Abstract
The present study focused on pollution status of groundwater in the industrial areas of Challawa and Sharada in Kano city based on pollution indices, statistical and spatial analyses. Twenty groundwater samples representing groundwater of the studied areas (Ten from each area) were analyzed for the presence of Cd, Cr, Ni, Fe, Mn and Zn using atomic absorption spectrophotometer. The result showed 95%, 5%, 60%, 15% and 25% of the analyzed water samples had detectable Cd, Cr, Ni, Fe, and Mn above the drinking water limits of both Nigerian standards for drinking water quality NSDWQ and World Health Organization (WHO) with Cd dominating other analyzed heavy metals in the groundwater. Evaluation of heavy metal pollution revealed a low polluted status based on the contaminant index (Cd), synthetic pollution index, heavy metals evaluation index, and heavy metal pollution index. Metal index categorized the groundwater as seriously polluted. The statistical evaluation gave strong and positive correlations between indices and a moderate one between the metallic ions. Component analysis revealed a strongly positive loading of Fe, Ni and Zn while Cd had a strong negative loading. Cr and Mn were positive and moderately loaded. Statistical analyses suggested both anthropogenic and geogenic sources for the heavy metals mainly from the industrial and agricultural practices and rock weathering processes, respectively. This study is expected to be a useful tool in the planning, monitoring and mitigation of pollution activities in the area.
Article Highlights
-
The pollution status of groundwater with respect to heavy metals was investigated in the Challawa and Sharada industrials zones in Kano city Nigeria
-
The concentration of Cd, Fe, Ni, Cr Mn and Zn was determined using the AAS
-
Different Pollution indices of HPI, HEI, SPI, Cd and MI were utilized to categorized the area as low, medium and highly polluted.
-
Spatial and temporal distribution maps demarcated based on the metal concentrations and computed indices in the area.
-
CA, PCA, and HCA were used to identified the geochemistry, relationship, sources and origin of heavy metals in groundwater.
-
The study revealed zones with low to high-risk groundwater in terms of toxic heavy metals and pollution status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The safety, purity and potability of water both surface and ground are constantly being threatened by both natural and anthropogenic processes [1]. These natural processes which are mostly geologic in nature include among others rock weathering, volcanic process. As reported by [2] pathways of heavy metals in the environment are mainly ground deposition, surface runoff, dry and wet depositions, air and gas exchange processes as well as groundwater. Anthropogenic processes capable of degrading the quality of water include among others Industrial processes such as the release of partially/untreated industrial effluents, mining and metal processing activities, sewage and waste disposal, agricultural activities like pesticides, herbicides application, the use of metal-based fertilizers, manures and poultry byproducts [3,4,5,6,7]. These processes and activities contribute large quantity of heavy metals into the water sources with some of these metals being toxic, poisonous and hazardous and hence life threatening to both humans and animals in the environment. When water containing these toxic trace metals is consumed, it can affect the well-being of people. Being non-biodegradable, the toxic metals when consumed remain in the body system through the process of bioaccumulation where they attack major and vital delicate organs which finally lead to their malfunctions [8]. Apart from being carcinogenic, the enrichment of trace heavy metals in water can lead to many ailments in humans. At very high concentrations trace metals can cause the malfunctions of many organs in the human body. The itai-itai disease which is Cd-related disease is very painful and can result in wastage and embrittlement of bones [9]. Chronic exposure to the metal can lead to kidney disorders, anemia, emphysema, anosmia (loss of sense and smell), cardiovascular diseases, renal problems, and hypertension. Chromium at low level of exposure can irritate the skin and cause ulceration. Long-term exposure can cause kidney and liver damage, as well as damage to both circulatory and nerve tissues, nose ulcers, asthma, change in DNA, hemolysis, carcinoma, damage to liver and kidney. Excess nickel can be mildly toxic. A long-term exposure to nickel can cause decrease in body weight, chronic bronchitis, heart and liver damage, skin irritation, impaired pulmonary function, fibrosis, and emphysema [9, 10]. High exposure to manganese through drinking water can cause adverse effects by causing neurological problems, tremor, psychological symptoms such as irritability. The deficiency of Mn can lead to a decreased ability to store and use thiamin which is vitamin B1. Some authors believed that Parkinson’s disease may be casually related to Mn [11].
According to [12] heavy metals like Pb, Cd, Hg, Zn, Cu, Cr, Ni, and As are common "pollution elements" produced through modern urban, industrial, and agricultural processes. The discharge of partial and untreated tannery effluents and associated toxic compounds into a river system can be a threat to public health and is an issue of concern to the environment. The human population living in close proximity to the Challawa river is affected by tannery wastes, plastic wastes and chemicals. Metals that are released by the industries in the area may pollute the soil, leach into the water table and contaminate the water to be used for drinking and irrigation purposes. The process can lead to the degradation and change in the quality of drinking water. [13] pointed out that with the increase in the concentrations of heavy metals in the environment, the capacity of soils towards retaining those metals decreases and thus will facilitate their leaching into groundwater and soil solution.
The presence of heavy metals in high concentrations in groundwater has necessitated the use of different mathematical and statistical models in the evaluation of the contamination and pollution levels as well as the evaluation of the associated health risk of such toxic heavy metal’s concentration levels in groundwater. All water samples would contain individual elements in different concentrations. Therefore, the quality of any water to be utilized for a particular purpose will depend on the concentration value of chemical parameters present in the water compared to their desirable and permissible limits as per national and World Health Organization standards [14]. A comprehensive quality assessment that will completely take into consideration all the effects of individual water chemical constituents can be provided by the Indexing approach.
