Abstract
Different phase compositions of mesoporous nanocrystalline TiO2 (meso-nc-TiO2), comprised of anatase (16–100%), rutile (0–70%) and brookite (0–52%) were obtained by sol–gel synthesis with or without hydrothermal treatment (HTT) by means of titanium tetrabutoxide and dibenzo-18-croun-6 as structure-forming agent in the presence of HCl. It was shown, that small amounts of surfactant and/or lanthanum salt as well as HTT determine phase composition and texture of meso-nc-TiO2. All samples were calcined at 500 оС and characterized by SEM, TEM, XRD and N2-adsorption/desorption isotherms. It has been established that photocatalytic properties of almost all obtained samples significantly exceed the photocatalytic activity of Evonik P-25 TiO2 in gas phase ethanol oxidation. The most active sample is characterized by phase composition of anatase (97%)-rutile (3%). It is obvious, that decrease of photocatalytic activity of sample was affected by decrease of anatase phase content. It was shown that the specific surface area of the sample is not a key factor affecting the activity of mixed-phase meso-nc-TiO2 samples in the process of ethanol oxidation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solving the global energy problems of modern society requires the development of various areas of “green technology”. Today, in the field of semiconductor photocatalysis, TiO2 is an undoubted leader both in terms of demand and research intensity (including the synthesis and testing of a wide range of new composite titania-based materials) due to the unique combination of its properties and, first of all, non-toxicity, chemical stability and low cost [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. The efficiency of TiO2 photocatalyst is determined by numerous parameters: crystallinity, phase composition, crystallite size, particle morphology, porosity, developed surface area, surface organization, etc. [16, 20,21,22,23,24]. The classic definition of “the ideal powder should be pure, stoichiometric, dense, spheroidal, and nearly monodisperse” was made by Brinker and Scherer back in 1990 [25]. 5 years later, Antonelli and Ying [26] proposed their own method of the sol–gel synthesis of mesoporous TiO2 with a high specific surface, thereby indicating the main direction of researchers’ efforts to improve TiO2 photocatalysts.
Undoubtedly, the sol–gel template method still remains one of the most advanced methods for producing mesoporous oxides. Its combination with widely used solvo (hydro) thermal processing of an intermediate amorphous organo-inorganic product allows controlling the processes of formation of unique TiO2 materials with a certain morphology and texture at the nanoscale level [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41].
Hydrothermal treatment at relatively low temperatures (< 200 °C) gives the possibility to obtain highly homogeneous nanocrystalline TiO2 powders [16, 29, 31, 35, 37, 42, 43]. However, in the process of manufacturing a highly efficient photocatalyst, the obtained powders, as a rule, require calcination at temperatures not lower than 500 °C to achieve full catalytic potential [23, 24, 29, 36, 40, 44,45,46,47].
TiO2 exists mainly in three different crystalline forms: anatase, rutile and brookite. Anatase has the highest photoactivity [48]. However, the interest in obtaining TiO2 nanocomposites, which consist of two or three polymorphs (relatively few works for latter), is not diminishing. The results obtained in these works sometimes lead to contradictory conclusions when comparing of the photocatalytic activity of the TiO2 mixed-phase in various redox processes. Many authors reported that the photocatalytic activity of mixtures of TiO2 phases in a number of processes is higher in comparison with the pure single polymorphs [14, 16, 20, 21, 24, 49,50,51]. It is attributed to the synergistic effect of different positions of the energy levels of different phases of titanium dioxide, which leads to the spatial separation of photogenerated charges in mixed-phase materials, decrease in the negative electron – hole recombination of charges, and increase in the activity of these materials in various redox processes [20, 31, 36, 52,53,54,55,56,57,58].
Earlier, under conditions of neutral hydrolysis, samples of thermally stable meso-nc-TiO2 with a pure anatase structure were obtained [59,60,61,62,63], demonstrating different textures with well-defined spherical morphology (micro- and nanospheres) and a narrow pore distribution. Calcined at 500 °C after hydrothermal treatment at 175 °C TiO2 samples had a radically more developed porous structure compared to samples without hydrothermal treatment [59]. Mesoporous hierarchical TiO2 microspheres ranging in size from 0.6 to 3.0 μm, were formed by aggregation and agglomeration of homogeneous nanoparticles (30–70 nm), in turn formed by primary spherical particles—anatase crystallites of about 10 nm (XRD). The introduction of small amounts of surfactant and a lanthanum salt into the reaction mixture led to increase in crystallinity, change in morphology, and development of the of meso-nc-TiO2 texture. Spherical particles of micrometer scale in the presence of La3+ ions are not formed. It should be emphasized that anatase is the only crystalline phase (in the absence of signs of phase transformation) of meso-nc-TiO2 formed under neutral conditions. Almost all the obtained samples are highly effective photocatalysts for water reduction, gas-phase oxidation of alcohol and benzene [61,62,63,64].
In the present work, the above approach was used for synthesis of meso-nc-TiO2 samples of both anatase and various phase compositions (anatase, brookite, rutile), as a combination of two or three phases, using hydrochloric acid (HC1) as a catalyst for hydrolysis of the TiO2 precursor. All samples were calcined at 500 оС.
2 Materials and methods
2.1 Materials
Titanium tetrabutoxide (IV), dibenzo-18-crown-6 (Sigma-Aldrich), dodecyldi- methylethylammonium bromide (Fluka), НCl acid, n-butanol, NaCl and ethanol (96%) (Himlaborreactiv) were used. Commercial TiO2 Evonik-P25 (Evonik Corp) was chosen as the standard photocatalyst.
