Abstract
ZnO nanoparticles have been synthesized and applied for the removal of different environmental pollutants in the present study. Combustion method is used for the preparation of ZnO NPs. X-Ray diffraction pattern reveals the crystallinity of the nanoparticles, where SEM and TEM images displayed that ZnO NPs were of size less than 100 nm and nearly spherical in shape. UV–Vis and IR spectra revealed the formation of ZnO NPs. Adsorption and advanced oxidation processes were employed for the removal/degradation of trace elements/pesticide. UV reactor containing 1 UV rod of 11 W (Philips) was used for the photocatalytic degradation of pesticide. ICP–OES and GC–MS techniques were used for the further quantitative analysis of trace elements and OP pesticide—monocrotophos, respectively. The analysis shows the 88% degradation of monocrotophos when subjected to UV light in the reaction chamber for 120 min at a pH 4 when 2 g of nanocatalyst is applied. However, the removal of trace element Arsenic shows linear adsorption as compared to Cd and Se. The removal efficiency of ZnO nanoparticles for Cd and Se was 36% and 64%, respectively, after 120 min. The synthesized nanoparticles are more effective than the commercially available ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pollution emerges as a serious environmental concern. It is a global issue faced by present generation and can be a huge threat to future generations. Different types of pollutants have been reported in the drinking water as well as surface water streams including physiochemical, biological, heavy metals (trace elements), chlorophenols, dyes and pesticides. Pesticides are used in agricultural as well as domestic field from so many decades. A few pesticides on account of their harmful nature are prohibited by the public authority, yet because of the persistency they stay in the climate for quite a long time. Thus, they can be entering in aquatic climate by means of soil permeation or surface spill over [1]. Some of the prohibited pesticides are reliably found in water, residue and soil [2]. Drinking water from numerous pieces of India has been accounted for with the presence of a few toxins including pesticides [3]. On the other hand, the presence of trace elements in drinking water samples is also not ignorable [4,5,6,7]. Pesticides are health hazardous to all living creature, so they should be taken out or corrupted from their source. There are several methods involved in the process of pollutants removal from river water as well as wastewater stream. Numerous treatment strategies have been accounted for the expulsion of poisons from water. Among these methods, advanced oxidation process (AOP) is best, modest and helpful. Numerous researchers have reported the photocatalytic reactant corruption of various pesticides by utilizing nanoparticle as an impetus. Nanomaterials like titanium dioxide, silver, zinc oxide and iron oxides were used as photocatalyst for the heterogeneous degradation of different pesticides [8].
In recent years, heterogenous photocatalysis utilizing a semiconductor and photon energy has been increasingly used as another methodology for the degradation of natural poisons. Many researchers have reported photocatalytic degradation of different pollutants from aqueous medium [9]. ZnO nanoparticles appear very useful for the degradation of the same. ZnO NPs have a wide gap of band energy, are biologically and chemically inert and are easy to be synthesized. ZnO formed a special wurtzite n-type semiconductor with a band gap of 3.37 eV [10]. ZnO nanoparticles are applicable in the various fields like drug delivery, cosmetic, solar cells and gas sensor. ZnO nanoparticle also removes heavy metals like cadmium from its aqueous solution using as an absorbent. Removal of cadmium can also be processed by ion-exchange method by using ZnO NPs [11]. The photocatalytic degradation of chlorpyrifos (organophosphate pesticide) by using ZnO as a catalyst was reported by Khan et al. [10]. ZnO nanoparticles were most conveniently synthesized by using coprecipitation method [12]. The coprecipitation method is simple and used for the rapid synthesis of huge amount of nanoparticles; also the particle size can be controlled easily. Unlike other procedures, the coprecipitation strategy does not need exorbitant equipment and tough response conditions, and sometimes the crystallinity can be acquired straightforwardly [13, 14].