Several heavy metals Indexing methods have been proposed and used by different scholars in the assessment of pollution status and quality of water in respect to heavy metals concentration in both surface and groundwater globally [6, 7, 15,16,17,18,19,20,21,22,23,24,25,26,27]. However, [16] showed that the indexing method can provide a composite picture on the aggregate impact of each heavy metal on the overall water quality. Pollution indices are effective tools used by executives, environmental managers, stake holders as well as decision makers for water quality assessment, because it has the advantage of measuring the combined influences of the entire detected chemical parameters in given water sample as it affects the quality of water. Statistical techniques have assisted greatly in the evaluation and interpretation of complex data matrices for understanding water quality and a variety of environmental factors as well as sources identifications of pollutants in groundwater. Multivariate Statistical approach is used to identify the origin or sources of pollution in groundwater, through the relationship that exists between the different chemical components of groundwater [28] used the hierarchical cluster and principal components analyses for the clustering of sampling sites and the identification pollution sources, respectively.
Several studies on the heavy metals’ concentrations in soils, surface water and industrials effluents have been conducted at different locations by several scholars within Kano town and environs including the industrials areas. Among these are the works of [8, 29,30,31,32,33,34,35,36,37,38,39,40,41]. It is on this background that the idea of this study was conceived, for the very first time in the area of present study, the pollution status of groundwater with respect to heavy metals will be attempted with the following objectives in mind 1. Assessment of heavy metals pollution by the indexing approach using indices like the heavy metal pollution index (HPI), heavy metal evaluation index (HEI), contamination degree (Cd), synthetic pollution index (SPI) and metal index (MI) 2. The use of multivariate statistical approach to show the relationship between heavy metals in groundwater, their sources as well as the prediction of their origin by using statistical tools such as the correlation matrix, component analysis and cluster analysis. 3. To show the spatial distribution of the heavy metals in groundwater of the area on the map of the study area as well as the spatial distribution of the pollution indices that will be used in the assessment of the intensity of groundwater pollution in the study area and the demarcation of zones with low and high groundwater pollution as it relates to heavy metals in the area. This work is expected to be added to the database on heavy metals composition of groundwater in these two industrial areas, and it will also be of assistance to professionals, stakeholders, managers and planners in designing, monitoring and planning of pollution control measures for groundwater in the area.
1.1 Study area, geology and hydrogeology
1.1.1 Study area
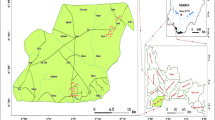
The two industries are located between latitudes 11°52′29.2″ N–11°57′44.9″ N and longitude 008°20′01.6″–008°31′04.2″ E. Highest elevation above sea level is recorded in Sharada area with 476 m and lowest elevation is recorded at Challawa area with 426 m. Other industries in Kano city include textiles, tanneries, chemicals and allied products. The study area is located on the main watershed which divides the two main river basins in the metropolitan city; the Jakara River to the north and Kano River to the south. Sharada, Challawa and Bompai industrial areas which are the main industrial estates in Kano city are situated within the two River basins [42]. There is a considerable distance of about 3–4 km between these two industrial areas. Untreated or partially treated effluents are discharged from 15 to 20 operational tanneries in Panshekara town down a canal which empties the effluents into river Challawa located about 1200 m downstream at “Yandanko settlement”. In Sharada area, effluents are discharged into a wide drainage canal which meanders into densely populated neighborhood of Garangamawa area, then goes down to Sabuwar Gandu and then collected in a pool at Gidan Maza area. Sharada industrial area has a good access route from the main road coming from Kofar Dan Agundi to Dorayi Sabontiti while Challawa industrial area is fairly accessible from Panshekara town, Fig. 1.
1.1.2 Geology and hydrogeology
Kano town and its environs are underlain by the rocks of the Crystalline Complex of Nigeria. Three rock types have been identified in the study area; gneiss, porphyritic granite and medium grained granite. [41, 42] The Older granite is the most common rock type in the area and is composed of coarse-grained granite, granodiorite, diorite and aplite [43, 44].
The groundwater aquifers in the study area are of fractured crystalline type. Groundwater in this setting is found in fissures, crevices, fractures and network of joints within the basement rocks. Shallow regolith, weathered overburden materials similarly have good water yield depending on seasonal variations, porosity and permeability.
2 Materials and methods
2.1 Groundwater sampling
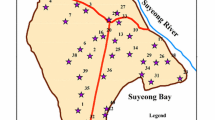
Twenty pieces of 1-L capacity polyethylene bottles were filled with representative groundwater samples collected from hand dug wells, boreholes and wash-bores ten each from Challawa and Sharada industrial areas in the study area. All samples were collected in accordance with [45] standard procedures for waste water sample collection. Figure 2 is the flowchart of the different methods used in this study. Few drops of Conc. HNO3 acid were added to lower the water pH to 2; this was done to stop any post-sampling reaction which could lead to the precipitation of metals out of solution. At the sampling points physical parameters of the groundwater that includes pH, TDS, EC and temperature were determined using the hand-held digital pH and 3 in 1 conductivity meters, respectively. The locations and elevations of the wells were taken using Global Positioning System (GPS) etrex Garmin model.