2.2 Synthesis
Samples of meso-nc-TiO2 with different phase compositions were obtained using the modified sol–gel method described in detail in our works [59, 60]. Dibenzo-18-crown-6 (in the form of the sodium complex [Na(DB18C6)]Cl due to the low solubility of crown ether in butanol (BuOH)) was used as a template in the presence (or in the absence) small additives of the cationic surfactant dodecyldimethylethylammonium bromide (DDMEABr) and/or lanthanum salt. The calculated amounts of reagents were sequentially dissolved in butanol (BuOH). Titanium tetrabutoxide (TBOT) was added dropwise under vigorous stirring. In contrast to the mentioned works, in this work, TBOT was hydrolyzed in an acidic medium using hydrochloric acid as catalyst.
The reaction mixture was left under a glass hood in air at room temperature (without stirring) until gel formation has stopped. HTT was carried out at 175 °C for 24 h. All samples (both treated and untreated hydrothermally) were calcined at 500 °C for 4 h.
Samples obtained under various conditions were designated as Tn and TnH, where n is the number of the sample and H denotes hydrothermal treatment. The molar ratios of the reaction mixture used for the synthesis are shown in Table 1:
2.3 2.3 Characterization
A.Dron-3.M diffractometer with Cu Kα (λ = 1.5406 Ȧ) radiation was used for the X-ray diffraction (XRD) analysis to determine the crystal structure and crystallinity of TiO2. The average size of TiO2 crystallites (2R) was estimated from the broadening of the anatase peak at 2Θ = 25.4o (101) in XRD-spectra using well-known Scherrer’s equation.
The morphology of the samples was observed using transmission electron microscopy (TEM) and scanning electron microscopy (SEM) оn a JEOL JEM 1230 and а JEOL JSM 6060 LA microscopes respectively. Energy dispersive X-ray (EDX) analysis were performed with a Tescan Measure 3 microscope, equipped with an Oxford X-max energy-dispersive X-ray assay for 80 mm2, for an accelerating voltage of 5–20 kV. Electron diffraction patterns were obtained on a transmission electron microscope Selmi TEM-125К with the accelerating voltage of 100 kV.
In some cases, the samples were pre-treated with an ultra sound disperser UZDN-A (radiation power 130 W) for 5 min.
N2 adsorption–desorption isotherms were recorded at 77 K using an Autosorb-6 automated gas adsorption analyzer (Quantachrome). Prior to the measurements, samples were degassed at 473 K for 12 h. The specific surface area (SBET) of the samples was determined by the BET method. The average pore size (Dp) was calculated using BJH method. The NLDFT method (cylindrical pore model) was used to calculate the pore size distribution from the desorption branch. The mesopore volume (Vp) was determined using amount of N2 adsorbed at relative pressure p/p0 of 0.997.
2.4 Photocatalytic oxidation studies
The photocatalytic activity of the obtained meso-nc-TiO2 samples was studied in the gas-phase reaction of ethanol oxidation. A 50 mg titanium dioxide sample pressed onto a 1 × 3 cm copper support was put into a 150 cm3 glass reactor, equipped with a magnetic stirrer and membrane for sampling. The required amount of alcohol was introduced into the reactor using a microsyringe. For complete evaporation of the alcohol and adsorption equilibrium establishing, the reactor was kept without irradiation with stirring of the gas mixture for ~ 2 h. The samples were irradiated with the focused light of a DRSh-1000 mercury lamp. A region with λ = 310–390 nm was isolated from the radiation spectrum by means of filters. The light intensity was measured using a ferrioxalate actinometer (I = 5 µmol of quants per min). The concentration of the starting substrate was determined using a CHROM 5 gas chromatograph equipped with a flame ionization detector and a column packed with Porapak Q. The initial oxidation rates were determined using the initial linear region over approximately 30 min of irradiation as the ratio of the change in ethanol concentration in the reactor to the irradiation time.
3 Results and discussion
Samples of meso-nc-TiO2 with different phase composition were obtained by a simple modification of the previously proposed approach [59] through changing the conditions of TBOT hydrolysis, namely, the transition from a neutral to an acidic environment using a well-known acid catalyst—hydrochloric acid [23, 53, 65]. HCl is the most commonly used due to the lower electronegativity of the chloride anion compared with others and bonding of the Cl- ion to the Ti atom. HCl promotes the hydrolysis reaction versus the condensation reaction [35] as well as serves as an electrolyte to prevent particle growth or agglomeration through electrostatic repulsion. The main concept of such optimization of the previous approach [59, 60] was to combine the factors: HTT, small additives of surfactant and (or) lanthanum salt, the influence of reagents concentration in the presence of HCl on the phase composition of meso-nc-TiO2 materials (at the same calcination temperature—500 оС).
Figure 1 shows the diffraction patterns of meso-nc-TiO2 samples obtained in an acidic medium in the presence of HCl. The phase content of each polymorph was determined by the main diffraction peaks of anatase, rutile and brookite, designated as (101), (110) and (121) reflexes, respectively, using the well-known calculation method [66]. The diffraction patterns of samples T2 and T4 contain weak narrow peaks at 32° and 45.5° (2Θ), which can be attributed to NaCl, occluded by the main product during the synthesis [60]. The content of NaCl in the samples was confirmed by the EDX (Table S1). Calculated from XRD date, size of anatase crystallites in samples under consideration is about 10 nm (see Table 2).
Figures 2 and S1 show the N2 adsorption-desorption isotherms and pore size distributions for meso-nc-TiO2 samples. All isotherms belong to type IV IUPAC classification with H1 and H2 hysteresis loops located at relative pressure p/po values higher than 0.6, indicating the formation of mesopores in the obtained samples [67, 68].
