Abstract
A set of mono-disperse aerosol generator was designed to meet the requirement of efficiency testing for high efficiency particle air filter. The aerosol generation tests and performance tests were conducted by using evaporation–condensation method, with NaCl solutions at different concentrations as the condensation nucleus and respectively using the DEHS, DOP, PAO–4 as reagents. The results show that three reagents can generate mono-disperse aerosol particles by strictly controlling various parameters which affects the aerosol performance, where the particle size range is 0.33–0.36 μm for DEHS, 0.35–0.37 μm for DOP and 0.34–0.36 μm for PAO–4 and the concentrations of the aerosols lager than 106 cm–3. The particle size characteristics and concentrations generated through such method basically conform to the requirements of efficiency testing for high efficiency particle air filter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The radioactive aerosols formed by suspension of radioactive materials in the air are one of the most important pollutant sources in the workplace environment, such as nuclear technology applications, nuclear facilities, and nuclear and radiation terrorist incidents [7, 15, 18, 19]. The radioactive aerosols can invade into the human body through the human breathing, skin absorption and other means, which is one of the main ways to cause the internal irradiation hazards in the human body, [1, 9, 12, 13]. The high efficiency particle air (HEPA) filter is usually used in nuclear facility air purification system to remove the radioactive aerosol. This type of filter is characterized by the filtration efficiency of not less than 99.97% for particles with particle size of 0.3 μm. According to the requirements of US Military Standards MIL-STD-282 [3], each HEPA filter is tested for its efficiency by using the mono-disperse DOP aerosol with particle size of 0.30 ± 0.03 μm before leaving the factory.
Aerosol generators are widely used in aerosol instrument calibration, aerosol movement law research, aerosol respiratory deposition law research, filter aerosol mechanism research, and aerosol filter testing. The test aerosol produced is mono-disperse or poly-disperse, spherical or non-spherical, solid or liquid particles [2]. The ideal aerosol generator is required to continuously produce stable spherical solid aerosol particles and conveniently control the particle size and concentration of the aerosol particles. The commonly used methods for producing spherical mono-dispersity aerosols include atomization, vibration, and condensation [4, 11, 16]. At present, the evaporation–condensation method is a commonly used generation method for mono-disperse aerosol [14, 21]. The condensation-type mono-disperse aerosol generator can quickly generate the sub-micron mono-disperse aerosol particles (geometric standard deviation σg ≤ 1.25) with adjustable concentration and suitable for HEPA filter efficiency testing. This method was first proposed by Sinclair and Lamer, employing high-voltage electric sparks to heat NaC1 to produce condensation nucleus. It presented extremely high electrical insulation requirements and poor stability. Prodi improved the above device, used a sprayer to generate condensation nucleus, and adjusted the particle size of condensation nucleus by controlling the pressure of compressed air and the dilution ratio of the solution. In the past 20 years, the research on this type of aerosol generator has primarily used electromigration technology to produce nanometer aerosol particles [6, 17]. A series of research results and patented technology have made the mono-dispersion aerosol generator a product. Besides, it has exhibited relatively mature technology, stable performance, and broad application after gradual improvement and innovation. However, it is expensive. In China, there are no reports on the development of this equipment. This subject aims to master the generation method of condensation type mono-disperse aerosol through exploration and research, laying the foundation for the development of mono-disperse aerosol generator for HEPA filter detection. Considering that the DOP (Di-n-octyl-o-phthalate and 1-decene) reagents are carcinogenic and strongly irritative, there is a trend to use the non-toxic PAO (polydecene) liquids to substitute DOP in the world. Therefore, we have also studied the method to generate mono-disperse aerosols by using PAO.
This paper mainly introduces the design and establishment of a mono-disperse aerosol generator and emphatically introduces the test process of generating DEHS (Di-2-ethyl hexyl sebacate), DOP and PAO–4 (1-decene, tetramer mixed with 1-decene, commonly known as Emery 3004) mono-disperse aerosols. The best process parameters and particle size characteristic parameters of generated aerosols under the existing conditions are selected. The geometric mean diameter of the generated aerosol particles is about 0.35 μm, σg ≤ 1.25 and its concentration is 106 cm–3 or over.
