Abstract
The structural characterization of Ni3−xCuxP2O8 (0.0 ≤ x ≤ 3.0) compositions was performed and the colour parameters of these materials measured. Solid solutions with Ni3P2O8, Ni2CuP2O8 and Cu3P2O8 structures were obtained and the compositional range in which they are formed was established. Structural distortion was detected in these solid solutions when x increases from the variation in the unit cell parameters and that of the interatomic distances. Solid solutions with Ni3P2O8 structure are stable at 1200 °C and may be used as ceramic pigments. Changes in the colour of these materials were related to the structural changes. Yellow materials are obtained from Ni3−xCuxP2O8 solid solutions with a Ni3P2O8 or Ni2CuP2O8 structure and a gradual change from yellow to brown is obtained with the introduction of Cu(II) in the Ni3P2O8 structure, 0.0 ≤ x ≤ 0.7 at 1200 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metal phosphates can be used to prepare cathode and anode materials, magnetically ordered at low temperature materials and pigments [1,2,3,4]. d–d electronic transitions within transition metal ions give rise to many colours. The absence of a charge transfer band in the visible wavelength range explains the pale blue or weak greenish-blue colourations in Cu2P2O7 and Cu3P2O8 compounds. Strong absorbance due to charge transfers is the most important component in the colouration of some materials [4,5,6]. The Ni(II) orthophosphate structure is stable with temperature and in a commercial glaze and can be used as a yellow ceramic pigment in industry [4]. The Ni3P2O8 compound melts at 1350 °C. Yellow colouration can be also stable in some commercial glazes or can change to beige in others commercial glazes. Dopant cations and changes in the structure of the compounds modify their physical properties. Phosphates have a high capacity to form solid solutions. For example, Mg0.5Cu1.5VxP2−xO7 (0 < x ≤ 2) solid solutions fired at 600 °C can be used in the preparation of yellow–orange–red paint pigments. The colour of the materials darkens with increasing temperature [6].
Information about Ni3P2O8, CuNi2P2O8 and Cu3P2O8 compounds can be found in the bibliography but information about solid solutions between Ni3P2O8—Cu3P2O8 compositions cannot be found. At 900 °C, mixtures of the Cu3P2O8 and CuNi2P2O8 or Ni3P2O8 and CuNi2P2O8 crystalline phases are obtained from Cu3−xNixP2O8 compositions when the content in Cu(II) or Ni(II) is high [7]. Information about the stability of these compounds and mixtures of compounds with the temperature is not included.
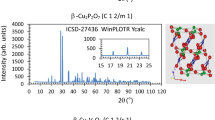
Ni3P2O8 crystallizes in the monoclinic system with space group P 1 21/c 1, a = 5.830(2) Å, b = 4.700(2) Å, c = 10.107(4) Å, β = 91.22(2)° and Z = 2 [8]. In the Ni3P2O8 crystal structure the Ni(II) ions are octahedrally coordinated at two types of sites: a special site (2a) and a general site (4e), with average Ni–O bond lengths of 2.081(2) Å and 2.067(2) Å. Groups of three octahedra (Ni3O14 edge-sharing octahedra) are interconnected with tetrahedra PO4. The phosphorus atoms occupy only one crystallographic site (4e). The mean P–O bond length is 1.547(2) Å, although the tetrahedron shows some unusually large distortions with bond lengths ranging from 1.521(2) to 1.595(2) Å. Four different sites are occupied by oxygen atoms (four 4e general positions) in this structure. Figure 1 shows the Ni3P2O8 structure from ICSD-153159 data [9]. The structure was drawn with the Studio program [10,11,12]. Yellow lines have been drawn between the oxygen atoms in a group of three octahedra and all the atoms in this trimer have been labelled. This compound exhibits three-dimensional antiferromagnetic couplings with a Neel temperature of 17.1 K and it is magnetically ordered at 1.5 K. It is an antiferromagnetic phase with the presence of ferromagnetic couplings in the trimer units [3].