Table 2 shows the phase composition and texture characteristics of meso-nc-TiO2 samples. As can be seen from Figs. 1 and 2, S1 and Table 2, the phase composition and texture characteristics of meso-nc-TiO2 samples were influenced by DDMEABr surfactant additive, lanthanum salt, and concentration of reagents in the initial reaction mixture during synthesis, as well as the heat treatment regime (HTT before calcination).
The efficiency of crown ether templating activity was improved by addition of minute amounts of surfactant using previously described method [60]. The possible mechanism of the process can be explained by the destructuring effect of the surfactant on the solvation shell of the crown ether complex. “Naked” oxygen atoms from the crown ether can react with structure-forming fragmens with Ti to form TiO2 mesophase framework. As can be seen from Table 2, the addition of DDMEABr lead to the decrease of anatase and brookite content and increase in the content of rutile, respectively (samples T1 and T2). The differences become more visible in a case of HTT processing of these samples (samples T1H and T2H). The presence of a surfactant additive opens possibility for the synthesis of T2H sample with the highest rutile phase content of 70%. The presence of a surfactant lead to a slight increase in the specific surface area (SBET) in the case of calcined samples T1 and T2 (39 m2/g and 48 m2/g), on the contrary, for the T1H and T2H samples (with preliminary hydrothermal treatment), there are almost twofold decrease of the corresponding SBET values (61 m2/g and 33 m2/g). However, surfactant did not affect the pore diameter of the specified pairs of samples (samples T1−T2 and T1H−T2H) in a great extent.
The addition of lanthanum salt promotes the increase the anatase content and decreases the brookite content regardless of the use of HTT (samples T1, T1H and T3, T3H), while the content of rutile decreases in the absence of HTT (samples T1 and T3) and increases at HTT (samples T1H and T3H). In the case of samples obtained with the simultaneous presence of DDMEABr and lanthanum salt (T1, T1H and T5, T5H, T6H), the three-fold increase of the anatase content is observed along with the a sharp drop of the brookite content and complete disappearance of the rutile phase. The phase composition of the sample undergoes especially profound changes when using HTT (samples T1H and T5H), when the three-phase composition turn into anatase monophase (Fig. 1; Table 2).
Addition of La3+ ions to the initial reaction mixture provided a significant increase in the specific surface area. So, for example, for samples T1 and T3, the value of SBET increases twofold (39 m2/g and 89 m2/g, respectively). Doping with small amounts of La3+ cations was successfully used for thermal stability increasing of mesoporous anatase [60]. The role of this cation in stabilizing the structure of mesoporous TiO2 has been explained by implantation of rare-earth cations into framework of the oxide system. Lanthanum is more electropositive than titanium and thus formation of the La–O bond causes partial transfer of electrons from the La–O bond to the Ti–O bond leading to strengthening of the latter. La3+ does not enter into the crystal lattice of TiO2 and was uniformly dispersed onto TiO2, supported by the results of EDX analysis (Table. S1). The temperature of anatase–rutile transformation significantly increases in the presence of La3+ as compared to pure TiO2.
Dilution of the initial reaction mixture in the presence of lanthanum salt (samples T3, T3H and T4, T4H, Table 1) lead to a twofold increase of the anatase content, along with the decrease of the content of brookite and rutile. For example, sample T3 comprises of anatase (55%), rutile (21%) and brookite (24%), and the corresponding sample T4 has only anatase (97%) and rutile (3%) phases. An increase of the concentration of reagents in the initial reaction mixture containing additives of DDMEABr and lanthanum salts (samples Т5Н, Т6Н and Т7Н) allows formation of one, two, and three phase TiO2 compositions (Fig. 1; Table 2). In the case of sample T7H (compared with samples T5H and T6H), additional amounts of Н2О and НСl, apparently, have a definite effect on the phase composition of this sample.
Upon dilution of the initial reaction mixture in 1.5 times, the specific surface area increases by a factor of ~ 1.5 (samples T3, T3H and T4, T4H), while the pore diameter decreases (samples T3H and T4H). The simultaneous introduction of surfactant additives and La3+ ions (samples T5 and T5H) lead to a tremendous increase in the specific surface area SBET of meso-nc-TiO2 samples compared to samples obtained only in the presence of surfactant (samples T2 and T2H) or La3+ ions (samples T3 and T3H).
As follows from a comparison of the texture characteristics of the samples presented in Table 2, the Т5Н sample has the largest specific surface area (SBET = 132 m2/g) and pore volume (Vp = 0.46 cm3/g). An increase of the concentration of the starting reagents (samples T5H and T6H) lead to a 1.5-fold decrease in the specific surface and a substantial increase in pore diameter (~ 25%) with a constant pore volume.
The values of the average sizes of anatase crystallites presented in Table 2 fall in the range of 7.0–10.4 nm, and the highest values are observed for samples T1 (9.8 nm) and T1H (10.4 nm) obtained without DDMEABr or lanthanum salt.
The choice of the heat treatment mode (calcination or calcination with preliminary hydrothermal treatment) significantly affects the phase composition of meso-nc-TiO2 nanocomposites (samples T1 and T1H, T2 and T2H, T3 and T3H, T4 and T4H, T5 and T5H) in all cases. For example, a T1 sample subjected to calcination only has a composition of anatase (29%), rutile (39%) and brookite (32%), and a T1H sample (preliminary hydrothermally treated)-anatase (34%), rutile (14%) and brookite (52%). It should be noted that among all meso-nc-TiO2 samples, the T1H sample (obtained in the absence of any additives) contains the highest amount of brookite.