2 Theory
2.1 Basic principle
The condensation-type mono-disperse aerosol generator generates the monodisperse aerosol by the principle of evaporation–condensation, that is, heating a liquid state aerosol substance (such as DEHS) to evaporates it into the supersaturated vapor, and then suddenly cooling to make the supersaturated vapor condense to form the aerosol particles. Based on the presence or absence of condensation nucleus in the condensation process, the condensation test can be divided into homogeneous condensation and heterogeneous condensation. The homogeneous condensation does not provide the additional condensation nucleus in the condensation process, therefore, the steam condensation environment is greatly different and the monodispersity is also poor; the heterogeneous condensation has the condensation nucleus to evenly condensate the steam and can generate the aerosols with good monodispersity, therefore, it is widely used.
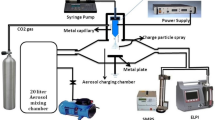
The basic principle of condensation-type mono-disperse aerosol generator is heterogeneous condensation and the source of condensation nucleus is generally the solid NaCl crystal particle. Figure 1 is the generation principle diagram. In Fig. 1, the clean nitrogen is used as the carrier gas and passes through the sprayer with inorganic salt solutions (generally NaCl solutions) at the certain pressure and flow rate, after the atomization, the small liquid drops are dried and crystallized into solid NaCl aerosol particle as condensation nucleus. The condensation nucleus particles are mixed with the aerosol substance evaporated from the saturator by heating and then enter into the reheater. The reheater heats the liquid drops in the mixture to completely evaporate into supersaturated vapor. Finally, the mixture (condensation nucleus particles, aerosol vapor, and nitrogen) enters into the condenser pipe and is suddenly cooled, and the vapor is condensed on the condensation nucleus to form the mono-disperse aerosol [5, 10, 20].
2.2 Technological process
According to the principle in Fig. 1, a process flow of mono-disperse aerosol generator as follows is designed (see Fig. 2). High-purity nitrogen enters into the sprayer under certain pressure after passing through the pressure regulating valve, and then sprays NaCl solution in the sprayer into fine NaCl drops which enter into the silica gel drying column and then form tiny NaCl solid crystal particles as the condensation nucleus after desiccation and crystallization. Later on, the condensation nucleus is divided into two parts: one part enters into the saturator containing liquid organic matters, and the other part directly enters into the reheater. The Liquid organic matters in the saturator are heated to evaporate and enter into the reheater under certain pressure and temperature, and those entering into the reheater are the mixture of condensation nucleus, nitrogen and vapor of organic matters. The mixture is further heated in the reheater to ensure no condensation prior to entry into condenser. Finally, in the condenser, the organic matters condensate on the condensation nucleus and form the mono-disperse aerosol.
2.3 Main technical specifications
Aerosols with particle size conforming to logarithmic normal distribution can be signified by geometric mean diameter dg and geometric standard deviation σg:
where di is the mean particle size of aerosol particle at the ith part; Ni is the number of aerosol particles at the ith part; Nt is total number of particles.
Aerosol dispersion is signified by relative standard deviation α, which is the ratio of the standard deviation of particle size to the mean radius of particles. Judgment standard for mono-disperse aerosol can be represented by α < 0.2 (lnσg < 0.2) or σg < 1.25. Hence, aerosol with σg < 1.25 is one condition for mono-dispersion, which is also main technical index of the designed device.
2.4 Particle size measurement
Particle size is the most attribute of aerosol behavior and characteristics. Different particle size testing technologies are the measurements on different particle characteristics, together with the measurement results to be different equivalent diameters. Common particle size measurement methods comprise: optical method, sedimentation method and scattering method, etc. Particle size measurement method used in this work is the scattering method.
Instrument used for measurement of aerosol particle size is Malvern Mastersizer2000 particle size measurer which measures the particle size by virtue of the scattering (diffraction) of particles to light, with particle size measurement in 0.02 μm ~ 2000 μm. Measurement principle of Mastersizer 2000 particle size measurer is as shown in Fig. 3. It consists of transmission, receiver and measuring window. Transmission part is constituted by the laser and beam processor, of which the laser can offer parallel lights of two different wave lengths. Measuring window is to obtain the particle size information of the sample. Receiver consists of Fourier lens and photodetector arrays. To enlarge the receiving angle of scattered light, a large-angle detection system is designed, which expands the lower detection limit of the instrument.