The only Ni(II) and Cu(II) orthophosphate structure described to date corresponds to the 2:1:2:8 stoichiometry. Ni2CuP2O8 crystallizes in the monoclinic P21/n space group, with cell parameters a = 6.393(1) Å, b = 9.325(1) Å, and c = 4.718(1) Å; β = 90.71(1); V = 281.24 Å3; and Z = 2 [9], standard cell 4.7180, 9.3250, 7.8983, 90.000, 125.967, 90.000 (space group P 1 21/c 1). The Cu(II) ions are in CuO4 planar square coordination (Cu1 at a special site (2a)) with an average Cu–O bond length of 1.990(2) Å. The Ni(II) ions are in square-planar pyramid coordination (Ni1 at a general site (4e)) with an average Ni–O bond length of 2.031(2) Å. Each two square-planar pyramids have two common oxygen atoms (Ni2O8 dimers) and are linked to CuO4 planar squares by one oxygen atom. The Ni2O8 dimers and CuO4 planar squares form chains connected with tetrahedra PO4. The phosphorus atoms occupy only one crystallographic site (4e). The mean P-O bond length being 1.538(2) Å with bond lengths ranging from 1.5055(2) Å to 1.5552(2) Å. Four different sites (four general positions (4e)) are occupied by oxygen atoms in this structure. Figure 2 shows the crystal structure of Ni2CuP2O8 with the labelled atoms for a planar square centred around Cu and a Ni2O8 dimer obtained from ICSD-245202 data [9]. The structure was drawn with the Studio program [10,11,12]. Yellow lines have been drawn between the oxygen atoms in a part of the -CuO4-Ni2O8-CuO4-Ni2O8- chain. The heterometallic phosphate Ni2CuP2O8 was obtained from the thermal treatment of the Cu(II) intercalated compound [13]. The Ni2CuP2O8 phosphate has also been synthesized via the ceramic method at 800 °C in air [14]. This compound exhibits three-dimensional antiferromagnetic couplings with a Neel temperature of 29.8 K and it is magnetically ordered at 2.0 K temperature. It is an antiferromagnetic phase with the presence of ferromagnetic couplings inside the Ni2O8 dimers [13, 14].
Cu3P2O8 crystallizes in the triclinic system with space group P − 1, a = 4.8537(7) Å, b = 5.2855(6) Å, c = 6.1821(8) Å, α = 72.35(1)°, β = 86.99(1)°, γ = 68.54(1)º and Z = 1 [15]. The Cu(II) ions are in planar squares coordination (Cu1 in CuO4) and square-planar pyramids coordination (Cu2 in CuO5) at two types of sites: a special site (1a) and a general site (2i), with average Cu–O bond lengths of 1.949(2) and 2.030(2) Å. Each two square-planar pyramids have two common oxygen atoms (Cu2O8 dimers) and are linked to CuO4 planar squares by one oxygen atom. Copper-oxygen layers are formed by zig-zag chains (–CuO4–CuO5–CuO5–CuO4–) which form four- and six-membered rings, the chains being aligned parallel to the [101] direction [15]. Only one crystallographic site is occupied by phosphorus atoms (2i), which are tetrahedrally coordinated with oxygen atoms. The mean P-O bond length is 1.540(2) Å, with bond lengths ranging from 1.510(2) to 1.572(2) Å (the tetrahedron distortions in Cu3P2O8 are smaller than in Ni3P2O8). Four different sites (four general positions (2i)) are occupied by oxygen atoms in the Cu3P2O8 structure [9]. Figure 3 shows the crystal structure of Cu3P2O8 with the labelled atoms for a planar square centred around Cu1 (CuO4) and a Cu2O8 dimer obtained from ICSD-68811 data [9]. The structure was drawn with the Studio program [10,11,12]. Yellow lines have been drawn between the oxygen atoms in a part of the –CuO4–Cu2O8–CuO4–Cu2O8– chain (repeating group). This compound orders antiferromagnetically at TN = 22 K [16].