Comparison of the texture characteristics of samples T2 and T2H (obtained in the presence of a surfactant), T3 and T3H, T4 and T4H (obtained in the presence of lanthanum salt), hydrothermal treatment before calcination lead to a decrease in the specific surface (~ 20–30%) and a significant increase in pore diameter (~ 20–80%) along with a change in pore volume (an increase of almost 1.5 times) only in the case of the T3H sample (Table 2). In the case of samples T5 and T5H (obtained with the simultaneous presence of La3+ ions and a surfactant), on the contrary, the T5H sample previously hydrothermally treated before calcination compared to a simply calcined sample T5 showed an increase in specific surface area (~ 35%) with a simultaneous increase in ~ 2.5 times the pore volume and in 2 times the pore diameter.
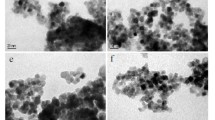
As follows from the TEM data (Figs. 3, S2–7), all samples of meso-nc-TiO2 has homogeneous, spherical primary particles (anatase phase). The sizes of anatase crystallites in the samples estimated from TEM are in good agreement with the corresponding values, calculated by the Scherrer formula (Table 2). Samples in which the percentage of the rutile and brookite phases are high (Т1, Т1Н, Т2, Т2Н, Т3, Т3Н, T7H), shows the presence of crystallites in the form of variously sized plates of hexagons and quadrangles shapes (Fig. 3a–d, Fig. S2–7). Electron diffraction patterns are in good agreement with the XRD (Fig. S8–9).
Figures 4, 5, 6 and 7, S10–18 show SEM images of meso-nc-TiO2 samples. As follows from Figs. 4, S10–12 there are no microspheres in samples of T1 and T1H, T2 and T2H obtained with and without HTT. Earlier [59,60,61, 63], we have showed that TiO2 samples obtained in a similar way under neutral conditions—microscopic particles with a well-defined spherical shape, were mesoporous TiO2 microspheres (1–3 μm), which consist of spherical homogeneous nanoparticles (secondary particles ) 30–50 nm in size, which in turn were formed by aggregation of anatase crystallites (primary particles) of about 10 nm in size. This led us to the conclusion that the presence of hydrochloric acid prevented the formation of microspheres. All meso-nc-TiO2 samples formed in acidic medium were aggregates of various sizes (from 100 to 500 nm). During ultrasonic treatment of samples T1H, T4, T4H, T5H, T6H, the aggregates break up into homogeneous nanoscale (40–100 nm) secondary particles of spherical or spheroidal shape (Figs. 4b, 6a, e, f, 7a, b, S15).
4 Photochemistry
For evaluation of photocatalytic activity of meso-nc-TiO2 samples, we used the gas-phase oxidation of ethanol as a model process for the oxidation of organic substrates [69,70,71,72,73].
Such an assessment is of particular interest because all titanium polymorph compositions were obtained within the same synthetic method using the same heat treatment mode (calcination at 500 °C) [74,75,76].
Histograms of the phase composition of meso-nc-TiO2 samples and the corresponding values of ethanol oxidation initial rates are shown in the Fig. 8a, b. As can be seen, all the samples significantly outperformed the commercial photocatalyst Evonik P25 in photocatalytic activity. The dependence of the photocatalytic activity of the obtained samples, except for T4 and T4H, in the process under study increases almost linearly with an increase in the content of the anatase phase (Fig. 9a). The activity of the T4 and T4H samples is significantly higher than the activity of the meso-nc-TiO2 samples with similar anatase content (T5, T5H, T6H) (Fig. 9a).
Sample T4 has a composition of anatase (97%)-rutile (3%) phases, and sample T4H-anatase (85%)-rutile (4%)-brookite (11%) phases. The Т5Н sample (anatase ~ 100%), was noticeably inferior in activity to the Т4 and Т4Н samples. Samples T5 and Т6Н are very close in composition (88% anatase and 90% anatase, 12% brookite and 10% brookite, respectively), but, unlike the T4 biphase sample and the T4H three-phase sample, did not contain rutile phase.
Samples T4 and T4H also significantly deviate from the dependence of the photocatalytic activity of samples on their specific surface area (Fig. 9b). In this case, the activity of the T4 and T4H samples is significantly higher than that of the samples with a similar specific surface area (T5, T5H, T7H).
The observed picture can apparently be explained by the synergistic effect [16, 19, 77] due to the combination of the anatase and rutile phases in the phase compositions of T4 (anatase (97%)-rutile (3%)) and T4H (anatase (85%)-rutile (4%)-brookite (11%)). The authors of [77] have showed that even 2% content of rutile in anatase-rutile mixed-phase promotes the photocatalytic activity of TiO2. A significant increase in the photocatalytic activity of anatase composites with a small amount of rutile can be associated with the different positions of the energy levels in anatase and rutile [78], which leads to spatial separation of photogenerated charges between the components of the composite and a decrease in the unwanted electron-hole recombination (Fig. S19). In the case of composites of anatase with brookite, the synergistic effect is not observed, which is associated with a higher level of the conduction band of brookite compared to anatase [78] and the absence of separation of charges photogenerated in anatase between the components of the composite (Fig. S19). In other samples obtained containing rutile, the positive effect of charge separation is compensated by the relatively low content of the active phase of anatase.
5 Conclusions
Binary and ternary phase mixtures of mesoporous nanocrystalline titanium dioxide samples with different phase composition and texture were obtained by sol–gel synthesis (with or without hydrothermal treatment). The anatase phase, the content of which in the samples ranged from 16 to ~ 100%, consisted of homogeneous spherical anatase crystallites ~ 10 nm in size.