The objectives of this laboratory study are (1) synthesis and characterization of ZnO nanoparticles, (2) photocatalytic degradation study of monocrotophos pesticide, (3) adsorption-based removal study of some trace elements, and (4) comparison of the effectiveness of commercially available and laboratory-synthesized ZnO nanoparticles for the removal of trace elements from drinking water. In this laboratory experiment, degradation and removal of pesticide and trace elements have been done simultaneously. This experimental work is extended to the comparative study of commercial and synthesized nanoparticles for the removal of selected trace elements which are reported in previous studies, to test their effectiveness [3,4,5,6].
2 Materials and methods
2.1 Chemicals
Zinc nitrate hexahydrate (Zn(NO)3·6H2O), sodium hydroxide (NaOH) pellets and ethanol (C2H5OH) of analytical grade were purchased from Merck, India. ZnO NPs were synthesized by using coprecipitation method, and commercially available technical grade monocrotophos is used for the degradation study. HPLC grade n-hexane was purchased from Merck, India, for the extraction procedure.
2.2 Structure of monocrotophos and mode of action
Monocrotophos is an organophosphate pesticide. It is widely used as an insecticide against a wide range of pest on cotton, sugarcane, potatoes, peanuts and tomatoes, which are abundantly harvested in Uttar Pradesh, India. It is highly toxic in nature and frequently used as a tool to commit suicide. It is a diabetogen, can cause cardiotoxicity and show various acute effects according to WHO [15]. The general structural formula of monocrotophos is dimethyl (E)-1-methyl-2-(methylcarbamoyl) vinyl phosphate (Fig. 1).
2.3 Synthesis of nanoparticles
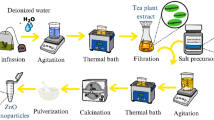
Different chemical synthesis methods, such as precipitation, sol–gel, hydrothermal, etc., are used for the synthesis of selective nanoparticles. According to Nabiyouni et al. [16] among the reported methods, the precipitation method is an efficient and economic way to mass production. The metal oxide nanoparticle is synthesized by coprecipitation method [17]. 14.8 g of (Zn(NO)3·6H2O) is dissolved in 50 mL of distilled water in a pre-cleaned borosilicate glass beaker with a continuous stirring to make homogenous 1 M concentration solution of zinc nitrate hexahydrate. 0.5 M NaOH solution was prepared by dissolving 1 g of NaOH in 50 mL of distilled water by using above method. 0.5 M NaOH solution was added drop wise in the 1 M (Zn(NO)3·6H2O) solution with a constant and continuous stirring until pH of solution reached at 10 and white precipitate form. The reaction vessels were rested apart for effective hours with a set rate of stirring. The precipitate was filtered and washed with distilled water and ethanol mixture. The washed precipitate was dried overnight at 110 °C in a laboratory oven and placed into a Muffle furnace at 450 °C for 3 h. The precipitate was stored for further characterization and experiments.
3 Nanoparticles characterization: results and discussions
3.1 X-ray diffraction (XRD) analysis
The X-ray diffraction pattern was recorded on Rikagu Ultima IV X-ray diffractometer using CuKα radiation (\(\lambda = 1.54 \) Å) at 40 kV. The scanning rate was 4/min in 2θ scale. The XRD spectrum of pure ZnO NPs is shown in Fig. 2. The recorded sharp and narrow diffraction confirmed the perfect size and crystallinity of the sample. These sharp and narrow peaks appeared at 2θ = 31.76, 34.42, 36.25, 47.53, 56.59, 62.85, 66.37, 67.94, 69.08 and 72.56 at a reflecting plane (100), (002), (101), (102), (110), (103), (200), (112), (201) and (004), respectively. Crystal structure of ZnO NPs is hexagonal with P63mc (186) space group, which is due to their resemblance with ICSD- 065,120 code reported result of powder diffraction by Albertsson et al. [18]. The crystallite size of the powdered sample was calculated by Debye–Scherrer’s equation as follows:
where D is an average size of crystallite; β is full width at half maximum of the peak in radians; λ is wavelength of X-ray; θ is Bragg’s angle; and K is constant (geometric factor = 0.94). It was found that the crystallite size of the nano-sample is less than 20 nm, i.e. in the nano range.