2.2 Samples preparation and analysis
50 mL of the water sample was taken in a conical flask; 10 m Conc HNO3 acid solution was added and heated on a hot plate to digest. This was allowed to cool and distilled water was added to make 50 mL volume and then filtered using a filter paper. Heavy metals (Cd, Cr, Fe, Ni, Mn, and Zn) were analyzed using atomic absorption spectrophotometer (AA 630 SCHIMADZU model) at the biomass instrumental laboratory of the Centre for energy research Usmanu Danfodiyo University, Sokoto. Turbidity was measured using Turbidimeter (HACH 2100P model); DO was determined using the different chemical reagents in the laboratory.
3 Pollution indices
3.1 Heavy metal pollution index (HPI)
This gives the aggregate influence of an individual heavy metal on the overall quality of sampled water, [46] the index was developed by [8], and this index is a mathematic model that is based on weighted arithmetic quality mean method. [20] Two steps are involved, the first is the development of a rating scale for the parameters and then allocation of weight (Wi); the second step is the selection of a pollution parameter which the calculated index will be based on. The equation of [16] was adopted and used in computing the HPI in this study which is given as
where \(Q_{i}\) is the Sub-index of the ith Parameter, \(W_{i}\) represent the weightage of the ith Parameter while n is the number of parameters been considered. To obtain the \(Q_{i}\) Sub-index Eq. 2 was used
\(M_{i}\), \(I_{i}\) and \(S_{i}\) represent the heavy metal’s ith parameter monitored, ideal and standard values, respectively, (−) sign in the equation stands for the numerical difference of the two values, and the algebraic sign is not considered. The critical value of 100 was adopted for drinking water in this study. \(W_{i}\) and \(S_{i}\) were obtained by taking the inverse of the MAC as given in (Table 1) for the metals considered.
3.2 Contamination index (Cd)
To calculate this index only heavy metals with concentrations that exceeded the upper permissible limits or guide values of the potentially harmful heavy elements were considered. This condition is adopted in this study. The contamination degree which is the sum of the contamination factors of the individual metallic component with concentration that exceeded the upper permissible value is calculated separately for each of the analyzed water sample while the contamination index gives the summary of all the parameters that are considered harmful in household water [47]. This index is calculated from (Eq. 3) below
\(Cf_{i}\) is the contaminant factor for the i-th component and is obtain using (Eq. 4)
\(CA_{i}\) is the analytical concentration value of the i-th parameter, \(CN_{i }\) represent the upper permissible concentration of the i-th parameter (N is the normative value).
3.3 Heavy metal evaluation index (HEI)
This index gives the composite quality of water in respect to heavy metal pollution. HEI is determined using (Eq. 5).
Hc is the measured concentration of i-th parameter measured in the sample water, Hmac the minimum admissible concentration of the i-th parameter.
3.4 Synthetic pollution index (SPI)
This model was previously used by many researchers among which were [26, 48,49,50,51]. The synthetic index of pollution is computed using the formula
The process involves 3 steps; the first step is computing the constant of proportionality \(K_{i}\) using (Eq. 7).
The second step is to calculate the weight coefficient (\(W_{i}\)) using (Eq. 8).
K is the constant of proportionality, \(V_{s}\) = standard for each of the parameters considered, in this case the Nigerian standards for drinking water quality [52] n = Total number of parameters considered, \(V_{o}\) = observed concentration of the individual parameters, Wi = weighted coefficient of each parameter.
3.5 Metal index (MI)
The metal index (MI) was proposed by [53] and later used by [53,54,55]. This index can be calculated using the expression given by (Eq. 9)
where MI is the metal index, C is the concentration of each element in the solution, MAC is the maximum allowed concentration of each element. The higher the concentration of a metal compared to its respective MAC value, the worse the quality of the water. MI value > 1 is a threshold of warning.
3.6 Statistical analysis
Both data of the heavy metals’ concentration in groundwater and calculated pollution indices were subjected to statistical evaluation in order to reveal the sources as well as any possible hidden association or relationship that existed among these metals and between the metals and the computed indices. Statistical tools of correlation analysis (CA), principal component analysis (PCA), and hieratical cluster analysis (HCA) were utilized to achieve these objectives.
The SPSS Statistical software package of IBM version 21 was used for the statistical evaluation of the data set.
3.6.1 Geo-spatial analysis
The spatial distribution maps of the heavy metals with concentration above the recommended standard limit were produced using the Inverse Distance Weighted (IDW) a geo-statistical-based method of the Arc GIS version 10.2, software using the spatial analysis tools function to present the results of interpolation in order to show how the heavy metals and pollution indices are spatially distributed across the study area.
4 Results and discussions
The concentrations of the heavy metals in the analyzed groundwater are presented in (Table 2), and the spatial distribution maps of all analyzed heavy metals detected in the groundwater samples are shown in Fig. 3a–f.
4.1 Cadmium (Cd)
The concentration of cadmium in the analyzed groundwater range between 2 and 39 μg/L with an average of 8.87 μg/L. Cd was detected in all the analyzed samples; the concentration of Cd in the area is higher in the Challawa area compared with the concentrations obtained at Sharada with maximum values of 39 and 8 μg/L recorded for both sites, respectively. Cadmium concentration exceeded both the national [52] and [56] permissible limit of 5 μg/L in 19 out of the 20 sampled groundwater analyzed in this study, with all the 10 samples taken from Challawa exceeding these two standards; spatial variation map of cadmium is shown in Fig. 3a.
4.2 Chromium (Cr)
Chromium was detected in only four out of the 20 groundwater samples analyzed from the area, and all the samples were taken from the Sharada area. Cr concentration ranges between 0 and 80 μg/L with a mean value of 7 μg/L. Only one sample had Cr concentrations exceeding the Nigerian standard of drinking water quality, NSDWQ [52] and the WHO [56]. The spatial map of Cr is given in Fig. 3b.