The phase composition of the meso-nc-TiO2 samples was formed by varying the concentrations of the reagents, using small additions of dodecyldimethylethylammonium bromide surfactant and/or lanthanum salt in the reaction mixture and hydrothermal treatment. All synthetized samples were calcined at the same temperature of 500 °C for 4 h. This is the crucial condition for formation a number of photocatalysts with different phase composition for a comparative assessment of their photocatalytic activity. Meso-nc-TiO2 samples with anatase content over 80% had the high level of photoactivity. The highest photocatalytic activity was demonstrated by the sample with an anatase phase (97%)-rutile (3%) composition.
References
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107(7):2891–2959. https://doi.org/10.1021/cr0500535
Nakata K, Fujishima A (2012) TiO2 photocatalysis: design and applications. J Photochem Photobiol C Photochem Rev 13(3):169–189. https://doi.org/10.1016/j.jphotochemrev.2012.06.001
Soler-Illia GJ, de AA, Sanchez, Lebeau C, Patarin B J (2002) Chemical strategies to design textured materials: from microporous and mesoporous oxides to nanonetworks and hierarchical structures. Chem Rev 102(11):4093–4138. https://doi.org/10.1021/cr0200062
Pelaez M, Nolan NT, Pillai SC, Seery MK, Falaras P, Kontos AG, Dunlop PSM, Hamilton JWJ, Byrne JA, O’Shea K, Entezari MH, Dionysiou DD (2012) A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Catal B Environ 125:331–349. https://doi.org/10.1016/j.apcatb.2012.05.036
Hagfeldt A, Graetzel M (1995) Light-induced redox reactions in nanocrystalline systems. Chem Rev 95(1):49–68. https://doi.org/10.1021/cr00033a003
Tong H, Ouyang S, Bi Y, Umezawa N, Oshikiri M, Ye J (2012) Nano-photocatalytic materials: possibilities and challenges. Adv Mater 24(2):229–251. https://doi.org/10.1002/adma.201102752
Fresno F, Portela R, Suárez S, Coronado JM (2014) Photocatalytic materials: recent achievements and near future trends. J Mater Chem A 2(9):2863–2884. https://doi.org/10.1039/C3TA13793G
Wang Y, Sun C, Zhao X, Cui B, Zeng Z, Wang A, Liu G, Cui H (2016) The application of nano-TiO2 photo semiconductors in agriculture. Nanoscale Res Lett 11(1):529. https://doi.org/10.1186/s11671-016-1721-1
Kumar N, Chauhan NS, Mittal A, Sharma S (2018) TiO2 and its composites as promising biomaterials: a review. Biometals 31(2):147–159. https://doi.org/10.1007/s10534-018-0078-6
Macwan DP, Dave PN, Chaturvedi S (2011) A review on nano-TiO2 sol–gel type syntheses and its applications. J Mater Sci 46(11):3669–3686. https://doi.org/10.1007/s10853-011-5378-y
Noman MT, Ashraf MA, Ali A (2019) Synthesis and applications of nano-TiO2: a review. Environ Sci Pollut Res 26(4):3262–3291. https://doi.org/10.1007/s11356-018-3884-z
Humayun M, Raziq F, Khan A, Luo W (2018) Modification strategies of TiO2 for potential applications in photocatalysis: a critical review. Green Chem Lett Rev 11(2):86–102. https://doi.org/10.1080/17518253.2018.1440324
Zhang W, Zou L, Wang L (2009) Photocatalytic TiO2/adsorbent nanocomposites prepared via wet chemical impregnation for wastewater treatment: a review. Appl Catal A Gen 371(1–2):1–9. https://doi.org/10.1016/j.apcata.2009.09.038
Verma R, Gangwar J, Srivastava AK (2017) Multiphase TiO2 nanostructures: a review of efficient synthesis, growth mechanism, probing capabilities, and applications in bio-safety and health. RSC Adv 7(70):44199–44224. https://doi.org/10.1039/C7RA06925A
Etacheri V, Di Valentin C, Schneider J, Bahnemann D, Pillai SC (2015) Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J Photochem Photobiol C Photochem Rev 25:1–29. https://doi.org/10.1016/j.jphotochemrev.2015.08.003
Bagheri S, Julkapli NM (2017) Mixed-phase TiO2 photocatalysis: correlation between phase composition and photodecomposition of water pollutants. Rev Inorg Chem 37(1):11–28. https://doi.org/10.1515/revic-2016-0001
Deng X, Wang Y, Chen Y, Cui Z, Shi C (2019) Yttrium-doped TiO2 compact layers for efficient perovskite solar cells. J Solid State Chem 275:206–209. https://doi.org/10.1016/j.jssc.2019.04.022
Wen J, Xie J, Chen X, Li X (2017) A review on g-C 3 N 4 -based photocatalysts. Appl Surf Sci 391:72–123. https://doi.org/10.1016/j.apsusc.2016.07.030
Paul KK, Giri PK (2018) Shape tailored TiO2 nanostructures and their hybrids for advanced energy and environmental applications: a review. J Nanosci Nanotechnol 19(1):307–331. https://doi.org/10.1166/jnn.2019.15778
Luo Z, Poyraz AS, Kuo C-H, Miao R, Meng Y, Chen S-Y, Jiang T, Wenos C, Suib SL (2015) Crystalline mixed phase (anatase/rutile) mesoporous titanium dioxides for visible light photocatalytic activity. Chem Mater 27(1):6–17. https://doi.org/10.1021/cm5035112