Ni (II) ions are octahedrally coordinated in the Ni3P2O8 structure and in square-planar pyramidal coordination in the Ni2CuP2O8 structure while Cu (II) ions are in planar square coordination in Ni2CuP2O8 and Cu3P2O8 structures and in square-planar pyramidal coordination in the structure Cu3P2O8. Therefore, the coordination of square-planar pyramids can be occupied by Ni (II) and Cu (II) ions and these ions could be substituted in the formation of possible solid solutions.
The aim of this study was to investigate the possible formation of Ni3−xCuxP2O8 (0.0 ≤ x ≤ 3.0) solid solutions with Ni3P2O8, Ni2CuP2O8 and Cu3P2O8 structures so as to establish the compositional limits they present and monitor the evolution of the colour of these materials with composition and temperature for the purpose of obtaining materials with different shades of yellow or brown that may be used as ceramic pigments.
2 Experimental
Ni(NO3)2·6H2O (Acros Organic, 99%), Cu(NO3)2·2.5 H2O (Sigma-Aldrich, 98%) and H3PO4 (Merck, 99%) were used to synthesize Ni3−xCuxP2O8 (0.0 ≤ x ≤ 3.0) compositions via the chemical co-precipitation method. The stoichiometric amount of Ni(NO3)2·6H2O, Cu(NO3)2·2.5 H2O and a 0.5 M solution of H3PO4 in water were added to water to obtain a final volume of 200 mL. Samples were vigorously stirred for 12 h at room temperature and then an ammonia aqueous solution (Panreac, 25%) was added until reaching pH 10. The materials co-precipitated were dried using IR-irradiation until to obtain the 100% in solid materials. The Ni:Cu:P molar ratio of the starting materials is preserved. Only water is evacuated with drying. The dry samples were fired at 300, 600, 800, 1000 and 1200 °C for 6 h at each temperature.
To study the development of the crystalline phases at different temperatures, the resulting materials were examined using a Panalytical X-ray diffractometer with CuKα radiation. Diffraction patterns ranging between 6 and 110o (2θ) were collected employing monochromatic CuKα radiation, a step size of 0.02o (2θ) and a sampling time of 10 s. Fullprof.2 k computer program based on the Rietveld method [10,11,12] was used to refine the structures by adjusting the diffraction profile. The unit cell parameters and interatomic distances in the developed structures were obtained to investigate the possible formation of solid solutions under these synthesis conditions. The initial structural information was obtained from the Inorganic Crystal Structure Database [9]. This database includes standard cell [17], standard space group, fractional atomic coordinates and other information on crystalline phases found in the literature.
The Ni(II) and Cu(II) sites and the transfer charge bands in the materials were studied by UV–Vis-NIR spectroscopy (diffuse reflectance). A Jasco V-670 spectrophotometer was used to obtain the ultraviolet visible near infrared (UV–Vis-NIR) spectra in the 200 to 2500 nm range.
The CIEL*a*b* colour parameters on the fired compositions were obtained with an X-Rite spectrophotometer (SP60, standard illuminant D65, an observer 10°, and a reference sample of MgO). L* is the lightness axis (black (0) → white (100)), a* is the green (−) → red ( +) axis, and b* is the blue (−) → yellow ( +) axis [18].
