Abstract
Porous carbons were prepared from the biomass waste rambutan peels using hydrothermal carbonization followed by the KOH activation process. Rambutan peel derived porous carbons (RPC) with high surface area of 2104 m2 g−1 and large pore volume of 1.2 cm3 g−1 were obtained at KOH/carbon ratio of 4 and activation temperature of 900 °C. The as-obtained porous carbons were capable of encapsulating sulfur with a high loading of 68.2 wt% to form RPC/S composite cathode for lithium sulfur (Li–S) battery. High specific discharge capacities of about 1275 mAh g−1 were demonstrated by the RPC/S composites at 0.1 C. After 200 cycles at 0.1 C, a high specific capacity of 936 mAh g−1 was maintained, showing an excellent capacity retention of about 73%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
To support the development of renewable energy and reduce the CO2 emissions, the role of electrochemical energy storage especially rechargeable battery is crucially required [1]. Lithium sulfur (Li–S) battery is one of the candidates to be developed as promising battery system due to the high energy density of 2500 Wh kg−1, abundant raw material and safety issues [2, 3]. However, there are some obstacles that should be handled well for further commercial and practical use of this battery system [4]. The specific issues are due to the low conductivity of sulfur and its alloying products with Li (Li2Sx), high solubility of intermediates polysulfide (Li2Sx, 4 ≤ x ≤ 8), enormous volume expansion during cycling of sulfur electrodes and serious shuttle effects [5,6,7,8]. All of these issues will cause poor electrochemical performance of sulfur cathodes in Li–S battery because of the rapid capacity loss and high irreversible capacity [9]. Hence, it is urgent to solve those problems, so more reliable Li–S battery system can be used for commercial applications.
The above mentioned problems can be effectively handled by using porous carbons as composite along with sulfur [10,11,12]. The presence of porous carbons may enhance the electrical conductivity of sulfur cathodes as well as their connecting pores can act as polysulfide reservoirs [13,14,15]. It can minimize the effect of polysulfide shuttle during cycle test of the composite electrodes. For production of the porous carbons, biomass can become one candidate of the most possible precursors because it is cheap, high abundant, renewable, green and unique porous structures [16,17,18,19,20,21,22,23,24]. Various of biomass has been used as carbons precursors for for Li–S battery system such as avocado shells [25], snake-skin fruit peel[26], hickory shell [27], rapeseed meal [28], moss [29], dandelion [30], banana peel [31], fiber of fern [32], wheat straw [33], mangosteen peel [34], xanthoceras sorbifolia husks [35], goat hair [36], rice husks [37], pistachio shell [38], almond shell [39], waste mandarin peels [17], waste tea [40] and many others [41].
Rambutan (Nephelium lappaceum L) is a popular tropical fruit that belongs to Sapindaceae family [42]. Indonesia produces many varieties of rambutan, there are more than 20 varieties can be found. Fruits are oval-shaped with the peel is green, yellow or red when ripe. Normally, after consuming the rambutan fruit, the peel is regarded as waste and disposal of it can cause environmental problem in the community. Therefore, conversion of rambutan peel into useful product such as porous carbons in Li–S batteries, is expected can reduce the processing cost of the peel waste. The main merit of using rambutan peel as raw materials for production of porous carbons is the huge abundant resources of it which can minimize the production cost of Li–S batteries.
In this work, rambutan peels were utilized to prepare porous carbons to encapsulate sulfur as cathodes for lithium sulfur battery. Porous carbons were initially synthesized by hydrothermal treatment of waste rambutan peel followed by the KOH chemical activation process by varying the impregnation ratio between KOH and the precursors. The pretreatment of hydrothermal converts rambutan peel into a solid intermediate product (hydro-char) at relatively low temperature (180–250 °C) and saturated pressure (2–10 MPa). The hydro-char has better physico-chemical characteristics than its carbon precursors [43]. The role of the next step of chemical activation is develop carbon materials with high surface area and porosity. In specific, the effect of impregnation ratio on the electrochemical performance of porous carbon–sulfur composite cathodes will be studied.