4.3 Iron (Fe)
Only 10 out of the 20 sampled groundwater samples analyzed for Fe had detectable iron in them, with six and four recorded at Sharada and Challawa areas, respectively. Fe concentrations in the analyzed water samples range between 20 and 700 μg/L with a mean value of 91 μg/L. The highest concentration was recorded at Challawa industrial area. Only one out of the 20 groundwater samples had concentration above the [52] and [56] limits of 300 μg/L in the area which was from Challawa, Fig. 3c.
4.4 Manganese (Mn)
Manganese concentration in the groundwater samples area was detected in 17 out of the 20 analyzed samples with a mean value of 75.9 μg/L and a range of 0 – 220 μg/L. Manganese concentration is above the permissible limit of 100 μg/L in four and one of the sampled groundwater from Challawa and Sharada industrial areas, respectively, Fig. 3d.
4.5 Nickel (Ni)
Nickel concentration in the area had a range of 0–700 μg/L with an average of 102 μg/L; only 12 samples had detectable Ni, with both Sharada and Challawa having six groundwater samples with detectable sample each. The highest nickel concentration of 700 μg/L was recorded at Challawa industrial site. All the recorded concentrations of this research exceeded the [52] and [56] allowable set limits of 20 μg/L, Fig. 3e.
4.6 Zinc (Zn)
Zinc was the least detected heavy metal in the area and was detected in only four out of the 20 samples analyzed in this study. Concentration range of 0–30 μg/L with an average of 4 μg/L which is very far below the 5000 μg/L limits of both [52] and [56] with no detectable Zn in the Challawa area, Fig. 3f.
4.7 Heavy metals pollution indices
The computed pollution indices of HPI, HEI, Cd, SPI and MI are presented in Table 3, while all spatial distribution maps are shown in Fig. 4a–e.
4.7.1 Heavy metal pollution index (HPI)
This index had an average value of 226.5 with values ranging between -34.7 and 1369.6. Based on the classification in Table 4, majority of the analyzed groundwater samples 75% had computed values of HPI that fall into the 100–300 class which is class of medium heavy metal pollution index, while the remaining three and two samples, respectively, distributed into the low and high categories of heavy metal pollution. The mean value of HPI given above in this study revealed the groundwater in the area to be of medium heavy metal pollution Fig. 4a.
4.7.2 Heavy metal evaluation index (HEI)
The computed HEI value in Table 3 gives a range of 1.68–37.9 with a mean of 8.1 which indicates the area to generally belong to the low HEI class with 14 samples belonging to that category. One sample had high HEI values of more than 20 while five samples were evaluated as having medium index Table 4 and Fig. 4b.
4.7.3 Contaminant index (Cd)
Calculated contaminant index (Cd) of heavy metals ranges between -4.32 and 31.9 with a mean value of 2.07 which classified the area to be of low heavy metals contaminant index where eighteen (18) of the total samples used, which represented 90% of the total groundwater samples used were within the low class of contaminant index of heavy metals while the remaining two samples fall into the groundwater with medium and high heavy metal contamination, respectively, Fig. 4c.
4.7.4 Synthetic pollution index (SPI)
The SPI values for this work range between 0.04 and 0.33 with an average of 0.084 according to the classification of [59] in Table 4, out of the 20 samples used, 19 samples (95%) were suitable for drinking and not polluted by heavy metals in terms of SPI, and the remaining one sample was slightly polluted with heavy metals. However, the overall average of 0.084 for the area revealed the groundwater in the area to belong to the suitable class of drinking water that are not polluted by heavy metals Fig. 4d.
4.7.5 Metal index (MI)
Computed MI values in the average give a mean of 10.12 with a range of 2.89 -40, with 55% representing 11 of the sampled groundwater belonging to the seriously affected groundwater with values that are greater than 6. Five out of the remaining samples were categorized as moderately affected while other four groundwater samples considered as being strongly affected with values that are within the class range of 4–6 as given in Table 4 by [56], Fig. 4e.
4.8 Multivariate statistical analysis
The analyzed data set and computed heavy metals indices were subjected to different statistical evaluation that include correlation, component and cluster analysis.
4.8.1 Correlation analysis
The Pearson correlation analysis was performed on both the analytical data set and computed metal indices contain all possible correlations between all pairs of variables being considered in this study using the significant correlation of 0.0. 13 pairs of significant correlations were obtained with values that range between 0.582 and 1.000. From Table 5, Fe had a moderate positive correlation of (r 0.582) with Mn and a negative correlation of (r − 0.660) with temperature; this could be suggesting formation under the same geochemical condition for both Fe and Mn with probably temperature controlling the formation or precipitation of Fe into the groundwater solution. Zn was moderately and negatively correlated to PH at (r − 0.602) which might be indicating a pH controlling reaction that led to the release of zinc into the groundwater from its different sources. TDS was strongly and positively related to EC with (r 1.000); this is an indication of their close relationship and dependency on each other. Two strong positive correlations were observed between cadmium and the computed pollution indices. Synthetic pollution index SPI and cadmium had positive strong correlation of (r 0.951), while HPI was strongly and positively correlated with cadmium (r 1.000) which is an indication of the dominant contribution made by cadmium to the values obtained for these pollution indices computed for the different groundwater samples in the area which were above the allowable limit in 95% of the analyzed samples. Ni had strong positive correlations of (r 0.981, r 0.981 and r 0.946) with Cd, HEI and MI, respectively, suggesting the significant contribution made by Ni to the pollution status of groundwater as regards to these indices. The correlation between the individual indices revealed a strong to moderate and positive correlations that range between 0.596 and 1.000 where MI was strongly and positively correlated to HEI and Cd, at (r 0. 991) and (r 0.991), respectively. Cd was strongly correlated to HEI at (r 1.000), SPI and HPI had a strong positive correlation of (r 0.950), while SPI correlation with MI was the list and a moderate one of (r 0.596). The strong and positive correlations observed between the heavy metal’s pollution indices suggested that the indices are related and are the ideal tools for assessing the level of toxicity and pollution level of groundwater in terms of the heavy metal’s concentration in the area.