3.2 Scanning electron microscopy (SEM) analysis
Synthesized ZnO NPs undergo SEM analysis for the study of their surface morphology. FE-SEM analysis was performed by MIRA3 TESCAN at 10 kV operating voltage. Figure 3 shows the SEM images of the ZnO NPs having size less than 100 nm.
3.3 Transmission electron microscopy (TEM) analysis
In addition to SEM analysis, the size of prepared NPs was confirmed by the TEM analysis. Figure 4 shows the formation of spherical aggregate of ZnO NPs having dimension between 19 and 31 nm. So the average size of the nanoparticle is 25 nm. The variation in the size of SEM and TEM results may be due to surface agglomeration as shown in Fig. 3.
3.4 Fourier transform-infrared spectroscopy (FT-IR) analysis
FT-IR range uncovered the bond structure and recognizable proof of utilitarian gathering in the nano-sample. Infrared spectra of ZnO NPs were recorded by PerkinElmer FT-IR spectrophotometer by using KBr tablets. The spectrum is recorded in the range of 4000–400 cm−1 wave number. A sharp band in the range 400–600 cm−1 (Fig. 5) is a direct result of the extending of Zn–O. One more groups at 3457 cm−1 and 1630 cm−1 are related with O–H extending and H–O–H bowing individually. These bands demonstrate the presence of modest quantity of water consumed on the outside of the nanoparticles as the nanoparticles were set up in aqueous arrangement utilizing coprecipitation technique [19, 20].
3.5 Ultraviolet spectroscopy (UV) analysis
Shimadzu UV-1800 UV–Vis spectrophotometer is used for recording this spectrum. The concentration of ZnO is estimated at most extreme absorbance with the assistance of plotting absorbance-concentration curve as per Beer–Lambert's law. An ingestion peak between 300 and 400 nm (Fig. 6) affirms the arrangement of ZnO nanoparticles of higher group measurements [21, 22].
4 Photocatalytic degradation using advance oxidation process (AOP)
Degradation of pollutants has been measured as heterogeneous photocatalysis process. Advanced oxidation measure utilizing nano-semiconductors as a photocatalyst for the debasement of pesticides is considered as generally proficient and climate amicable method [10]. The photocatalytic degradation experiments were carried out in a wooden box reactor at atmospheric pressure. The reactor comprised of an UV rod (for UV illumination), a borosil glass container (for the sample) and a stirrer (for good mass exchange). Few holes were drilled on the reactor to keep up the correct air circulation and temperature. The schematic diagram of the photochemical reactor is shown in Fig. 7. A known amount of ZnO was added in the vessel containing 500 mL of monocrotophos aqueous solution. Place this vessel into the photochemical reactor chamber under UV irradiation. The aqueous solution was ceaselessly stirred at a pace of 400 rpm by utilizing a stirrer and for a fixed time. Ten ml of aliquot was taken at a regular interval of time, i.e. 15 min., 30 min,. 45 min., 60 min., 120 min., and the liquid–liquid extraction process follows as used by Kaushik et al. [23]. At that point, these samples were concentrated with nitrogen purging, and further examination was done utilizing GC–MS. The percent removal effectiveness was evaluated by the accompanying the expression:
where RE is the removal efficiency (%); Co and C are initial and final concentration of pollutants (pesticides).
4.1 Degradation study of pesticide
Pesticides are broadly utilized as they are viable and modest. As indicated by Pimentel [24], just a little amount (0.3%) of applied pesticides goes into target bug while 99.7% head off to someplace else into climate. They can be reached to groundwater spring through soil permeation or SW–GW interaction. Water is the second natural resource without which the endurance of living creatures is impractical after air. It should be clean and potable. So, numerous treatment strategies have been accounted for the removal of pesticide from surface water and groundwater. Among these techniques, advanced oxidation process (AOP) is best, modest, helpful and successfully utilized for the current investigation. Under the illumination of UV rays, ZnO nanoparticles assimilate the photon energy, and photocatalytic reactions were initiated. The valence band (VB) electron of ZnO got promoted to the conduction band (CB) by leaving a positive hole behind in valance band. The photo-generated hole and the electron both induced the following redox reactions as:
The batch experiment was used for the degradation of monocrotophos in aqueous medium via photocatalytic degradation process.