2 Experimental
2.1 Preparation process of porous carbons
The preparation process of rambutan peel based porous carbons was done by hydrothermal carbonization and then followed by the chemical activation process using KOH. The rambutan peel was collected from the local market in Cimahi, Indonesia. After grounded by mortar and pestle pestle, the peel powders were screened by sieve screening to have uniform particle size distribution. After screening, the peel powder was dispersed in distilled water and then was loaded into a Teflon-lined stainless steel autoclave and hydrothermally treated at 200 °C for 20 h. After the reaction completed, the autoclave was cooled down into room temperature. The resulting solid carbons was separated by centrifuge and then washed with ethanol and distilled several times. The washed solids were then dried in an oven at 80 °C overnight. The dried hydrochar was then separately mixed with KOH, respectively with different impregnation ratio of 1:1, 1:2 and 1:4 (mass ratio between of tea powder and KOH). Finally, further pyrolysis carbonization was applied to the impegnated hydrochar samples at temperature of 900 °C for 2 h with heating rate of 5 °C/min in N2 atmosphere. The resulting solid carbon was washed with 1 M HCl solution and distilled water several times until the filtrate became neutral in pH. The obtained wet carbon was finally dried at 80 °C overnight. The carbon samples were denoted as RPC-1 which was obtained by impregnation ratio of 1:1, RPC-2 for impregnation ratio of 1:2 and RPC-4 for impregnation ratio of 1:4.
2.2 Preparation process of porous carbon–sulfur composites
The porous carbon–sulfur composites were prepared by a standard melt-diffusion method. 3 samples of porous carbons (RPC-1, RPC-2 and RPC-4) and elemental sulfur (Sigma Aldrich) were mixed with the weight ratio of 1:3 by using pestle and mortar followed by heating under vacuum at 155 °C for 10 h in a crucible to produce carbon–sulfur composites. The choice of this temperature because it is slightly above the melting point of sulfur, where solid orthorhombic α-S melts into liquid cyclic α-S8 and its viscosity is the lowest. The melted sulfur infiltrates into the pore structures of the carbon by capillary forces [44]. The carbon–sulfur composites were denoted as RPC/S-1, RPC/S-2 and RPC/S-4.
2.3 Structural characterizations
The textural, structural and morphological properties of rambutan peel porous carbon (RPC) and their corresponding sulfur composites (RPC/S) were investigated by scanning electron microscope (SEM, HITACHI), energy dispersive X-ray spectroscopy (EDXS) and X-ray diffraction (XRD, Bruker). The N2 adsorption–desorption isotherm was obtained by surface area analyzer (Micromeritics). Pore size distributions were estimated using density functional theory (DFT) for micropores and the Barrett-Joyner-Halenda (BJH) model for mesopores. Thermogravimetric analysis was conducted to measure the sulfur content in the composites.
2.4 Electrochemical characterizations
The electrochemical performance of composite electrodes was investigated by coin cells fabricated in an Ar-filled glove box. The used electrolyte was 1 M LiN(CF3SO2)2 (LiTFSI) dissolved in a mixture of 1,3-dioxolane (DOL)/dimethoxyethane (DME) (1:1 volume ratio) containing 0.1 M LiNO3 and Li metal was used as reference/counter electrode. Electrode containing RPC/S, Super P conducting carbon and poly(vinylidene fluoride) (PVDF) binder were made by casting slurry onto Al foil, in the weight of 80:10:10. The ratio of electrolyte/sulfur composite ratio was set about 40 µL/mg. The typical mass loading of sulfur in the electrode was 1.1 mg cm−2. Charge–discharge measurements were conducted on battery tester (MACCOR) at 1.5–3 0.0 V vs. Li/Li+.
3 Results and discussion
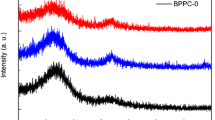
The structure of rambutan peel-based carbon (RHC) was then investigated by X-ray diffraction (XRD), as seen in Fig. 1. Two broad peaks are identified at around 23° and 23° for all RHC samples. This suggests that the RHC samples possess are turbostratic carbon structures. After mixing with sulfur, no apparent XRD peak for elemental sulfur can be found in RPC/S composite, suggesting that the sulfur into carbon pores has been infiltrated well into the carbon pores. The lack of crystallinity of the sulfur in its composites (XRD pattern) implies that sulfur exists in a highly dispersed state inside the pores of carbon [45].
