the reference dose and reference concentration processes. Prepared for the Risk Assessment Forum U.S. Environmental Protection Agency, Washington, DC, 192 pp
Hang X, Wang H, Zhou J, Ma C, Du C, Chen X (2009) Risk assessment of potentially toxic element pollution in soils and rice (Oryza sativa) in a typical area of the Yangtze River Delta. Environ Pollut 157(8–9):2542–2549. https://doi.org/10.1016/j.envpol.2009.03.0022542-2549
Walpole SC, Prieto-Merino D, Edwards P, Cleland J, Stevens G, Roberts I (2012) The weight of nations: an estimation of adult human biomass. BMC Public Health. https://doi.org/10.1186/1471-2458-12-439
Gupta S, Jena V, Jena S, Davić N, Matić N, Dragan R, Solanki JS (2013) Assessment of heavy metal contents of green leafy vegetables. Croat J Food Sci Technol 5(2):53–60
Najib Z, Wahidatul N, Syakirah M, Saffaatul I, Ahmad W, Amneera WA (2012) Assessment of heavy metal in soil due to human activities in Kangar, Perlis, Malaysia. IJCEE-IJENS 12(6):28–33
Yunus SM, Hamzah Z, Ariffin NAN, Muslim MB (2014) Cadmium, chromium, copper, lead, ferum and zinc levels in the cockles (Anadara granosa) from Kuala Selangor, Malaysia. Malay J Anal Sci 18:514–521
Tang Y, Qiu R, Zeng X, Ying R, Yu F, Zhou X (2009) Lead, zinc, cadmium hyperaccumulation and growth stimulation in Arabis paniculata Franch. Environ Exp Bot 66(1):126–134. https://doi.org/10.1016/j.envexpbot.2008.12.016
Ong G, Yap C, Maziah M, Tan S (2011) Heavy metal accumulation in a medicinal plant Centella asiatica from peninsular Malaysia. J Biol Sci 11(2):146–155. https://doi.org/10.3923/jbs.2011.146.155
Ong GH, Wong LS, Tan AL, Chee KY (2016) Effects of metal-contaminated soils on the accumulation of heavy metals in gotu kola (Centella asiatica) and the potential health risks: a study in Peninsular Malaysia. Environ Monit Assess 188:40. https://doi.org/10.1007/s10661-015-5042-0
Sulaiman FR, Hamzah HA (2018) Heavy metals accumulation in suburban roadside plants of a tropical area (Jengka, Malaysia). Ecol Process 7:28. https://doi.org/10.1186/s13717-018-0139-3
Amin H, Arain BA, Jahangir TM, Abbasi MS, Amin F (2018) Accumulation and distribution of lead (Pb) in plant tissues of guar (Cyamopsis tetragonoloba L..) and sesame (Sesamum indicum L.): profitable phytoremediation with biofuel crops. Geol Ecol Landsc 2(1):51–60. https://doi.org/10.1080/24749508.2018.1452464
Yang Y, Nan Z, Zhao Z (2014) Bioaccumulation and translocation of cadmium in wheat (Triticum aestivum L.) and maize (Zea mays L.) from the polluted oasis soil of Northwestern China. Chem Spec Bioavail 26(1):43–51
Negussie AB, Endale TM (2015) Quantitative determination of the heavy metal levels in the wild edible plant parts and their corresponding soils of the central and western regions of the Oromia state, Ethiopia. J Environ Anal Toxicol. https://doi.org/10.4172/2161-0525.1000299
Srivastava DK, Prakash S, Adhish V, Nair KS, Gupta S, Nandan DA (2009) study of interface of ASHA with the community and the service providers in Eastern Uttar Pradesh. Indian J Public Health 53(3):133–136
Usman K, Al-Ghouti MA, Abu-Dieyeh MH (2019) The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci Rep. https://doi.org/10.1038/s41598-019-42029-9
Prabasiwi DS, Sukirno MS, Rozana K (2020) Transfer factor as indicator of heavy metal content in plants around adipala steam power plant. IOP Conf Ser J Phys 1436:012133. https://doi.org/10.1088/1742-6596/1436/1/012133
Acknowledgements
The authors wish to acknowledge the Department of Zoology and Environmental Management, University of Kelaniya, Sri Lanka and International Foundation for Science (IFS), Sweden.
Funding
The research project was funded by Grant I-3-E-6048-1 by the International Foundation for Science (IFS), Sweden.
Author information
Authors and Affiliations
Contributions
WMDNW designed the experiment, analyzed the data and wrote the manuscript. EACSK assisted in experimental design, collected and analyzed the samples and assisted in writing the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wijeyaratne, W.M.D.N., Kumari, E.A.C.S. Heavy metal concentrations in the edible portions of Centella asiatica: Health risk toward chronic kidney disease of uncertain etiology. SN Appl. Sci. 3, 658 (2021). https://doi.org/10.1007/s42452-021-04666-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04666-6