Qiu Y, Ouyang F, Zhu R (2017) A facile nonaqueous route for preparing mixed-phase TiO2 with high activity in photocatalytic hydrogen generation. Int J Hydrog Energy 42(16):11364–11371. https://doi.org/10.1016/j.ijhydene.2017.03.047
Chang JA, Vithal M, Baek IC, Seok S, Il (2009) Morphological and phase evolution of TiO2 nanocrystals prepared from peroxotitanate complex aqueous solution: influence of acetic acid. J Solid State Chem 182(4):749–756. https://doi.org/10.1016/j.jssc.2008.12.024
Cano-Casanova L, Amorós-Pérez A, Lillo-Ródenas M, Román-Martínez M (2018) Effect of the preparation method (sol–gel or hydrothermal) and conditions on the TiO2 properties and activity for propene oxidation. Materials (Basel) 11(11):2227. https://doi.org/10.3390/ma11112227
Di Paola A, Bellardita M, Palmisano L (2013) Brookite, the least known TiO2 photocatalyst. Catalysts 3(1):36–73. https://doi.org/10.3390/catal3010036
Brinker CJ, Scherer GW (1990) Sol–gel science: the physics and chemistry of sol–gel processing. Academic Press, Boston. https://doi.org/10.1002/adma.19910031025
Antonelli DM, Ying JY (1995) Synthesis of hexagonally packed mesoporous TiO2 by a modified sol–gel method. Angew Chemie Int Ed English 34(18):2014–2017. https://doi.org/10.1002/anie.199520141
Yang P, Zhao D, Margolese DI, Chmelka BF, Stucky GD (1998) Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks. Nature 396(6707):152–155. https://doi.org/10.1038/24132
Ismail AA, Bahnemann DW (2011) Mesoporous titania photocatalysts: preparation, characterization and reaction mechanisms. J Mater Chem 21(32):11686. https://doi.org/10.1039/c1jm10407a
Chen L-H, Li X-Y, Deng Z, Hu Z-Y, Rooke JC, Krief A, Yang X-Y, Su B-L (2013) Hydrothermal and surfactant treatment to enhance the photocatalytic activity of hierarchically meso–macroporous titanias. Catal Today 212:89–97. https://doi.org/10.1016/j.cattod.2012.07.021
Hung I-M, Wang Y, Huang C-F, Fan Y-S, Han Y-J, Peng H-W (2010) Effects of templating surfactant concentrations on the mesostructure of ordered mesoporous anatase TiO2 by an evaporation-induced self-assembly method. J Eur Ceram Soc 30(10):2065–2072. https://doi.org/10.1016/j.jeurceramsoc.2010.04.015
Shen X, Tian B, Zhang J (2013) Tailored preparation of titania with controllable phases of anatase and brookite by an alkalescent hydrothermal route. Catal Today 201:151–158. https://doi.org/10.1016/j.cattod.2012.04.038
Mohamed MA, Wan Salleh WN, Jaafar J, Yusof N (2014) Preparation and photocatalytic activity of mixed phase anatase/rutile TiO2 nanoparticles for phenol degradation. J Teknol (Sciences Eng) 70(2):65–70. https://doi.org/10.11113/jt.v70.3437
Wu M, Lin G, Chen D, Wang G, He D, Feng S, Xu R (2002) Sol-hydrothermal synthesis and hydrothermally structural evolution of nanocrystal titanium dioxide. Chem Mater 14(5):1974–1980. https://doi.org/10.1021/cm0102739
Yang X, Konishi H, Xu H, Wu M (2006) Comparative Sol–hydro(solvo)thermal synthesis of TiO2 nanocrystals. Eur J Inorg Chem 2006(11):2229–2235. https://doi.org/10.1002/ejic.200500855
Wang C-C, Ying JY (1999) Sol–gel synthesis and hydrothermal processing of anatase and rutile titania nanocrystals. Chem Mater 11(11):3113–3120. https://doi.org/10.1021/cm990180f
Castrejón-Sánchez V, López R, Ramón-González M, Enríquez-Pérez Á, Camacho-López M, Villa-Sánchez G (2018) Annealing control on the anatase/rutile ratio of nanostructured titanium dioxide obtained by sol–gel. Crystals 9(1):22. https://doi.org/10.3390/cryst9010022
Kumar A (2018) Different methods used for the synthesis of TiO2 based nanomaterials: a review. Am J Nano Res Appl 6(1):1. https://doi.org/10.11648/j.nano.20180601.11
Bamne J, Sharma PK, Haque FZ (2018) Effect of solvent mixing and calcination temperature on the growth of TiO 2 nanoparticle prepared via sol–gel method. Mater Focus 7(2):232–241. https://doi.org/10.1166/mat.2018.1502
Carrera-López R, Castillo-Cervantes S (2012) Effect of the phase composition and crystallite size of sol–gel TiO 2 nanoparticles on the acetaldehyde photodecomposition. Superf y Vacio 25(2):82–87
Sheikhnejad-Bishe O, Zhao F, Rajabtabar-Darvishi A, Khodadad E, Mostofizadeh A, Huang Y (2014) Influence of temperature and surfactant on the photocatalytic performance of TiO2 Nanoparticles. Int J Electrochem Sci 9(8):4230–4240
Masolo E, Meloni M, Garroni S, Mulas G, Enzo S, Baró M, Rossinyol E, Rzeszutek A, Herrmann-Geppert I, Pilo M (2014) Mesoporous titania powders: the role of precursors, ligand addition and calcination rate on their morphology, crystalline structure and photocatalytic activity. Nanomaterials 4(3):583–598. https://doi.org/10.3390/nano4030583