The structure of RPC and RPC/S samples was also studied by using Raman spectroscopy, as shown by Fig. 2, where two distinctive peaks can be seen namely the defect-induced D peak at around 1327 cm − 1 and crystalline graphite G peak located at 1594 cm − 1. The intensity ratio of D and G peaks (ID/IG) can be also estimated. The relative intensity (ID/IG) of RPC-1, RPC-2 and RPC-4 can be estimated about 1.12, 1.13 and 1.15, respectively. It seemed that the degree of disordered carbon structure has increased by increasing the impregnation ratio from 1:1 to 1:4 in the carbon preparation process. After impregnating with sulfur, the relative intensity of RPC/S-1, RPC/S-2 and RPC/S-4 is about 1.19,1.27 and 1.33, respectively. It can be said that the degree of disordered structure is increased by impregnating carbons with sulfur. Furthermore, we did not find any possible signals in the range of 100–500 cm−1, caused by the presence of sulfur [46, 47].
The textural properties of RPC/S composites such as BET surface areas and pore size distribution were investigated by N2 adsorption–desorption experiments at 77 K. We can see in Fig. 3 that the N2 adsorption desorption isotherms of RPC and its composites are similar in shape which can be categorized as type IV isotherm of IUPAC classification. The presence of microporous structure can be detected from the high adsorption amount in low relative pressure. The textural properties of all samples are shown in Table 1. The BET surface areas of the RPC-4, RPC-2 and RPC-1 are about 2104, 1975 and 1527 m2 g−1, respectively. While the total pore volume of RPC-4, RPC-2 and RPC-1 are estimated as 1.2, 1.07 and 0.89 cm3 g−1and their pore size distribution (Fig. 1d) suggest dominant mesopores. After mixing with sulfur, the surface area of RPC-4, RPC-2 and RPC-1 are decreasing to 40.7, 35.2 and 22.7 m2 g−1. and the total pore volume also decreases from 0.64, 0.52 and 0.41 cm3 g−1, showing the successful infiltration of sulfur into carbon matrix using melt-diffusion process.
Figure 4 shows the TGA measurements of RPC/S composites to estimate the exact sulfur content in an argon flow. As seen in Fig. 4, the content of sulfur in the RPC/S-1, RPC/S-2 and RPC/S-4 composites was about 55.2, 61.9 and 68.2 wt%.
Figure 5 presents the SEM images of the rambutan peel based porous carbons and their corresponding composites with sulfur. We can observe that no apparent aggregation of bulk sulfur on the surface of porous carbons, meaning that the sulfur has been infused perfectly into the pores of the carbons matrix. Table 2 shows the energy dispersive X-ray spectroscopy measurements of all carbon samples. It suggests that there are no contaminants found in the carbon samples.
The electrochemical performances of the RPC/S composite cathodes for LiS batteries were studied by coil cells. Figure 6a shows the comparison of cycle performance of all RPC/S composite cathodes at current density of 0.2 C between 1.5 and 3.0 V vs Li/Li+ in which the specific capacity of LiS cells were determined using the sulfur mass. All the samples show almost the same trend where all the capacities are decaying in the first 10 cycles and starting to have a stable cycle performance until 100th cycle. The RPC/S-4 sample demonstrated the most superior performance among others until 100 cycles of charge/discharge of LiS cells. The initial specific discharge capacity of RPC/S-4 samples is 1275 mAh g−1 and the capacity is remaining at 944 mAh g−1 at the end of 100th cycle. While for the RPC/S-2 and RPC/S-1 samples, they showed more inferior performance in which their initial capacities are about 1100 and 910 mAh g−1 and their final capacities at 100th cycle are around 824 and 657 mAh g−1, respectively. The superior performance of RPC/S-4 is attributed to the highest BET surface area which can allow more sulfur to be encapsulated in the porous carbon matrix of RPC/S-4. In addition, the large pore volume of RPC/S-4 can accommodate the volume expansion occurred during the cycles of LiS cells.
Figure 6b shows the long cycle test of RPC/S-4 sample measured at current density of 0.2 C between 1.5 and 3.0 V vs Li/Li+. It can be seen that the capacity at end of 200th cycle is about 936 mAh g−1, reflecting a capacity retention of 73%. The columbic efficiency is about 89% in the first cycle and it is increasing in the subsequent cycles at about 99%. This excellent long cycle performance is also due to the high surface area and large pore volume of porous carbons derived from the rambutan peel obtained by the impregnation ratio of 4.