4.2 Batch experiment
This investigation was done by dissolving the standard amount of pesticide standard into 500 mL of distilled water and sonicate for 2 h, with the goal that all the pesticides totally blended in water. Since monocrotophos is highly soluble in water, there is no need for the addition of any surfactant [25]. This sample was separated for around 45 min., with the goal that both the layers got isolated totally. The organic layer was dispose off and the aqueous layer taken for additional investigation. The complete batch experiment procedure followed by us is given in Table 1. Two µL of aliquot was injected in GC–MS, and the degradation results are shown in Fig. 8. The consequences of this examination indicated that zinc oxide nanoparticles are equipped for debasing organophosphate pesticide—monocrotophos successfully.
One gram zinc oxide nanoparticle was added to photocatalytic vessels containing known convergence of 250 mL pesticide fluid arrangement with consistent blending. When the nano-impetus was added to the fluid arrangement under UV illumination, corruption measure begins.
4.3 Optimization of operating parameters
To accomplish most extreme corruption, reactant debasement conditions were upgraded, and these boundaries are portion of inmetus (nanocatalyst), impact of pH and UV Irradiation time, which are talked about below.
-
(i)
Effect of catalyst dose: Efficiency of degradation depends upon the dose of catalyst. Therefore, optimization was achieved by using 0.5 g, 1 g, 2 g and 5 g of catalyst during the process. The results of these finding are shown in Fig. 9a. It is clear that maximum degradation of pesticide monocrotophos was in order of 2 g ˃ 5 g ˃ 1 g ˃ 0.5 g ˃ 0 g. Of course, 2 g nanocatalyst in 500 mL aqueous solution gave maximum degradation efficiency. When high dose of nanocatalyst was applied, the degradation tendency decreased; it is because of the turbidity created by more amount of nanocatalyst. In the absence of nanocatalyst no degradation takes place.
-
(ii)
Effect of pH: The effectiveness of nanocatalyst relies on its ability to be protonated, which is controlled by pH up to a decent degree. The results of pH variation on the percentage degradation efficiency towards the monocrotophos are shown in Fig. 9b. It is obvious from the figure that most extreme corruption effectiveness of nano-impetus was accomplished at pH 4. Maximum degradation of monocrotophos was achieved in order of pH 4 ˃ pH 5 ˃ pH 6 ˃ pH 7 ˃ pH 8, when the catalyst quantity was taken as 2 g/ 500 mL of sample.
-
(iii)
Effect of UV Irradiation time: The debasement effectiveness of nano-impetus likewise relies on the time of UV Irradiation under the batch analysis. The optimization was achieved by using 15, 30, 45, 60 and 120 min of irradiation time. It is clear from Fig. 9c that the maximum efficiency of nanocatalyst was in order 120 min ˃ 60 min ˃ 45 min ˃ 30 min ˃ 15 min, when the catalyst dose is 2 g/500 mL of sample as taken. It appears that after 120 min of irradiation time, nanocatalyst degraded maximum amount of pesticide monocrotophos.
The after effects of the above examination demonstrated that zinc oxide nanoparticles are fit for corrupting monocrotophos in our therapy process. In this manner, ZnO NPs were unmistakably liable for the debasement of organophosphate pesticide—monocrotophos.
5 Trace elements (heave metals) removal study using synthesized ZnO nanocatalyst as an adsorbent
The term heavy metals refers to those metallic elements which have a high density and toxicity at low concentrations like Cd, As, Pb, Hg, Cr, etc. [26]. Cd and As both are found in the drinking water samples as well as Krishni river sample in our study area. In addition to both, Se (metalloid) also is found in high concentrations in the same. So we are focussing on the term trace elements in place of heavy metals for the removal purposes. Zinc oxide and other nanoparticles have been reported to be used as an adsorbent for the effective removal of trace elements [27, 28].