3 Experimental
3.1 Aerosol experiment with DEHS
According to Ref. [8], the mass concentration of NaCl solution C1, total flow Qt, saturator flow Qb, bypass flow Qp and saturator temperature T1 are the main parameters influencing the geometric mean diameter dg of aerosol particle.
Select the operating parameters recommended by Ref. [8]: concentration of NaCl solution C1 = 20 mg L–1, total flow Qt = 210 L h–1, saturator flow Qb = 150 L h–1, bypass flow Qp = 60 L h–1 and saturator temperature T1 = 120 ~ 200 °C, adjust the reheater temperature T2 (100 ~ 400 °C) and then generate the aerosol with DEHS. Measurement on the samples under various conditions shall be made with Mastersizer2000 particle size measurer, and dg and σg of samples are calculated.
3.2 Aerosol experiment with DOP
With different concentrations of NaCl solution selected, under C1 respectively at (1) 10 mg L–1; (2) 20 mg L–1; (3) 40 mg L–1; (4) 250 mg L–1, total flow Qt = 210 L h–1, saturator flow Qb = 150 L h–1, bypass flow Qp = 60 L h–1, and T1 = 100 ~ 160 °C, T2 (from 100 ~ 400 °C) is adjusted, and aerosol is generated by DOP. Measurement is made to the samples under various conditions with Mastersizer2000 particle size measurer, and dg and σg of various samples are calculated.
3.3 Aerosol experiment with PAO–4
With different concentrations of NaCl solutions selected, under C1 respectively at (1) 10 mg L–1; (2) 20 mg L–1; (3) 250 mg L–1, total flow Qt = 210 L h–1, saturator flow Qb = 150 L h–1, bypass flow Qp = 60 L h–1, and T1 = 100 ~ 190 °C, T2 (from 100 ~ 400 °C) is adjusted, and aerosol is generated by PAO–4. Measurement is made to the samples under various conditions with Mastersizer2000 particle size measurer, and dg and σg of various samples are calculated.
4 Results and discussion
4.1 Experiment results of DEHS
According to the results (see Table 1) of measurement samples, T1 = 150 °C is the optimal condition generating the mono-disperse DEHS aerosol, and T2 is of a wide requirement range within 150 ~ 325 °C, with particle size range at 0.33 ~ 0.36 μm, σg ≤ 1.25, and the concentrations of aerosol generated under various temperature conditions above 106 cm–3. Figure 3 is the particle size distribution diagram of one representative sample, and from the diagram, it can be seen that concentration of particle is up to 3.0 × 106 cm–3.
4.2 Experiment results of DOP
According to the results of measurement samples, it can be obtained that, upon T1 = 120 °C, DOP aerosols generated by NaCl solutions of four different concentrations are of best mono-dispersion, and all σg are basically less than 1.20 (except for very few samples), so that T1 = 120 °C is the optimal condition.
According the data calculated from measured samples, dg and σg of DOP aerosols generated by NaCl solutions of four different concentrations change with increase of T2 (see Figs. 4 and 5). From Figs. 4 and 5, it can be seen that, when DOP aerosol with NaCl solutions of four different concentrations as condensation nucleus is at T1 = 120 °C: (1) dg of aerosol generated under different concentrations are within 0.34 ~ 0.38 μm; (2) When C1 = 10, 20, 40 mg L–1, dg increases with the increase of T2 at first and then decreases; Upon C1 = 250 mg L–1, from T2 = 150 °C, dg decreases with the increase of T2, and in case of T2 = 400 °C, dg is minimum, at 0.34 μm; (3) All σg of aerosols generated under various concentrations are almost less than 1.25 (except for two points); (4) Upon T2 ≥ 200 °C, all σg of aerosols generated under various concentrations are less than 1.20; (5) When T2 changes in 100 ~ 400 °C, all σg of aerosol generated upon C1 = 250 mg L–1 are less than 1.20, which is the optimal condition for the generation of the mono-disperse aerosol.