Yue Y, Gao Z (2000) Synthesis of mesoporous TiO2 with a crystalline framework. Chem Commun 18:1755–1756. https://doi.org/10.1039/b004124f
Byrappa K, Adschiri T (2007) Hydrothermal technology for nanotechnology. Prog Cryst Growth Charact Mater 53(2):117–166. https://doi.org/10.1016/j.pcrysgrow.2007.04.001
Luís AM, Neves MC, Mendonça MH, Monteiro OC (2011) Influence of calcination parameters on the TiO2 photocatalytic properties. Mater Chem Phys 125(1–2):20–25. https://doi.org/10.1016/j.matchemphys.2010.08.019
Agarwala P, Makkar P, Sharma S, Garg R (2014) The effect of heat treatment of TiO2 nanoparticles on photovoltaic performance of fabricated DSSCs. J Mater Eng Perform 23(10):3703–3709. https://doi.org/10.1007/s11665-014-1131-4
Yu JC, Yu J, Zhang L, Ho W (2002) Enhancing effects of water content and ultrasonic irradiation on the photocatalytic activity of nano-sized TiO2 powders. J Photochem Photobiol A Chem 148(1–3):263–271. https://doi.org/10.1016/S1010-6030(02)00052-7
Pulido Melián E, González Díaz O, Doña Rodríguez JM, Colón G, Navío JA, Pérez Peña J (2012) Effect of hydrothermal treatment on structural and photocatalytic properties of TiO2 synthesized by sol–gel method. Appl Catal A Gen 411–412:153–159. https://doi.org/10.1016/j.apcata.2011.10.033
Luttrell T, Halpegamage S, Tao J, Kramer A, Sutter E, Batzill M (2015) Why is anatase a better photocatalyst than rutile? Model studies on epitaxial TiO2 films. Sci Rep 4(1):4043. https://doi.org/10.1038/srep04043
Lei J, Li H, Zhang J, Anpo M (2016) mixed-phase TiO2 nanomaterials as efficient photocatalysts. In: Ünlü H, Horing NJM, Dabowski J (eds) Low-dimensional and nanostructured materials and devices. NanoScience and Technology. Springer, Cham. https://doi.org/10.1007/978-3-319-25340-4_17
Fischer K, Gawel A, Rosen D, Krause M, Abdul Latif A, Griebel J, Prager A, Schulze A (2017) Low-temperature synthesis of anatase/rutile/brookite TiO2 nanoparticles on a polymer membrane for photocatalysis. Catalysts 7(7):209. https://doi.org/10.3390/catal7070209
Mahshid S, Askari M, Sasani Ghamsari M, Afshar N, Lahuti S (2009) Mixed-phase TiO2 nanoparticles preparation using sol–gel method. J Alloys Compd 478(1–2):586–589. https://doi.org/10.1016/j.jallcom.2008.11.094
Connelly K, Wahab AK, Idriss H (2012) Photoreaction of Au/TiO2 for hydrogen production from renewables: a review on the synergistic effect between anatase and rutile phases of TiO2. Mater Renew Sustain Energy 1(1):3. https://doi.org/10.1007/s40243-012-0003-9
Kaplan R, Erjavec B, Dražić G, Grdadolnik J, Pintar A (2016) Simple synthesis of anatase/rutile/brookite TiO2 nanocomposite with superior mineralization potential for photocatalytic degradation of water pollutants. Appl Catal B Environ 181:465–474. https://doi.org/10.1016/j.apcatb.2015.08.027
Baghernejhad B (2016) Nano-TiO2: an efficient and useful catalyst for the synthesis of 3-acetyl- coumarin derivatives. Res J Pharm Biol Chem Sci 7(2):1248
Kumar SG, Devi LG (2011) Review on modified TiO 2 photocatalysis under UV/visible light: selected results and related mechanisms on interfacial charge carrier transfer dynamics. J Phys Chem A 115(46):13211–13241. https://doi.org/10.1021/jp204364a
Boppella R, Basak P, Manorama SV (2012) Viable method for the synthesis of biphasic TiO 2 nanocrystals with tunable phase composition and enabled visible-light photocatalytic performance. ACS Appl Mater Interfaces 4(3):1239–1246. https://doi.org/10.1021/am201354r
Boehme M, Ensinger W (2011) Mixed phase anatase/rutile titanium dioxide nanotubes for enhanced photocatalytic degradation of methylene-blue. Nano-Micro Lett 3(4):236–241. https://doi.org/10.1007/bf03353678
Mutuma BK, Shao GN, Kim WD, Kim HT (2015) Sol–gel synthesis of mesoporous anatase–brookite and anatase–brookite–rutile TiO2 nanoparticles and their photocatalytic properties. J Colloid Interface Sci 442:1–7. https://doi.org/10.1016/j.jcis.2014.11.060
Ermokhina NI, Nevinskiy VA, Manorik PA, Ilyin VG, Shcherbatyuk NN, Klymchyuk DO, Puziy AM (2012) Synthesis of large-pore mesoporous nanocrystalline TiO2 microspheres. Mater Lett 75:68–70. https://doi.org/10.1016/j.matlet.2012.01.133
Ermokhina NI, Nevinskiy VA, Manorik PA, Ilyin VG, Novichenko VN, Shcherbatiuk MM, Klymchuk DO, Tsyba MM, Puziy AM (2013) Synthesis and characterization of thermally stable large-pore mesoporous nanocrystallineanatase. J Solid State Chem 200:90–98. https://doi.org/10.1016/j.jssc.2012.12.034