Figure 6c then shows the voltage profiles of RPC/S-4 sample at the 1st, 10th, 50th, 100th and 200th cycle. For all cycles, we can observe that there are two voltage discharge plateaus and one voltage charge plateau at 2.6 V. The charge plateau is related to the oxidation from S2− to Sx2− and finally to produce S8 [48]. The first discharge plateau located at about 2.3 V represent the oxidation of elemental sulfur to long-chained polysulfides (Li2Sn for n ≥ 4) while the second plateau at 2.0 V is due to the decomposition of the long chained polysulfides to the insoluble products of Li2S2 and Li2S [49].
The cyclic voltammetry (CV) profiles with RPC/S-4 samples as composite cathode materials for Li–S cells in the first three cycles are illustrated in Fig. 7a. There are two apparent reduction peaks at about 2.3 and 2.0 V which are due to the subsequent reduction of elemental sulfurto soluble lithium polysulfides (Li2Sn, 4 ≤ n ≤ 8) and finally, the conversion to insoluble Li2S2 and Li2S, confirmed by the voltage profiles in Fig. 6c. The broad oxidation peak located at 2.6 V is related to the the oxidation of Li2S2 and Li2S into long-chained and soluble polysulfides. Furthermore, we can see that the CV profiles in the next cycles are overlapped and similar in shape. It shows that the reversibility the composite cathode materials has been enhanced during cycle tests.
Figure 7b depicts the CV measurements of RPC/S-4 samples by varying the scan rate from 0.1 to 0.5 mV s−1. The redox anodic (A1) and cathodic (C1 and C2) peak currents follow the linear relationship with square root of scan rates, as shown by the Randles Sevcik equation:
where, \({I}_{p}\) is the corresponding cathodic/anodic peaks current, \(n\) is the numbers of electron transfer, \(A\) is the active electrode area, \({D}_{Li+}\) is the lithium ions diffusion coefficient, \(C\) is the concentration of lithium ions, and ν is the scan rate. The lithium-ion diffusion coefficient of RPC/S-4 can be estimated by using the linear regression, as shown in Fig. 7c. It is about \(3.2\times {10}^{-10}\) cm2s−1. This value is lower than those found in the literature such as graphene based composite [47, 50].
In order to study in detail about the electrochemical performance, the rate capability of all RPC/S electrodes were also conducted, as shown in Fig. 8. The three samples were measured with various C-rate varying from 0.2 to 2 C (1 C = 1675 mA g−1). The stable specific discharge capacities of RPC/S-4 composite at 0.2 C, 0.3 C, 0.4 C, 0.5 C, 1 C, 2 C and 3 C are 1100, 875, 750, 625, 500 and 375 mAh·g−1, respectively. Furthermore, the discharge capacity of 1000 mAh·g−1 can still be reached when the current density returned back to 0.2 C. It shows that the RPC/S-4 composite has a the most stable and robust cycle performance among others. The main role of porous carbon with large pore volume is to contain more amount of active sulfur as well as to accommodate the volume expansion of sulfur during discharge process. In addition, the interconnected micro-mesoporous structures of carbons can provide diffusion channels for facile Li+ diffusion. The electrochemical characteristics comparisons among biomass based carbon–sulfur composites are shown in Table 3.
4 Conclusions
In summary, waste rambutan peels were employed as carbons precursors to derive porous carbon materials to encapsulate sulfur cathodes for Li − S batteries. Due to the high surface area and large pore volume, the RPC/S electrodes demonstrated superior rate capacity and stable cycle performance. The large pore volume enables the porous carbons to load high content of sulfur. As a results, the best RPC/S cathodes with sulfur loading of 68.2 wt% possesses a high initial specific capacity of about 1275 mAh g−1 at 0.2 C rate with high Coulombic efficiency above 97% and a high capacity of 936 mAh g−1 after 200 cycles. The Li+ diffusion coefficient in the RPC/S composites has been estimated as \(3.2\times {10}^{-10}\) cm2 s−1, implying a fast transport rate of Li.