3 Results and discussion
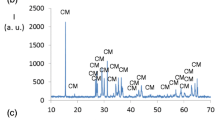
Table 1 shows the evolution of the crystalline phases with composition and temperature in the Ni3−xCuxP2O8 (0.0 ≤ x ≤ 3.0) samples. At 600 °C, the Ni3P2O8 composition is not crystalline under the synthesis conditions employed in this study. The Ni3P2O8 crystalline phase can be detected with a weak or medium diffraction intensity when 0.5 ≤ x ≤ 2.5 at this temperature. At temperatures above 600 °C, the Ni3P2O8 crystalline phase is developed only when x ≤ 1.0. At 600 °C, the Ni2CuP2O8 phase can be detected when 1.5 ≤ x ≤ 2.5. At temperatures above 600 °C, this phase is detected in all compositions including Ni(II) and Cu(II), 0.5 ≤ x ≤ 2.5, and it is the only crystalline phase when 1.5 ≤ x ≤ 2.0 at 800 °C and when x = 0.20 at 1000 °C. The Cu3P2O8 crystalline phase can be detected with medium and strong diffraction intensities in compositions when 1.5 ≤ x ≤ 3.0 at 600 °C. At temperatures above 600 °C, the Cu3P2O8 crystalline phase is detected when x ≥ 2.5. At 1200 °C, x ≥ 1.5 compositions melt and the diffraction intensities show strong preferred orientations. When 1.5 ≤ x ≤ 2.0, a crystalline phase M3P2O8 (M = Ni, Cu) with Z = 4 and S.G. P − 1 (structure type “triclinic Mg3P2O8” (ICSD-84710)), develops at 1200 °C under the experimental conditions employed in this study. The coordination number of M is 4, 5 and 6 in this last structure. The evolution of the crystalline phases with temperature from x = 1.0 composition (Ni2CuP2O8) is shown in Fig. 4.
Table 2 shows the unit cell parameters in the Ni3P2O8 structure obtained from Ni3−xCuxP2O8 (0.0 ≤ x ≤ 1.0) fired compositions, while the values at 800, 1000 and 1200 °C are represented in Fig. 5. The slight increase in the a unit cell parameter of the Ni3P2O8 structure with increasing x is in accordance with the partial substitution of Ni(II) by Cu(II), as the radius of Cu(II) is slightly larger than that of Ni(II). No significant variation in the b unit cell parameter is observed and the decrease in the c unit cell parameter is not according with the ionic radius. These inverse variations of the a and c parameters can be attributed to the deformation of the Ni3P2O8 structure when Cu(II) is incorporated and the increase in the unit cell volume is very slight (Table 3). This structure is not compact throughout its volume; it includes less dense areas that allow this deformation. From Fig. 5, the x = 0.7 composition can be established as the composition with the maximal distortion in the Ni3P2O8 structure and this composition is seen to be the limit composition in the formation of solid solutions with this structure. At 800–1200 °C, Ni3−xCuxP2O8 solid solutions are formed when x ≤ 0.7.
The inverse variation in the a and b parameters in the Ni2CuP2O8 structure were obtained from the Ni3−xCuxP2O8 (1.5 ≤ x ≤ 2.5) compositions fired at 800 and 1000 °C (Table 4 and Fig. 6). As the radius of Cu(II) is slightly larger than that of Ni(II), the increase in the a unit cell parameter is in accordance with the partial substitution of Ni(II) by Cu(II), but the decrease in b cannot be explained by the radius values. The aforementioned increase can be explained by the deformation of the Ni2CuP2O8 structure when Cu(II) is incorporated, forming solid solutions. Hence, the increase in the unit cell volume is very slight (Table 3). The variation in the c parameter is the smallest and the β angle increases with x. A composition limit with x cannot be established from Fig. 6. The variation in the unit cell parameters is more evident when x ≥ 1.0 and the Ni3−xCuxP2O8 solid solutions with a Ni2CuP2O8 structure may well have been formed when 1.0 ≤ x ≤ 2.5 at 800 and 1000 °C.
In the Cu3P2O8 structure, structural deformation was obtained with decreasing x (Table 5). A slight decrease in the a and b unit cell parameters of the Cu3P2O8 structure with decreasing x is in accordance with the partial substitution of Cu(II) by Ni(II), as the radius of Ni(II) is slightly smaller than that of Cu(II). The slight increase in the c unit cell parameter is not in accordance with the ionic radius. At 600 °C, a tendency for the volume of this structure to decrease with x can be detected in the 3.0 ≥ x ≥ 1.5 compositional range (Table 3). At T > 600 °C, the Cu3P2O8 structure is developed in the compositions with x ≥ 2.5.