4.8.2 Principal components analysis
The component analysis differentiated five important principal components extracted with only component with eigenvalue of 1 and above considered based on [60]. The [60] classifications of loading values of “strong”, “moderate”, and “weak” for values > 0.75, 0.50–0.75, and 0.30–0.50, respectively, were adopted for this study. All significant contributions made to each component are boldly represented in Table 6. A total of five components were extracted with a total variance of 86.45 (Table 6). The first component contributed 31.95% of the total variance with an eigenvalue of 5.43 and with a positive moderate to strong loading for Ni, HEI, Cd, MI and SPI of 0.911, 0.954, 0.953, 0.960 and 0.699; this can be favorably compared with the correlation analysis results. The occurrence of Ni alongside these indices suggests a leading role played by this metal in the pollution of groundwater. The second PC with eigenvalue of 3.42 and a variance percentage of 20.11% with positive moderate loadings of DO, temperature, pH, Cr, and Zn of − 0.688, 0.548, 0.584, 0.551 and − 0.613, respectively. The loading of pH, temperature alongside DO in the same PC could probably be suggesting that temperature did not play any significant role in the enrichment of Cr in the groundwater, while DO on the other hand did play role in the reactions that lead to Cr enrichment which could be due to oxidation redox reaction. PC 3 was positively and negatively moderately loaded with HPI, Cd, Fe and Mn of 0.624, 0.625, 0.574 and − 0.686. The third PC contributed 15% of the total variance with an eigenvalue of 2.55, the composition of this PC indicates the roles of Cd in the HPI computation as well as its contribution in the groundwater pollution in the area.
Component 4 had positive strong loading of TDS and EC with a moderate loading for turbidity of 0.794, 0.789 and 0.504, respectively. The PC had an eigenvalue of 2.04 and accounted for 12.024% of the total variance. The fifth PC had a moderate negative loading for temperature of − 0.609, with a moderate positive loading for Cd of 0.521, an eigenvalue of 1.25 and a variance of 7.37% were contributed by this PC.
4.8.3 Cluster analysis
The dendrogram Fig. 5 was used to demarcate 4 different clusters for the heavy metals and physical parameters of groundwater in this study where the R- mode was used; this was used to study the relationship as well as the origin and sources of the heavy metals in the groundwater of the study area. The first cluster consisted of a total of 12 parameters that include DO, PH, turbidity, SPI, HEI, MI, Cd, Cd, Zn, Cr, Mn and temperature. The cluster contained four of the heavy metals analyzed in the groundwater which probably originates from anthropogenic sources specifically due to the tanning, textiles and plastic industrial activities in Sharada and Challawa areas from which untreated and partially treated effluents are being constantly discharged into the rivers and soils in these two industrial areas. A second possible anthropogenic source can be linked to agricultural activities in these areas that involve the application of fertilizers, animal waste and agrochemicals on farmlands for the purpose of increasing crop yield, control of weeds and pests on grown crops, respectively. The second cluster comprises of five variables Cr, temperature, Mn, which is probably suggesting a common geochemical situation and sources for these metals that was probably favored under a certain temperature range. The other probable sources of these heavy metals in the groundwater can be natural through geochemical processes of weathering and dissolution of the rocks in the area. The third cluster contained Ni, Fe, Cr, with TDS HPI alongside some of the elements that appeared in the second cluster, the occurrence of Ni and Fe in this cluster is suggesting common sources and also their contributions to the total dissolved solid of the groundwater with their enrichment in the groundwater probably from mixed processes of anthropogenic activities and rock weathering processes. The fourth cluster singly contained only EC with no significant contribution to the pollution of groundwater.
The scree plot of the five components against the eigenvalues is shown in Fig. 6a with the red line drawn separating the first five important PCs with eigenvalues of 1 and above. Similarly, the rotated component plot in space is presented in Fig. 6b which shows the five components plotted in rotated space.
5 Conclusions
The heavy metals concentrations and pollution levels of groundwater from the industrial areas of Challawa and Sharada in Kano municipal have been investigated using five different pollution indices, multivariate statistical tools, and geospatial methods; the major conclusions that were drawn from this study are as follows.
-
Measured Physical parameters of groundwater that include temperature, pH, EC, TDS, DO, turbidity average PH of 6 confirmed a slightly acidic groundwater for the area with both measured electrical conductivity and (EC) and total dissolved solid (TDS) averages were within the Nigerian standards for drinking water quality (NSDWQ) and World Health Organization (WHO) permissible limits for drinking water.