In summary, in the test of DOP aerosol generated with NaCl solutions of four different concentrations as condensation nucleus, in case of controlled flow Qt = 210 L h–1, Qb = 150 L h–1 and Qp = 60 L h–1, C1 = 250 mg L–1 and T1 = 120 °C are the optimal conditions for the generation of mono-disperse DOP aerosol, and T2 is of a wide requirement range within 100 ~ 400 °C, with particle size range at 0.34 ~ 0.36 μm, σg ≤ 1.20, and corresponding particle concentration more than 106 cm−3.
4.3 Experiment results of PAO–4
According to the measurement results, upon T1 = 150 °C, PAO–4 aerosols generated by NaCl solutions of three different concentrations are of best mono-dispersion, and all σg are less than 1.20 except for individual samples, so that T1 = 150 °C is a optimal condition.
According the data calculated from measured samples, dg and σg of PAO–4 aerosols generated by NaCl solutions of three different concentrations change with increase of T2 (see Figs. 6 and 7). From Figs. 6 and 7, it can be seen that, when PAO–4 aerosol generated with NaCl solutions of three different concentrations as condensation nucleus is at T1 = 150 °C: (1) Upon C1 = 250 mg L–1 and T2 = 100 °C, dg is the maximum at 0.425 μm, and meanwhile, σg is the maximum at 1.65. Later on, dg decreases with increase of T2. Upon C1 = 10, 20 mg L–1, from T2 = 100 °C, dg increases at first and then decreases, so as the change trend of σg; (2) Upon T2 ≥ 150 °C, dg and σg curve of aerosols generated under various concentrations basically coincide, indicating small influence of NaCl solution concentration on dg and σg upon T2 within 150 ~ 400 °C; (3) Upon T2 within 150 ~ 400 °C, all σg of aerosols generated under various concentrations are less than 1.20; Especially upon T2 = 200 °C, dg is about 0.35 μm, with minimum σg about 1.16, but best mono-dispersion.
In summary, in the test of PAO–4 aerosol generated with NaCl solutions of three different concentrations as condensation nucleus, in case of controlled flow Qt = 210 L h–1, Qb = 150 L h–1 and Qp = 60 L h–1, T1 = 150 °C is the optimal condition for the generation of mono-disperse PAO–4 aerosol, and T2 is of a wide requirement range within 150 ~ 400 °C, with particle size range at 0.34 ~ 0.36 μm, σg < 1.20, and corresponding particle concentration more than 106 cm–3.
5 Conclusions
A set of aerosol generation experimental device is design according to the aerosol generation principle of evaporation–condensation method. On the experimental device, with parameters able to affect aerosol performance under strict control, aerosols with good mono-dispersion (σg ≤ 1.25) and particle concentration more than 106 cm–3 can be generated with DEHS, DOP and PAO–4 reagents. The optimization conditions are slightly different, upon use of DOP, the saturator temperature is at 120 °C, while it is at 150 °C in using DEHS and PAO–4; concentrations of NaCl are also different, but which is easy to achieve. These three reagents all can be taken as reagents for efficiency testing of nuclear HEPA filter, of which DOP has been extensively applied in the production practices, but PAO-4 is of a better comprehensive performance. Particle size range of mono-disperse aerosols generated by the three reagents respectively is 0.33 ~ 0.36 μm, 0.35 ~ 0.37 μm, 0.34 ~ 0.36 μm and large than (0.30 ± 0.03) μm, but has a little influence on testing result; Mono-disperse aerosol particles (0.30 ± 0.03) μm generated from further experiment is our next assumption and target.
The evaporation–condensation method can produce aerosols with acceptable mono-dispersity, providing technical support for the efficiency testing of the HEPA filter. Besides, the secondary waste generated during the method operation is less, generating social and economic benefits. The built aerosol generator presents low cost, reliable performance, and flexible use. Moreover, the generated particles can meet the simulation of radioactive aerosol characteristics and the research on filtration purification technology. However, the aerosol generator needs to use high-pressure nitrogen as a carrier gas, limiting it to the laboratory environment. The use of the device can be effectively expanded if the sprayer device can be improved and the ultrasonic atomization method is used to achieve the atomization of the NaCl solution.