Figure 7 shows the variation in the M–O and P–O distances in the Ni3P2O8 structure. This variation is in accordance with the variation in the unit cell parameters (Fig. 5). The increase in the M2–O3b and M2-O4b distances with increasing amounts of Cu(II) ions (0 ≤ x ≤ 0.7) lengthens the a unit cell parameter because the Ni2–O4b direction is near to that of the a axis. The Ni1–O and Ni2–O distances and the position of the labelled atoms for a Ni2–Ni1–Ni2 trimer are shown in Fig. 1. The O1–M2–O2 direction is parallel to the c axis and the decrease in the M2–O1 and M2–O2 distances shortens the c unit cell parameter with increasing x. The O4b–M1–O4b direction is near to the direction of the c axis and the slight decrease in the M1–O4b distance contributes to the decrease in the c unit cell parameter. An increase in the covalent component in the M1–O4b bonds in the c direction might explain this variation, which is not in accordance with the variation in the ionic radius. The longest M2–O4a distance (the O4a oxygen atoms are bonded to two trimers) does not vary with x. The variation in the obtained distances could be explained by the tendency to square plane coordination of Cu(II), a plane of the octahedron with smaller distances and the other two oxygen atoms situated at a longer distance. The Ni2CuP2O8 and Cu3P2O8 structures with Cu(II) in square plane coordination are obtained when x ≥ 1.0 at 1000 °C (Table 1). No significant change in the three similar P–O distances around 1.54 Å and a slight increase in the four distances around 1.60 Å are detected at 1000 °C.
From the variation in the M(II)–O bond lengths (M = Ni, Cu) with x in the Ni2CuP2O8 structure at 1000 °C (Fig. 8), a decrease in most of the bonds and an increase in the M1-O4 and M2-O3 (M1 = Cu, M2 = Cu, Ni) distances can be observed. These last bonds are aligned in the direction of the a axis (Fig. 2) and their increase with increasing amounts of Cu(II) ions (1.0 ≤ x ≤ 2.5) lengthens the a unit cell parameter (Fig. 6). This increase is in accordance with the partial substitution of the Ni(II) ion by the slightly larger Cu(II) ion. All the other M–O distances decrease when x increases. This decrease causes a decrease in the b unit cell parameter and may be due to the increased covalent character of the bond when the increasing amount of Cu(II) ions in the compositions is replaced by Ni(II) ions. Deformation of the tetrahedra around the P atoms is detected in this structure with increasing x. The P–O3 distance (O3 is coordinated to P and M2) decreases and the P-O1 distance (O1 is coordinated to P, Cu1 and M2) increases with x.
The presence of Ni(II) in the Cu3P2O8 structure decreases the M2–O2 and M1–O3 distances (M2–O2 is considerably longer than the other distances) and increase the other distances with decreasing x (there is Ni(II) in the compositions). It is in accordance with the decrease in the a and b parameters and the increase in the c unit cell parameter when x decreases (Table 5 and Fig. 3). This phase is only developed when x ≥ 2.5 at T > 600 °C. The presence of Ni(II) weakens most of the bonds while strengthening the longest bond. Hence, distortion occurs with the formation of solid solutions in the three structures to accommodate the cations.