References
Deng D (2015) Li-ion batteries: basics, progress, and challenges. Energy Sci Eng 3:385–418. https://doi.org/10.1002/ese3.95
Kaiser MR, Han Z, Liang J et al (2019) Lithium sulfide-based cathode for lithium-ion/sulfur battery: recent progress and challenges. Energy Storage Mater 19:1–15. https://doi.org/10.1016/j.ensm.2019.04.001
He J, Manthiram A (2019) A review on the status and challenges of electrocatalysts in lithium-sulfur batteries. Energy Storage Mater 20:55–70. https://doi.org/10.1016/j.ensm.2019.04.038
Kang W, Deng N, Ju J et al (2016) A review of recent developments in rechargeable lithium–sulfur batteries. Nanoscale 8:16541–16588. https://doi.org/10.1039/C6NR04923K
Nara H, Tsuda S, Osaka T (2017) Techniques for realizing practical application of sulfur cathodes in future Li-ion batteries. J Solid State Electrochem 21:1925–1937. https://doi.org/10.1007/s10008-017-3629-9
Fotouhi A, Auger D, O’Neill L et al (2017) Lithium-sulfur battery technology readiness and applications—a review. Energies 10:1937. https://doi.org/10.3390/en10121937
Zeng LC, Li WH, Jiang Y, Yu Y (2017) Recent progress in Li–S and Li–Se batteries. Rare Met 36:339–364. https://doi.org/10.1007/s12598-017-0891-z
Ma L, Hendrickson KE, Wei S, Archer LA (2015) Nanomaterials: science and applications in the lithium-sulfur battery. Nano Today 10:315–338. https://doi.org/10.1016/j.nantod.2015.04.011
Fu C, Guo J (2016) Challenges and current development of sulfur cathode in lithium–sulfur battery. Curr Opin Chem Eng 13:53–62. https://doi.org/10.1016/j.coche.2016.08.004
Borchardt L, Althues H, Kaskel S (2017) Carbon nano-composites for lithium–sulfur batteries. Curr Opin Green Sustain Chem 4:64–71. https://doi.org/10.1016/j.cogsc.2017.02.008
Bandosz TJ, Ren T (2017) Porous carbon modified with sulfur in energy related applications. Carbon 118:561–577
Li Z, Huang Y, Yuan L (2015) Status and prospects in sulfur – carbon composites as cathode materials for rechargeable lithium – sulfur batteries. Carbon N Y 92:41–63. https://doi.org/10.1016/j.carbon.2015.03.008
Liang J, Sun ZH, Li F, Cheng HM (2016) Carbon materials for Li-S batteries: functional evolution and performance improvement. Energy Storage Mater 2:76–106. https://doi.org/10.1016/j.ensm.2015.09.007
Wang M, Xia X, Zhong Y et al (2019) Porous carbon hosts for lithium-sulfur batteries. Chem - A Eur J 25:3710–3725. https://doi.org/10.1002/chem.201803153
Le SJ, Tang C, Huang JQ et al (2018) Effective exposure of nitrogen heteroatoms in 3D porous graphene framework for oxygen reduction reaction and lithium–sulfur batteries. J Energy Chem 27:167–175. https://doi.org/10.1016/j.jechem.2017.09.014
Zhao Y, Ren J, Tan T et al (2017) Biomass waste inspired highly porous carbon for high performance lithium/sulfur batteries. Nanomaterials 7:260. https://doi.org/10.3390/nano7090260
Półrolniczak P, Nowicki P, Wasiński K et al (2016) Biomass-derived hierarchical carbon as sulfur cathode stabilizing agent for lithium-sulfur batteries. Solid State Ion 297:59–63. https://doi.org/10.1016/j.ssi.2016.10.012
Imtiaz S, Zhang J, Zafar ZA et al (2016) Biomass-derived nanostructured porous carbons for lithium-sulfur batteries. Sci China Mater 59:389–407. https://doi.org/10.1007/s40843-016-5047-8
Kaiser MR, Ma Z, Wang X et al (2017) Reverse microemulsion synthesis of sulfur/graphene composite for lithium/sulfur batteries. ACS Nano 11:9048–9056. https://doi.org/10.1021/acsnano.7b03591
Rejaul Kaiser M, Wang J, Liang X et al (2015) A systematic approach to high and stable discharge capacity for scaling up the lithium-sulfur battery. J Power Sour 279:231–237. https://doi.org/10.1016/j.jpowsour.2014.12.098