5.1 Chemicals used in the removal study
The chemicals used for the removal studies are NaAsO2 (for the arsenic removal study), Cd(NO3)2 (for the cadmium removal study) and Na2SeO4 (for the selenium removal study) of analytical grade purchased from Sigma-Aldrich. Laboratory-synthesized ZnO nanoparticles were used as an adsorbent for the removal study. 1 M HCl and 1 M NaOH solutions were used for adjusting the pH. Commercially available ZnO nanoparticles purchased from Sigma-Aldrich for the comparative study of trace elements removals.
5.2 Batch experiment used for the removal study
A known amount of zinc oxide nanoparticles was added in a conical flask containing 100 ml aqueous solution of known concentration of particular trace elements. This aqueous solution was continuously shaken at a rate of 80–100 rpm by using a rotary shaker for a fixed period of time. 10–15 mL of aliquot was taken at a regular interval of time, i.e. 15 min, 30 min, 60 min, 90 min and 120 min. Then these samples were filtered with micro-filter syringe, and further analysis was done using ICP-OES. All the samples were performed in triplet, and the complete batch experiment procedure followed by us is given in Fig. 10, and trace elements removal results are shown in Fig. 11. The results of this study showed that synthesized zinc oxide nanoparticles are capable of removing several trace elements from their aqueous solutions.
5.3 Optimization of operating conditions
Maximum adsorption can be achieved by adopting some optimized conditions such as pH, dosage of nano-adsorbent and contact time.
-
(i)
Effect of pH: pH plays an important role in trace elements removal from aqueous solution using nano-adsorbent. The effect of pH on the removal of As, Cd and Se was investigated in 4–10 range of pH. The variations in the adsorption capacity of these elements are shown in Fig. 12a. It is clear from the figure that As and Se removal decreases with the increase in pH from acidic to basic range, while Cd removal increases with increase in pH. The orders of their removal change from As > Se > Cd to Se > As > Cd as a function of acidic to basic range.
-
(ii)
Effect of nano-adsorbent dosage: The removal of trace elements directly depends upon the dose of adsorbent. This may be due to more available site for adsorption. But an optimum shaking leads to maximum adsorption and hence maximum removal take place. The effect of adsorbent on the adsorption phenomenon is depicted in Fig. 12b. The order of these elements removal is in the range of Se > As > Cd to As > Se > Cd from lower dose to higher dose.
-
(iii)
Effect of contact time: The maximum adsorption could be achieved when both the adsorbent and heavy metals are in contact with each other to perform adsorption. The contact time was varied from 15 to 120 min. It is clear from Fig. 12c that maximum adsorption takes place at initial stage, and after 120 min the maximum removal is obtained in case of Se and Cd. The removal of As is about linear from lower to higher adsorption dose. This may be due to higher adsorption of arsenic at initial stage. The orders of their removal are in As > Se > Cd. It is clear from the figure that cadmium adsorbs in smaller quantity. This may be due to its photocatalytic adsorption tendency [29].
6 Comparative study of synthesized as well as commercially available ZnO nanoparticles for the removal of Trace elements
To test the effectiveness of synthesized nanoparticles, comparative study was used for the removal of selected trace elements from their aqueous solution. Five mg dose of nanoparticles was added in each conical flask containing 100–100 mL aqueous solution of different trace elements. The six conical flasks (3 trace elements × 2 nanoparticles) were shaken at a speed of 80–100 rpm by using rotary shaker for a fix period of time, i.e. 15 min, 30 min, 60 min, 90 min and 120 min. The complete batch experiment done by us is shown in Fig. 10. Under the selected conditions, both the nanoparticles examined and results are shown in Fig. 13. The comparative results obtained with other works already reported are shown in Table 2.
It is clear from the figure that removal efficiency of synthesized as well as commercially available ZnO nanoparticles towards different trace elements seems very good and equally effective. The removal efficiency of synthesized nanoparticles for cadmium is time dependent, and a maximum of 37% removal takes place after 120 min of adsorption process, as the removal of Cd increases with increase in time. On the other hand, commercially available nanoparticles showed approximate linear removal towards cadmium.