-
Detected heavy metals concentration in the analyzed groundwater samples revealed Cd was present in all the 20 samples collected with 19 out of the total samples had Cd concentration above allowable limit. Chromium (Cr) was detected in only four samples all of which were from Sharada area with concentration range of 10–80 μg/L; however, only one sample had concentration above the prescribed limits of [52] and [56]. Ni was detected in 12 samples, with six samples each for both Challawa and Sharada having concentrations exceeding the Nigerian and WHO standard limits. This had a range of between 30 and 700 μg/L. Based on the above, the area can be classified as having cadmium and nickel polluted groundwater. However, the highest concentrations of all detected heavy metals in groundwater in this study area were recorded from the Challawa industrial area which categorized the groundwater in that area as more polluted compared to the groundwater from Sharada area.
-
The pollution status of groundwater from these industrial areas using heavy metal indices revealed the groundwater in the area to be of low contamination degree (Cd) in 18 samples, while one sample each was of medium and high Cd all of which are located in Challawa. In terms of heavy metals pollution index (HPI), 15 samples (75%) belong to the medium pollution index class with only two samples from those in the Challawa area falling into the high pollution index category of groundwater. HEI values computed for the groundwater categorized 14 samples (70%) as low polluted, four samples as medium and two samples from Challawa as highly polluted. Metal index (MI) gave a seriously polluted category in (55%) of the analyzed groundwater; four samples were strongly affected. In terms of synthetic pollution index, the study revealed the groundwater of the area to be suitable for drinking and unpolluted in 95% of the analyzed sample with only one sample being slightly polluted. The groundwater pollution in terms of pollution indices can generally be classified as low polluted based on the HPI, HEI, Cd and SPI while MI values have rated the groundwater as seriously polluted
-
Statistical evaluation of heavy metal data set and computed pollution indices gave a moderate positive correlation between the heavy metals and a strong positive correlation between the computed indices. Component analysis performed on the heavy metals data gave five PC with a total of 86% variance extracted where majority of the variables had a moderate to strong positive loading. Cluster analysis identified 4 different clusters which grouped the metals based on their sources in the groundwater. Anthropogenic sources were identified as the major source of input of heavy metals like Cd, Cr Ni and Mn into the groundwater, these were principally contributed by the effluents discharge from the tannery, textile and plastic industries as well as agricultural practices in the area, while a few percentages were contributed by geogenic sources which were basically from rock weathering and leaching from minerals and soils within the aquifers in the area.
-
Geospatial analysis was used to show the aerial variations and distribution of both heavy metals and pollution indices in order to demarcate the areas of low, medium and high metals concentrations as well as areas of high, medium and low groundwater pollution based on pollution indices.
-
The findings of this study will be very useful to professionals and stakeholders in monitoring, controlling and mitigation of pollution in these industrialized areas of Challawa and Sharada.
References
Islam MS, Ahmed MK, Raknuzzaman M, Habibullah-Al-Mamun M, Islam MK (2015) Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Ecol Ind 48:282–291
Navrátil T, Minařík L (2002) Trace elements and contaminants. In: Cílek V, Smith RH (eds) Earth’s System: history and natural variability. EOLSS-UNESCO Eolss Publishersx, Oxford
Ukah BU, Ameh PD, Egbueri JC, Unigwe CO, Ubido OE (2020) Impact of effluent-derived heavy metals on the groundwater quality in Ajao industrial area, Nigeria: an assessment using entropy water quality index (EWQI). Int J Energy Water Resour. https://doi.org/10.1007/s42108-020-00058-5
Egbueri JC, Mgbenu CN (2020) Chemometric analysis for pollution source identification and human health risk assessment of water resources in Ojoto Province, southeast Nigeria. Appl Water Sci 10:98. https://doi.org/10.1007/s13201-020-01180-9
Kumar M, Ramanathan A, Tripathi R, Farswan S, Kumar D, Bhattacharya P (2017) A study of trace element contamination using multivariate statistical techniques and health risk assessment in groundwater of Chhaprola Industrial Area, Gautam Buddha Nagar, Uttar Pradesh, India. Chemosphere 166:135–145
Wang X, SunLi YS, Wang H (2019) Spatial distribution and ecological risk assessment of heavy metals in soil from the Raoyanghe Wetland,China. PLoS ONE 14(8):e0220409. https://doi.org/10.1371/journal.pone.0220409
Dash S, Borah SS, Kalamdhad A (2019) A modified indexing approach for assessment of heavy metals contamination in Deepor Beel. India Ecol Indic 106:105444. https://doi.org/10.1016/j.ecolind.2019.105444
Akan JC, Ogugbuaja VO, Abdulrahman FI, Ayodele JT (2007) Determınatıon of pollutant levels ın water of river Challawa and ın tap water from Kano ındustrıal area. Res J Environ Sci 1(5):211–219