References
Boulyga SF, Lomonosova EM, Zhuk IV, Yaroshevich OI, Kudrjashov VP, Mironov VP (1999) Experimental study of radioactive aerosol in the vicinity of the Chernobyl Nuclear Power Plant. Radiat Meas 30:703–707
Cheng YS, Chen BT (2008) Aerosol sampler calibration. air sampling instruments Committee. ACGIH. Inc, Cincinnati, pp 165–186
Department of defense (1956). MIL-STD -282 Filter units, protective clothing, gas-mask components and related products: performence test methds. USA. Washington
Fuchs NA, Sutugin AG (1966) Aemsd science. Academic Press, London, pp 1–31
Geryes T, Monsanglant-Louvet C, Gehin E (2009) Experimental and simulation methods to evaluate the alpha self-absorption factors for radioactive aerosol fiber filters. Radiat Meas 44:763–765
Haas V, Birringer R, Gleiter H et al (1997) Synthesis of nanostructured powders in an aerosol flow condenser. J Aerosol Sci 28:1443–1453
Henry B, Ewa MB, Malgorzata K, Monika O (2002) Determination of radioactivity in air filters by alpha and gamma spectrometry. Nukleonika 47:87–91
Horton K, Miller R, Mitchell J (1991) Characterization of a condensation-type mono-disperse aerosol generator (MAGE). J Aerosol Sci 22:347–363
Kim S, Lee HY, Song JS (2018) A study on characteristics and internal exposure evaluation of radioactive aerosols during pipe cutting in decommissioning of nuclear power plant. Nucl Eng Technol 50:1088–1098
Klett A, Reuter W, De ML (1997) Dynamic calibration of an aerosol monitor with natural and artificial alpha-emitters. IEEE T Nucl Sci 44:804–805
Mercer T (1973) Production and characterization of aerosols. Arch Int Med 131:39–50
Muramatsu H, Kawasumi K, Kondo T, Matsuo K, Itoh S (2015) Size-distribution of airborne radioactive particles from the Fukushima accident. J Radioanal Nucl Ch 303:1459–1463
Neroda AS, Mishukov VF, Goryachev VA, Simonenkov DV, Goncharova AA (2014) Radioactive isotopes in atmospheric aerosols over Russia and the Sea of Japan following nuclear accident at Fukushima Nr. 1 Daiichi Nuclear Power Station in March 2011. Envion Sci Pollut Res 21:5669–5677
Niu FL, Du XC, Qi HB, Yi MQ, Yang X (2016) Modeling analyses of radioactive aerosol flow and collection in mesoscopic impactor filters. Prog Nucl Energ 88:147–155
Peter HM (2000) A review of atmospheric aerosol measurments. Atmos Environ 34:1959–1999
Raabe O (1976) The generation of aerosols ofthe fine partides In fine particles: aerosol generation, measurement, sampling and analysis. Academic Press, New York, pp 57–110
Singh Y, Javier JR, Ehrman SH et al (2002) Approaches to increasing yield in evaporation/condensation nanoparticle generation. J Aerosol Sci 33:1309–1325
Suzuki T, Swift DL, Wagner HN (1982) A new apparatus for generating hygroscopic radioactive aerosols for inhalation studies. Eur J Nucl Med Mol 7:474–479
Thakur P, Ballard S, Nelson R (2013) An overview of Fukushima radionuclides measured in the northern hemisphere. Sci Total Environ 460:577–613
Vargas A, Arnold D, Ortega X, Parages C (2008) Influence of natural radioactive aerosols on artificial radioactivity detection in the Spanish surveillance networks. Appl Radiat Isotopes 66:1627–1631
Xu HK, Huang ZH, Wang G, Mu CL, Yin Y (2017) Design of a new comprehensive continuous monitoring system for environmental radioactive aerosol. Appl Radiat Isotopes 120:82–88
Acknowledgements
This work was supported by the Natural Science Foundation of Chinese Program (No. 41804114) and Shenyang Science and Technology Bureau (No. 20-206-4-03). The authors would like to express thanks to the China Institute of Atomic Energy for its support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, G., Pan, L., Xu, Y. et al. Design of a mono-disperse aerosol generator for efficiency testing of HEPA filter. SN Appl. Sci. 3, 472 (2021). https://doi.org/10.1007/s42452-021-04480-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04480-0