Figure 9 shows the UV–V spectra obtained from the Ni3P2O8 and Cu3P2O8 compositions (raw material and fired composition). Three absorption bands assigned to Ni2+ at an octahedral site are detected from Ni3P2O8 fired at T ≥ 800 ºC. Maximum values of these bands are observed around 1300 nm (3A2g → 3T2g(F), first transition), 800 nm (3A2g → 3T1g(F), second transition) and 450 nm (3A2g → 3T1g(P), third transition). The [200–650] wavelength range includes the absorption band associated with the third d-d transition and the charge transfer band of Ni(II)–O. The three spin-allowed transitions of Ni2+ at an octahedral site generally fall within the 1400–800, 900–500 and 550–370 nm ranges, respectively, in octahedral systems [19]. The broad band observed in Cu3P2O8 fired at T ≥ 800 ºC within the 550–1900 nm wavelength range with the absorption maximum at 800–900 nm is assigned to the d-d transition of the Cu2+ ion (d9). A d–d transition is allowed from the Cu(II) ion. At 1200 °C, the charge transfer band of Cu(II)–O is observed around 400 nm. The high asymmetry observed in all the bands is due to the difference in Ni–O (between 1.982 and 2.181 Å) and Cu–O distances (between 1.924 and 2.259 Å) in the Ni3P2O8 and Cu3P2O8 structures (ICSD-153159 and ICSD-1143 [5]). Structural distortion due to the Jahn–Teller effect also contributes to the asymmetry in the observed bands obtained from the Cu3P2O8 composition.
The UV-V spectra obtained from the Ni3−xCuxP2O8 (0.0 ≤ x ≤ 3.0) compositions fired at 800, 1000 and 1200 °C are shown in the Fig. 10. The three absorption bands assigned to Ni2+ at an octahedral site and the d-d transition band of the Cu2+ ion can be observed when x ≤ 2.0 and x ≥ 2.5, respectively. Slight variations are observed with the change in the Ni2+:Cu2+ ratio. An increase in absorbance around 1700 nm can be detected. In the Ni3P2O8 composition, a shoulder in the band assigned to the first Ni(II) transition band appears at this wavelength. The maximum value of this band is not observed around 1300 nm in 1.5 ≤ x ≤ 2.0 compositions at 800 °C. At this temperature, the variation with composition in the inflection point between maximum and minimum absorbance within the 650–1000 nm wavelength range (second Ni(II) transition band) can be observed in 0.0 ≤ x ≤ 2.0 compositions. The wavelength at these inflection points increases with the amount of copper in the samples (x). No noticeable changes in the position of the Ni(II) third transition with x can be detected. These slight changes in spectra with composition are related to the variation in the Ni–O and Cu–O distances, which involve changes in the relative position of the oxygen atoms around M(II) (M = Ni, Cu) with x in the crystalline phases detected by XRD. With increasing temperature, the increase in absorbance around 1100 nm makes it difficult to distinguish the first and second Ni(II) transition bands. The lower definition of the absorption bands at T ≥ 1000 °C modifies the colouration of the samples.
Figure 11 and Table 6 show the observed colour of the compositions at 70, 600, 800, 1000 and 1200 °C. The yellowish green (x = 0.0), bluish green (x = 0.5) and light blue colouration (1.0 ≤ x ≤ 3.0) of the materials at 25 °C changes to yellow (0.0 ≤ x ≤ 2.0), pale green (x = 2.5) and bluish green (x = 3.0) at 800 °C. The Ni3P2O8 (0.0 ≤ x ≤ 1.0), Ni2CuP2O8 (0.5 ≤ x ≤ 2.5) and Cu3P2O8 (2.5 ≤ x ≤ 3.0) crystalline phases were detected at this temperature. Thus, the main change in colour with increasing Cu(II) in the samples is detected together with the presence of the Cu3P2O8 structure at 800 °C. Yellow colouration is also obtained in 0.0 ≤ x ≤ 1.0 compositions at 1000 °C. Brown colouration in x = 1.5 at 1000 °C (not obtained at 800 °C) and in x = 1.0 or 2.0 at 1200 or beige colouration in x = 0.5 at 1200 °C is associated with the lower definition of the Ni(II) transition bands and can be related to the observed structural distortion when solid solutions are formed or to the presence a different crystal structure. Both circumstances produce changes in the M–O (M = Cu, Ni) interatomic distances and the position of the bands in the spectra. The formation of solid solutions with presence of a small amount of Cu(II) in Ni3P2O8 structure modifies the colour of the material from yellow to beige. At 1200 °C, the change from yellow (x = 0.0) to beige-brown is obtained when the diffraction intensity of Ni3P2O8 crystalline phase is strong (Table 1) and the Ni3−xCuxP2O8 (0.0 ≤ x ≤ 0.7) solid solutions with this structure are stable (Fig. 5). Because of this stability at the work temperature in the ceramic industry (the Ni3P2O8 compound melts at 1350 °C), these materials may be used as ceramic pigments.