Plant JA, Ornton I (1983) Geochemistry applied to agriculture. In: Ornton I (ed) Applied environ mental geochemistry. Academic Press, London
Carla WM (2002) Environmental geology. WMC Brown Publishers, Dubugue
Tait L, Nation JR, Krebs NF, Bellinger DC (2005) Neurotoxicants, micronutrients, and social environments: individual and combined effects on children’s development. Psychol Sci Public Interest 6(3):57–121
Zhang H, Liu Y, Qi D, Wang Y, Hou L (2017) Pollution characteristics and ecological risk assessment of heavy metals in the surface layer sediments of Yangzonghai lakeside wetland. J Yunnan Univ 39(3):494–506. https://doi.org/10.7540/j.ynu.20160328
Zamani AA, Yaftian MR, Parizanganeh A (2012) Multivariate statistical assessment of heavy metal pollution sources of groundwater around a lead and zinc plant. J Environ Health Sci Eng 9:29
Eldaw E, Huang T, Elubid B, Khalifa A, Mahamed KA, Mahama Y (2020) A Novel approach for indexing heavy metals pollution to assess groundwater quality for drinking purposes. Int J Environ Res Public Health 17:1245. https://doi.org/10.3390/ijerph17041245
Horton RK (1965) An index number system for rating water quality. J Water Poll Control Fed 37(3):300–305
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metal in drinking water and development of heavy metal pollution index. J Environ Sci Health A 31:283–289
Prasad B, Jaiprakas KC (1999) Evaluation of heavy metals in ground water near mining area and development of heavy metal pollution index. J Environ Sci Health A 34(1):91–102
Prasad B, Bose JM (2001) Evaluation of the heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environ Geol 41:183–188
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring: a study case from Akpabuyo-Odukpani area, lower cross river Basin (southeastern Nigeria). GeoJ 57:295–304
Kwaya MY, Hamidu H, Mohammed AI, Abdulmumini YN, Adamu IH, Grema HM, Dauda M, Halilu FB, Kana AM (2019) Heavy metals pollution indices and multivariate statistical evaluation of groundwater quality of maru town and environs. J Mater Environ Sci 10(1):32–44
Ibrahim HA, Abdulkarim M, Grema HM, Abdullahi MI, Hamidu H (2020) Physico-chemical assessment of water quality in the Gidan Gulbi shallow floodplain aquifer, northwestern Nigeria. Am J Water Resour 8(4):155–163
Dash S, Borah SS, Kalamdhad AS (2020) Application of positive matrix factorization receptor model and elemental analysis for the assessment of sediment contamination and their source apportionment of Deepor Beel, Assam. India Ecol Indic 114:106291. https://doi.org/10.1016/j.ecolind.2020.106291
Dash S, Borah SS, Kalamdhad AS (2021) (2021) Heavy metal pollution and potential ecological risk assessment for surficial sediments of Deepor Beel. India Ecol Indic 122:107265. https://doi.org/10.1016/j.ecolind.2020.107265
Bhardwaj S, Soni R, Gupta SK, Shukla DP (2020) Mercury, arsenic, lead and cadmium in waters of the Singrauli coal mining and power plants industrial zone, Central East India. Environ Monit Assess 192:251. https://doi.org/10.1007/s10661-020-8225-2
Lotfi S, Chakit M, Belghyti D (2020) Groundwater quality and pollution index for heavy metals in saïs plain Morocco. J Health Pollut 10:26
Egbueri JC (2020) Heavy metals pollution source identification and probabilistic health risk assessment of shallow groundwater in Onitsha Nigeria. Anal Lett. https://doi.org/10.1080/00032719.2020.1712606
Rahman MATMT, Paul M, Bhoumik N, Hassan M, Alam MK, Aktar Z (2020) Heavy metal pollution assessment in the groundwater of the Meghna Ghat industrial area, Bangladesh, by using water pollution indices Approach. Appl Water Sci 10:186. https://doi.org/10.1007/s13201-020-01266-4
Dash S, Borah SS, Kalamdhad AS (2020) Application of environmetrics tools for geochemistry, water quality assessment and apportionment of pollution sources in Deepor Beel, Assam India. Water Pract Technol 15(4):973–992. https://doi.org/10.2166/wpt.2020.078
Egwuonwu GN, Olabode VO, Bukar PH, Okolo VO, Odunze AC (2011) Characterızatıon of topsoıl and groundwater at leather ındustrıal area, Challawa, Kano, Northern Nıgerıa. Pacıfıc J Sci Technol 12(1):628
Uzairu A, Okunola OJ, Wakawa RJ, Adewusi SG (2014) Bioavailability studies of metals in surface water of River Challawa, Nigeria. J Appl Chem Hindawi Publish Corp. https://doi.org/10.1155/2014/648453
Idris MB, Khalid DK, Abdullahi Z (2015) Comparative assessment of heavy metals concentration in the soil in the vicinity of tannery industries, Kumbotso Old Dump Site and River Challawa, conference at Challawa Industrial Estate, Kano State, Nigeria. Int J Innov Res Dev 4(6):122–128
Koki IB, Jimoh WLO (2015) Assessment of heavy metals in tannery solid waste from Challawa Industrial Estate, Kano State, Nigeria. Int J Res Environ Stud 2:33–40
Koki IB, Bayero AS, Umar A, Yusuf S (2015) Health risk assessment of heavy metals in water, air, soil and fish. Afr J Pure Appl Chem 9(11):204–210. https://doi.org/10.5897/AJPAC2015.0654
Udiba UU, Odey MO, Anyim B (2018) Heavy metals profile of Challawa River Basin around Challawa industrial layout, Kano and Its implications for cultivated vegetables. Sumerianz J Sci Res 1(1):8–15
Musa DM, Sadiya M, Yusuf MA, Garba YI, Gimba EB (2018) Study of water and soil contamination by heavy metal from industrial effluents at Bompai industrial area Kano, Nigeria. FUW Trend Sci Technol J 3(1):234–238