Liang X, Kaiser MR, Konstantinov K et al (2016) Ternary porous sulfur/dual-carbon architectures for lithium/sulfur batteries obtained continuously and on a large scale via an industry-oriented spray-pyrolysis/sublimation method. ACS Appl Mater Interf 8:25251–25260. https://doi.org/10.1021/acsami.6b07012
Cengiz EC, Demir-Cakan R (2020) TiO2 embedded hydrothermally synthesized carbon composite as interlayer for lithium-sulfur batteries. J Solid State Electrochem 24:2469–2478. https://doi.org/10.1007/s10008-020-04785-x
Kaiser MR, Han Z, Wang J (2019) Electro-polymerized polypyrrole film for fabrication of flexible and slurry-free polypyrrole-sulfur-polypyrrole sandwich electrode for the lithium-sulfur battery. J Power Sour 437:226925. https://doi.org/10.1016/j.jpowsour.2019.226925
Kaiser MR, Liang X, Konstantinov K et al (2015) A facile synthesis of high-surface-area sulfur-carbon composites for Li/S batteries. Chem - A Eur J 21:10061–10069. https://doi.org/10.1002/chem.201500429
Fawaz W, Mosavati N, Abdelhamid E, Simon Ng KY (2019) Synthesis of activated carbons derived from avocado shells as cathode materials for lithium–sulfur batteries. SN Appl Sci 1:1–10. https://doi.org/10.1007/s42452-019-0300-3
Arie AA, Kristianto H, Cengiz EC, Demir-Cakan R (2019) Activated porous carbons originated from the Indonesian snake skin fruit peel as cathode components for lithium sulfur battery. Ionics (Kiel) 25:2121–2129. https://doi.org/10.1007/s11581-018-2712-2
Leng S, Chen C, Liu J et al (2019) Optimized sulfur-loading in nitrogen-doped porous carbon for high-capacity cathode of lithium–sulfur batteries. Appl Surf Sci 487:784–792. https://doi.org/10.1016/j.apsusc.2019.05.206
Xia L, Song Z, Zhou L et al (2019) Nitrogen and oxygen dual-doped hierarchical porous carbon derived from rapeseed meal for high performance lithium–sulfur batteries. J Solid State Chem 270:500–508. https://doi.org/10.1016/j.jssc.2018.12.031
Lei W, Liu H, Xiao J et al (2019) Moss-derived mesoporous carbon as bi-functional electrode materials for lithium-sulfur batteries and supercapacitors. Nanomaterials 9:84. https://doi.org/10.3390/nano9010084
Ren J, Zhou Y, Wu H et al (2019) Dandelion derived nitrogen-doped hollow carbon host for encapsulating sulfur in lithium sulfur battery. ACS Sustain Chem Eng 121:3042–3051. https://doi.org/10.1021/acssuschemeng.8b04648
Xue M, Lu W, Chen C et al (2019) Optimized synthesis of banana peel derived porous carbon and its application in lithium sulfur batteries. Mater Res Bull 112:269–280. https://doi.org/10.1016/j.materresbull.2018.12.035
You X, Liu L, Zhang M et al (2018) Novel biomass derived hierarchical porous carbon for lithium sulfur batteries. Mater Lett 217:167–170. https://doi.org/10.1016/j.matlet.2018.01.096
Chen F, Ma L, Ren J et al (2018) Wheat straw-derived N-, O-, and S-tri-doped porous carbon with ultrahigh specific surface area for lithium-sulfur batteries. Materials (Basel) 11:1–15. https://doi.org/10.3390/ma11060989
Xue M, Chen C, Tan Y et al (2018) Mangosteen peel-derived porous carbon: synthesis and its application in the sulfur cathode for lithium sulfur battery. J Mater Sci 53:11062–11077. https://doi.org/10.1007/s10853-018-2370-9
Zhou X, Lu X, Zhang Z et al (2018) Xanthoceras sorbifolia husks-derived porous carbon for sodium-ion and lithium-sulfur batteries. Diam Relat Mater 85:104–111. https://doi.org/10.1016/j.diamond.2018.04.007
Ren J, Zhou Y, Wu H et al (2019) Sulfur-encapsulated in heteroatom-doped hierarchical porous carbon derived from goat hair for high performance lithium–sulfur batteries. J Energy Chem 121:121–131. https://doi.org/10.1016/j.jechem.2018.01.015