4 Conclusions
Ni3−xCuxP2O8 (0.0 ≤ x ≤ 3.0) compositions were synthesized via the chemical co-precipitation method. Structural characterization of these materials and the variation in the unit cell parameters with composition in Ni3P2O8, Ni2CuP2O8 and Cu3P2O8 structures confirm the formation of solid solutions in these structures in a partial compositional range. Between 800 and 1200 °C, Ni3−xCuxP2O8 solid solutions with a Ni3P2O8 structure were formed when x ≤ 0.7. Ni3−xCuxP2O8 solid solutions with the Ni2CuP2O8 structure were formed when 1.0 ≤ x ≤ 2.5 at 800 and 1000 °C. At T > 600 °C, the Cu3P2O8 structure is developed in the compositions with x ≥ 2.5. The variation in volume with x in these structures is slight. The variation in the unit cell parameters in the same structure is the opposite because of structural distortion with inverse variation in the interatomic distances. These structures are not compact throughout their volume; they include less dense areas that allow their deformation.
A gradual change from yellow to brown is obtained when the Ni3P2O8 crystalline phase is obtained with a strong diffraction intensity (0.0 ≤ x ≤ 1.0) at 1200 °C. These compositions are yellow at 800 and 1000 °C. Under the conditions employed in this study, the broadest compositional range (0.0 ≤ x ≤ 2.0) within which the materials are yellow is obtained at 800 °C. At this temperature, yellow materials are obtained from Ni3−xCuxP2O8 solid solutions with a Ni3P2O8 or Ni2CuP2O8 structure. Ni3−xCuxP2O8 (0.0 ≤ x ≤ 0.7) yellow–brown solid solutions with Ni3P2O8 structure are stable at 1200 °C and may be used as ceramic pigments.
References
Zhao W, Zhong G, Jian Z, Jianming Z, Song J, Gong Z, Chen Z, Zheng G, Jiang Z, Yang S (2018) Insights into the electrochemical reaction mechanism of a novel cathode material CuNi2(PO4)2/C for Li-ion batteries. ACS Appl Mater Interfaces 10:3522–3529. https://doi.org/10.1021/acsami.7b15086
Zhong G, Bai J, Duchesne PN, McDonald MJ, Li Q, Hou X, Tang JA, Wang Y, Zhao W, Gong Z, Zhang P, Fu R (2015) Copper phosphate as a cathode material for rechargeable Li batteries and its electrochemical reaction mechanism. Chem Mater 27:5736–5744. https://doi.org/10.1021/acs.chemmater.5b02290
Escobal J, Pizarro JL, Mesa JL, Rojo JM, Bazan B, Arriortua MI, Rojo T (2005) Neutron diffraction, specific heat and magnetic susceptibility of Ni3(PO4)2. J Solid State Chem 178:2626–2634. https://doi.org/10.1016/j.jssc.2005.06.022
Tena MA, Mendoza R, García JR, García-Granda S (2017) Structural characterization and colour of Ni3VxP2-xO8 (0 ≤ x ≤ 2) and Ni2VyP2-yO7 (0 ≤ y ≤ 2) materials. Results in Physics 7:1095–1105. https://doi.org/10.1016/j.rinp.2017.02.021
Tena MA, Mendoza R, Trobajo C, García JR, García-Granda S (2018) Co2P2O7–Ni2P2O7 solid solutions: Structural characterization and color. J Am Ceram Soc 00:1–10. https://doi.org/10.1111/jace.16158
Tena MA, Mendoza R, Martinez D, Trobajo C, García JR, García-Granda S (2020) Characterization of yellow and red inorganic pigments from Mg0.5Cu1.5VxP2−xO7 (0 ≤ x ≤ 2) solid solutions. SN Appl Sci 2:1–10. https://doi.org/10.1007/s42452-020-2917-7
Weimann I, Feller J, Žak Z (2017) Phase equilibria in the system CuO–NiO–P4O10 and synthesis, crystal structure, and characterization of the new copper nickel oxide phosphate Cu3NiO(PO4)2. Z Anorg Allg Chem 643:299–305. https://doi.org/10.1002/zaac.201600403
Calvo C, Faggiani R (1975) Structure of nickel orthophosphate. Can J Chem 53:1516–1520. https://doi.org/10.1139/v75-210
Inorganic Crystal Structure Database (ICSD web). Fachinformationszentrum (FIZ, Karlsruhe, Germany).