Synthesized ZnO showed best removal efficiency towards arsenic as it removes ˃ 95% arsenic ions from its aqueous solution only after 15 min. of batch process and approximate linear after full batch process of 120 min. It also seems that commercially available ZnO nanoparticles also perform like the synthesized ones.
On the other hand, the removal efficiency of both the nanoparticles seems equally effective towards selenium, as they remove 64% (Synthesized) and 69% (Commercially) of selenium after complete batch experiment of 120 min.
7 Conclusions
In this study, characterization of nanoparticles was performed via techniques like XRD, SEM, TEM, FT-IR and UV–visible spectroscopy. Well-defined zinc oxide nanoparticles were acquired, which were utilized for the degradation study of monocrotophos. The removal study of trace elements was performed comparatively with the commercially available as well as laboratory-synthesized ZnO nanoparticles. However, our investigation demonstrated that the degradation efficiency of synthesized nanoparticles towards monocrotophos pesticide was very good. On the other hand, trace elements adsorption takes place effectively with laboratory-synthesized nanoparticle as compared to commercially available nanoparticles.
References
Mutiyar PK, Mittal AK, Pekdeger A (2011) Status of organochlorine pesticides in the drinking water well-field located in the Delhi region of the flood plains of river Yamuna. Drink Water Eng Sci 4:51–60
Sankararamakrishnan N, Sharma AK, Sanghi R (2005) Organochlorine and organophosphorous pesticide residues in groundwater and surface waters of Kanpur, Uttar Pradesh, India. Environ Int 31:113–120
Bharti, Jangwan JS, Kumar A, Kumar V (2020) Analysis of organochlorine pesticides in drinking water and their degradation by synthesized iron oxide nanoparticles. Asian J Chem 32:1177–1182
Bharti, Jangwan JS, Kumar A, Kumar V (2020) Water quality of an Indian tributary affected by various industrial effluents—a case study. Adv Environ Res 9:41–54
Bharti, Jangwan JS, Kumar V, Kumar A (2019) Assessment and reason of drinking water quality in the catchment area of River Krishni, West U. P. India Int J Res Anal Rev 6:823–833
Jangwan JS, Bharti, Kumar V, Kumar A (2019) Drinking water monitoring in catchment area of river Krishni, Baghpat, Uttar Pradesh. India J Appl Chem 8(2):873–883
Mizan A, Juel MAS, Ahmed T (2020) Environmental and health risk of metal contaminated soil in the formar tannery area of Hazzribagh. Dhaka SN Appl Sci 1915(2):1–17
Firozjaee TT, Mehrdadi N, Baghdadi M, Bidhendi GRN (2017) Application of Nanotechnology in pesticides removal from aqueous solutions—a review. Int J Nanosci Nanotechnol 14:43–56
Guo J, Zhang Y, He Y-C, Shan J (2020) Photocatalytic performance of Co3O4/C based on ZIF-67/C composite material. Polyhedron 175:1–8
Khan SH, Suriyaprabha R, Pathak B, Fulekar MH (2015) Photocatalytic degradation of organophosphate pesticides (Chlorpyrifos) using synthesized zinc oxide nanoparticle by membrane filtration reactor under UV irradiation. Front Nanosci Nanotechnol 1:23–27
Kumar R, Chawla J (2014) Removal of cadmium ion from water/wastewater by nano-metal oxides: review. Water Qual Expo Health 5:215–226
Ghorbani HR, Mehr FP, Pazoki H, Rahmani BM (2015) Synthesis of ZnO nanoparticles by precipitation method. Orient J Chem 31(2):1219–1221
Nguyen TA, Pham V, Pham TH, Ngguyen LTT, Mittova IY, Mittova VO, Vo LN, Nguyen BTT, Bui VX, Viryutina EL (2020) Simple synthesis of NdFeO3 nanoparticles by the co-precipitation menthod based on a study of thermal behaviours of Fe(III) and Nd(III) hydroxides. Crystals 10:1–9