Stroyuk OL, Ermokhina NI, Korzhak GV, Andryushina NS, Shvalagin VV, Kozytskiy AV, Manoryk PA, Barakov RY, Kuchmiy SY, Shcherbatyuk M, Sapsay VI, Puziy AM (2017) Photocatalytic and photoelectrochemical properties of hierarchical mesoporous TiO2 microspheres produced using a crown template. J Photochem Photobiol A Chem 334:26–35. https://doi.org/10.1016/j.jphotochem.2016.10.039
Stroyuk AL, Ermokhina NI, Korzhak AV, Andryushina NS, Kozytskiy AV, Manorik PA, Ilyin VG, Puziy AM, Sapsai VI, Shcherbatyuk NN (2015) Photocatalytic and photoelectrochemical characteristics of mesoporous titanium dioxide microspheres. Theor Exp Chem 51(3):183–190. https://doi.org/10.1007/s11237-015-9414-x
Shvalagin V, Ermokhina N, Romanovska N, Barakov R, Manorik P, Sapsay V, Shcherbakov S, Poddubnaya O, Puziy A (2019) Mesoporous TiO2 microspheres with improved efficiency for photooxidation of volatile organic compounds. Res Chem Intermed 45(8):4133–4148. https://doi.org/10.1007/s11164-019-03896-z
Ermokhina NI, Shvalagin VV, Romanovska NI, Sydorova NA, Manoryk PA, Barakov RY, Shcherbatyuk MM, Klymchuk DO, Puziy AM (2018) Photocatalytic activity of mesoporous titanium dioxide stabilized with lanthanum in the gas-phase oxidation of ethanol. Theor Exp Chem 53(6):395–401. https://doi.org/10.1007/s11237-018-9537-y
Zhou J, Song B, Zhao G, Han G (2012) Effects of acid on the microstructures and properties of three-dimensional TiO2 hierarchical structures by solvothermal method. Nanoscale Res Lett 7(1):217. https://doi.org/10.1186/1556-276X-7-217
Zhang H, Banfield JF (2000) Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: insights from TiO 2. J Phys Chem B 104(15):3481–3487. https://doi.org/10.1021/jp000499j
Gregg SJ, Sing KSW (1982) Adsorption, surface area and porosity. 2. Auflage. Academic Press, London. https://doi.org/10.1002/bbpc.19820861019
Kruk M, Jaroniec M (2001) Gas adsorption characterization of ordered organic–inorganic nanocomposite materials. Chem Mater 13(10):3169–3183. https://doi.org/10.1021/cm0101069
Bianchi CL, Pirola C, Galli F, Stucchi M, Morandi S, Cerrato G, Capucci V (2015) Nano and micro-TiO 2 for the photodegradation of ethanol: experimental data and kinetic modelling. RSC Adv 5(66):53419–53425. https://doi.org/10.1039/C5RA05385D
Dionysiou DD, Puma GLi, Ye J, Schneider J, Bahnemann D (2016) Photocatalysis: applications. Royal Society of Chemistry, Cambridge. https://doi.org/10.1039/9781782627104
Schneider J, Matsuoka M, Takeuchi M, Zhang J, Horiuchi Y, Anpo M, Bahnemann DW (2014) Understanding TiO 2 photocatalysis: mechanisms and materials. Chem Rev 114(19):9919–9986. https://doi.org/10.1021/cr5001892
Anpo M, Kamat PV (2010) Environmentally benign photocatalysts. Springer, New York. https://doi.org/10.1007/978-0-387-48444-0
Shayegan Z, Lee C-S, Haghighat F (2018) TiO2 photocatalyst for removal of volatile organic compounds in gas phase: a review. Chem Eng J 334:2408–2439. https://doi.org/10.1016/j.cej.2017.09.153
Hoque M, Guzman M (2018) Photocatalytic activity: experimental features to report in heterogeneous photocatalysis. Materials (Basel) 11(10):1990. https://doi.org/10.3390/ma11101990
Wu M, Long J, Huang A, Luo Y, Feng S, Xu R (1999) Microemulsion-mediated hydrothermal synthesis and characterization of nanosize rutile and anatase particles. Langmuir 15(26):8822–8825. https://doi.org/10.1021/la990514f
Andersson M, Österlund L, Ljungström S, Palmqvist A (2002) Preparation of nanosize anatase and rutile TiO 2 by hydrothermal treatment of microemulsions and their activity for photocatalytic wet oxidation of phenol. J Phys Chem B 106(41):10674–10679. https://doi.org/10.1021/jp025715y
Tobaldi DM, Lajaunie L, Rozman N, Caetano APF, Seabra MP, Sever Škapin A, Arenal R, Labrincha JA (2019) Impact of the absolute rutile fraction on TiO2 visible-light absorption and visible-light-promoted photocatalytic activity. J Photochem Photobiol A Chem 382:111940. https://doi.org/10.1016/j.jphotochem.2019.111940
Schneider J, Bahnemann D, Ye J, Puma, GLi, Dionysiou DD (2016) Photocatalysis: fundamentals and perspectives. Royal Society of Chemistry, Cambridge. https://doi.org/10.1039/9781782622338
Acknowledgements
This research was supported by the General Research Project of the L.V. Pisarzhevskii Institute of Physical Chemistry NAS of Ukraine (funded by the National Academy of Sciences of Ukraine).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ermokhina, N.I., Shvalagin, V.V., Romanovska, N.I. et al. Synthesis and characterization of different binary and ternary phase mixtures of mesoporous nanocrystalline titanium dioxide. SN Appl. Sci. 3, 491 (2021). https://doi.org/10.1007/s42452-021-04474-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04474-y