Rietveld HM (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2:65–71. https://doi.org/10.1107/S0021889869006558
Rodriguez-Carvajal J (September 2018-ILL-JRC), Fullprof.2k computer program, version 6.50, France.
Chapon L (Rutherford Appleton Laboratory, UK) and Rodriguez-Carvajal J (Institut Laue Langevin, France) (August 2008). FPStudio computer program, version 2.0
Goñi A, Lezama L, Pizarro JL, Escobal J, Arriortua MI, Rojo T (1999) Intercalation of Cu2+ in the HNiPO4·H2O layered phosphate: study of the structure, spectroscopic, and magnetic properties of the intercalated derivative and the related CuNi2(PO4)2 compound. Chem Mater 11:1752–1759. https://doi.org/10.1021/cm980785w
Escobal J, Pizarro JL, Mesa JL, Larrañaga A, Rodriguez Fernandez J, Arriortua MI, Rojo T (2006) Magnetic susceptibility, specific heat and magnetic structure of CuNi2(PO4)2. J Solid State Chem 179:3052–3058. https://doi.org/10.1016/j.jssc.2006.05.034
Forsyth JB, Wilkinson C, Paster S, Effenberger H (1990) The antiferromagnetic structure of triclinic copper(II) phosphate. J. Phys.: Condens. Matter 2:1609–1617. https://doi.org/10.1088/0953-8984/2/6/019
Shvanskaya LV, Volkova OS, Vasiliev AN (2020) A review on crystal structure and properties of 3d transition metal (II) orthophosphates M3(PO4)2. J Alloy Compd 835(155028):1–4. https://doi.org/10.1016/j.jallcom.2020.155028
Tena MA (2012) Characterization of MgxM2−xP2O7 (M = Cu and Ni) solid solutions. J Eur Ceram Soc 32:389–397. https://doi.org/10.1016/j.jeurceramsoc.2011.09.018
Commission Internationale del’Eclairage (1971) In: Recommendations on Uniform Color Spaces, Color Difference Equations, Phychometrics Color Terms. 1978. Supplement No. 2 of CIE Publication No. 15 (E1–1.31). Bureau Central de la CIE, Paris.
Lever ABP (1977) Inorganic electronic spectroscopy (second edition). Elsevier, Amsterdam, pp 507–511
Acknowledgements
We gratefully acknowledge the financial support provided by Spain’s Ministerio de Ciencia, Innovación y Universidades, Project MAT2016-78155-C2-1-R.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tena, M.A., Mendoza, R., Trobajo, C. et al. Structural characterization and colour of Ni3−xCuxP2O8 (0 ≤ x ≤ 3) materials. SN Appl. Sci. 3, 373 (2021). https://doi.org/10.1007/s42452-021-04348-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04348-3