Wang F, Liu X (2011) Rare-earth doped upconversion nanophosphors. Compr Nanosci Nanotechnol 1(2):359–384
World Health Organization (2009) Health implications from monocrotophos use: a review of the evidence in India. WHO Regional Office for South-East-Asia
Nabiyouni G, Sharifi S, Ghanbari D, Niasari MS (2014) A simple precipitation method for the synthesis CoFe2O4 Nanoparticles. J Nanostruct 4:317–323
Mueting AM, Alexander BD, Boyle PD, Casalnuovo AL, Ito LN, Johnson BJ, Pignolet LH (1992) Synthesis and characterization of cationic gold-rhodium phosphate cluster complexes. X-Ray crystal and molecular structure of {Au4Rh(H)2[P(O-iso-C3H7)3]2(PPh3)4}PF6. In: Grimes RN (ed) Inorganic syntheses, vol 29. Wiley, New York, p 280
Albertsson J, Abrahams SC, Kvick A (1989) Atomic displacement anharmonic thermal vibration, expansivity and pyroelectric coefficient thermal dependences in ZnO. Acta Crystallogr Sect B Struct Sci 45:34–40
Lassoued A, Dkhil B, Gadri A, Ammar S (2017) Control of shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys 7:3007–3015
Manyasree D, Kiranmayi P, Kolli VR (2018) Characterization and antibacterial activity of ZnO nanoparticles synthesized by co precipitation method. Int J Appl Pharm 10(6):224–228
Hedayati K (2015) Fabrication and optical characterization of Zinc oxide nanoparticles prepared via a sol–gel method. J Nanostruct 5:395–401
Yu K, Shi J, Zhang Z, Liang Y, Liu W (2013) Synthesis, characterization and photocatalysis of ZnO and Er-doped ZnO. J Nanomater 1–5
Kaushik CP, Sharma HR, Kaushik A (2012) Organochlorine pesticide residue in drinking water in the rural areas of Harayana. Environ Monit Assess 184:103–112
Pimentel D (1995) Amounts of pesticides reaching target pests: Environmental impacts and ethics. J Agric Environ Ethics 8:17–29
Kaur P, Bansal P, Sud D (2013) Heterostructured nanophotocatalysts for degradation of organophosphate pesticides from Aqueous streams. J Korean Chem Soc 57:382–388
Duruible JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2:112–118
Danish EY, Marwani HM, Almoslehi KF, Bakhsh EM (2020) Adsorptive removal of lanthanum based on hydrothermally synthesized iron oxide-titanium oxide nanoparticles. Environ Sci Pollut Res 27:5408–5417
Khan SB, Marwani HM, Asiri AM, Bakhsh EM (2016) Exploration of calcium doped zinc oxide nanoparticles as selective adsorbent for extraction of lead ion. Desalin Water Treat 57:19311–19320
Le AT, Pung SY, Sreekantan S, Matsuda A, Huynh DP (2019) Mechanism of removal of heavy metals ions by ZnO particles. Heliyon 5:1–27
Mahdave S, Jalali M, Afkhami A (2012) Removal of heavy metals from aqueous solutions using Fe3O4, ZnO, and CuO nanoparticles. J Nanopart Res 14:1–18
Muensri P, Danwittayakul S (2017) Removal of Arsenic from groundwater using Nano-Metal Oxide Adsorbents. Key Eng Mater 751:766–772
Bakather OY, Fard AK, Ihsanullaha, Khraisheh M, Nasser MS, Atieh MA (2017) Enhanced adsorption of selenium ions from aqueous solution using iron oxide impregnated carbon nanotubes. Bioinorgan Chem Appl pp 1–12
Acknowledgements
One of the authors (Bharti) is thankful to Dr. Vivek Kumar, Assoc. Prof., Centre for Rural Development and Technology, IIT Delhi, India, for providing necessary facilities to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bharti, Jangwan, J.S., Kumar, G. et al. Abatement of organic and inorganic pollutants from drinking water by using commercial and laboratory-synthesized zinc oxide nanoparticles. SN Appl. Sci. 3, 311 (2021). https://doi.org/10.1007/s42452-021-04294-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04294-0