Rybarczyk MK, Peng HJ, Tang C et al (2016) Porous carbon derived from rice husks as sustainable bioresources: Insights into the role of micro-/mesoporous hierarchy in hosting active species for lithium-sulphur batteries. Green Chem 18:5169–5179. https://doi.org/10.1039/c6gc00612d
Benítez A, Morales J, Caballero Á (2020) Pistachio shell-derived carbon activated with phosphoric acid: a more efficient procedure to improve the performance of li–s batteries. Nanomaterials. https://doi.org/10.3390/nano10050840
Benítez A, González-Tejero M, Caballero Á, Morales J (2018) Almond shell as a microporous carbon source for sustainable cathodes in lithium-sulfur batteries. Materials (Basel). https://doi.org/10.3390/ma11081428
Arie AA, Kristianto H, Cengiz EC, Demir-Cakan R (2020) Waste tea-based porous carbon–sulfur composite cathodes for lithium–sulfur battery. Ion 26:202–212. https://doi.org/10.1007/s11581-019-03196-x
Wang J, Nie P, Ding B et al (2017) Biomass derived carbon for energy storage devices. J Mater Chem A 5:2411–2428. https://doi.org/10.1039/C6TA08742F
Mahmood K, Kamilah H, Alias AK, Ariffin F (2018) Nutritional and therapeutic potentials of rambutan fruit (Nephelium lappaceum L.) and the by-products: a review. J Food Meas Charact 12:1556–1571. https://doi.org/10.1007/s11694-018-9771-y
Kumar M, Olajire Oyedun A, Kumar A (2018) A review on the current status of various hydrothermal technologies on biomass feedstock. Renew Sustain Energy Rev 81:1742–1770. https://doi.org/10.1016/j.rser.2017.05.270
Oh C, Yoon N, Choi J et al (2017) Enhanced Li-S battery performance based on solution-impregnation-assisted sulfur/mesoporous carbon cathodes and a carbon-coated separator. J Mater Chem A 5:5750–5760. https://doi.org/10.1039/c7ta01161j
Zhang B, Qin X, Li GR, Gao XP (2010) Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy Environ Sci 3:1531–1537. https://doi.org/10.1039/c002639e
Elazari R, Salitra G, Garsuch A et al (2011) Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li-S batteries. Adv Mater 23:5641–5644. https://doi.org/10.1002/adma.201103274
Kim H, Lim HD, Kim J, Kang K (2014) Graphene for advanced Li/S and Li/air batteries. J Mater Chem A 2:33–47. https://doi.org/10.1039/c3ta12522j
You X, long, Liu L jun, Zhang M yuan, et al (2018) Novel biomass derived hierarchical porous carbon for lithium sulfur batteries. Mater Lett 217:167–170. https://doi.org/10.1016/j.matlet.2018.01.096
Zhao Y, Zhang X, He Y et al (2017) Biomass derived nitrogen-doped highly porous carbon material with a hierarchical porous structure for high-performance lithium/sulfur batteries. Materials (Basel). https://doi.org/10.3390/ma10101158
Benítez A, Caballero A, Morales J et al (2019) Physical activation of graphene: an effective, simple and clean procedure for obtaining microporous graphene for high-performance Li/S batteries. Nano Res 12:759–766. https://doi.org/10.1007/s12274-019-2282-2
Acknowledgement
This study is supported by the “Direktorat Riset dan Pengabdian Masyarakat Kementerian Riset dan Teknologi/Badan Riset dan Inovasi Nasional” of Ministry of Research and Technology of Republic Indonesia (KEMENRISTEK/BRIN) through “Hibah Penelitian Dasar Sentralisasi Nasional” (Contract No: 020/SP2H/LT-AMAND/LL4/2020) in 2020) and Korea Institute of Science and Technology (KIST) Institutional Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arie, A.A., Kristianto, H., Susanti, R.F. et al. Rambutan peel derived porous carbons for lithium sulfur battery. SN Appl. Sci. 3, 541 (2021). https://doi.org/10.1007/s42452-021-04540-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04540-5