Adejube AAH, Garba PY, Halid A, Yakubu A, Anteyi A, Ovye A (2018) Analysis of heavy metals concentration in effluents, groundwater and top soil at textile and tanneries waste dumped Site located in Challawa industrial estate Kano-State, Nigeria. Int J Chem Chem Process 4(2):62–70
Amoo AO, Gambo YH, Adeleye AO, Amoo NB (2018) Assessment of groundwater quality in Sharada industrial area of Kano, North-Western Nigeria. FUW Trend Sci Technol J 3(2A):407–411
Sani A, Darma AI, Rukayya AA, Namadina MM (2019) A study on physicochemical parameters and heavy metal in Sharada industrial effluents, Kano. Nigeria Innov J Life Sci 7(3):1–4
Falalu B H, Hamidu H, Kwaya M Y, Abdullahi S (2019) Heavy metals concentration in groundwater of Challawa and Sharada industrial areas Kano metropolis, Kano State, Northwestern Nigeria. Book of proceeding of the 10th NAHS International conference Sokoto, 192–196
Mustapha A, Sagagi BS, Daura MM, Tanko AI, Eze PP, Isiyaka A (2019) Geochemical evolution and quality assessment of groundwater resources at the downstream section of the Kano-Challawa River system Northwest Nigeria. Int J River Basin Manag. https://doi.org/10.1080/15715124.2019.1606817
Dan’azumı S, Bıchı MH (2010) Industrıal pollutıon and heavy metals profıle of Challwa rıver ın Kano, Nıgerıa. J Appl Scı Envıron Sanıt V:56–62
Magdi O (1999) Geological and geochemical investigation of lateritic profiles around Kano with Special reference to the distribution of base metals (V, Cr, Mn, Co and Ni). Unpublished M Sc. Thesis. Department of geology, Ahmadu Bello University Zaria
MacDonald and Partners Rural Water Supplie Report (Published).(1986) Sir MacDonald Press Ltd, Deterner, England. Vol. 1
Oyawoye MO (1972) The basement complex of Nigeria. In: Dessauvagie F, Whiteman J (eds) Africa geology. Uinversity Press, Ibadan, pp 18–27
APHA Standards methods for examination of water and wastewater. American Public Health Association. Washington DC. 1998, 20th edition
Afonne JO, Chukwuka UJ, Ifediba CE (2020) Evaluation of drinking water quality using heavy metal pollution indexing model in an agrarian, non-industrialized area of South-Eastern Nigeria. J Environ Sci Health part A. https://doi.org/10.1080/10934529.2020.1796402
Backman B, Bodiš D, Lahermo P, Rapant S, Tarvainen T (1998) Application of a groundwater contamination index in Finland. Environ Geol 136(1–2):55–64. https://doi.org/10.1007/s002540050320
Singh SK, Srivastava PK, Singh D, Han D, Gautam SK, Pandey AC (2014) Modeling groundwater quality over a humid subtropical region using numerical indices, earth observation datasets, and X-ray diffraction technique: a case study of Allahabad district, India. Environ Geochem Health. https://doi.org/10.1007/s10653-014-9638-z
Gautam SK, Maharana C, Sharma D, Singh AK (2015) Evaluation of groundwater quality in the Chotanagpur Plateau region of the Subarnarekha River Basin, Jharkhand State, India. Sustain Water Quality Ecol. https://doi.org/10.1016/j.swaqe.2015.06.001
Solangi GS, Siyal AA, Babar MM et al (2019) Evaluation of surface water quality using the water quality index (WQI) and the synthetic pollution index (SPI): a case study of the Indus Delta region of Pakistan. Desaline Water Treat 118:39–48
Solangi G, Siyal AA, Babar MM, Siyal P (2019) Groundwater quality evaluation using the water quality index (WQI), the synthetic pollution index (SPI), and geospatial tools: a case study of Sujawal district, Pakistan. Hum Ecol Risk Assess Int J. https://doi.org/10.1080/10807039.2019.1588099
Nigerian Standard for Drinking Water Quality (NSDWQ) Nigerian Industrial Standard (NIS) (2015) 554, Standard Organization of Nigeria. 30p
Tamasi G, Cini R (2013) Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena. Sci Total Environ 327:41–51. https://doi.org/10.1016/j.scitotenv.2003.10.011
Abdullah EJ (2013) Quality assessment for Shatt Al-Arab River using heavy metal pollution index and metal index. J Environ Earth Sci 3(5):114–120
Rezaei A, Hassani H, Jabbari N (2017) Evaluation of groundwater quality and assessment of pollution indices for heavy metals in North of Isfahan Province, Iran. Water Resour Manag Sustain. https://doi.org/10.1007/s40899-017-0209-1
World Health Organization (WHO) (2017) Guidelines for drinking water quality. World Health Organization, Geneva
Caerio S, Costa MH, Ramos TB, Fernandes F, Silveira N, Coimbra A, Painho M (2005) Assessing heavy metal contamination in Sado estuary sediment: an index analysis approach. Ecol Ind 5:155–169
Lyulko I, Ambalova T, Vasiljeva T (2001) Integrated water quality assessment in Latvia. MTM (Monitoring Tailor-Made) III. In: Proceedings of international workshop on information for sustainable water management. Netherlands. 449–452
Xiao C (1996) `Application of the equalized synthetic pollution index method to the assessment of groundwater quality pollution in the former Guoguan district. Jilin Water Conserv 11:33–35
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a black foot disease area in Taiwan. Sci Total Environ 313:77–89
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamidu, H., Halilu, F.B., Yerima, K.M. et al. Heavy metals pollution indexing, geospatial and statistical approaches of groundwater within Challawa and Sharada industrial areas, Kano City, North-Western Nigeria. SN Appl. Sci. 3, 690 (2021). https://doi.org/10.1007/s42452-021-04662-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04662-w