Abstract
Manganese chlorophosphate NaPO3–MnCl2 glasses were prepared via two synthetic routes and their thermal and spectroscopic properties were analyzed in order to draw a comparison between the conventional method of glass preparation, i.e. melting and quenching of the precursor salts and the so-called “coacervate route” for glass preparation. In this procedure, polyphosphate coacervates of the desired glass compositions are prepared by the mixture of an aqueous solution of the salts, separation and drying of the denser separated-phase, the coacervate. The coacervates are then melted and quenched to yield glasses. Differential Scanning Calorimetry (DSC), Differential Thermal Analysis (DTA) and Raman Spectroscopy results lead to a description of the “coacervate-derived glasses” as a system in which the polyphosphate chains are longer and mostly interconnected, when compared to the traditional glasses.
Similar content being viewed by others
1 Introduction
Sodium polyphosphate, (NaPO3)n, also known as Graham’s salt, is a water-soluble glassy product obtained from the melting of sodium dihydrogen phosphate, NaH2PO4 [1]. The polymeric structure of Graham’s salt is built by large polyphosphate chains, with reported average lengths attaining up to 30 PO3 units [1,2,3,4], leading to the general formula (NaPO3)n. Changes in the chemical properties of the aqueous (NaPO3)n solution, such as addition of electrolytes or changes in the dielectric constant, e.g. by the addition of methanol, lead to the coacervation process through the formation of colloidal vesicles followed by their aggregation, leading to liquid–liquid phase separation, being the coacervate the denser phase [1,2,3,4,5]. Drying procedures of the coacervates in silica gel vacuum desiccator lead to solid transparent glassy materials.
Silva and collaborators in a previous work reported a short-range ordering structure of Ni+2 and Co+2 polyphosphate coacervates by EXAFS measurements and the role of these ions in the coacervation process was described [6]. In low metal concentrations (phosphorus to metal ratio P/M2+ > 6) the ions are coordinated inside cages, formed by the polyphosphate chains wounded up around them. In this composition region, the samples can be treated as simple colloidal systems. In the concentration range 2 < P/M < 6, the cage sites have been saturated, and the metallic ions occupy external sites, linking two adjacent polyphosphate chains, giving rise to the coacervation process. After saturation of these external sites, for P/M < 2, the additional metallic ions are mainly present as hydrated complexes. In all situations, the metallic ions are highly susceptible to coordination with water molecules and the octahedral coordination is related both to coordination with oxygen atoms from water molecules and with oxygen atoms from the polyphosphate chains. Figure 1 shows the proposed structural characteristics of the polyphosphate coacervates, derived from the conclusions of reference [6].
Structural properties of polyphosphate coacervates, as proposed in [6]. Two sites for the metallic ions are present. Metallic ions M are trapped inside cages formed by long polyphosphate chains, originating colloidal vesicles. In this picture, only one metallic ion is present inside the cages, but several ions can be coordinated in this way. When the sites inside the cages are saturated, metallic ions M’ are prone to link adjacent vesicles, leading to the coacervation process
The melting of the polyphosphate coacervates to produce polyphosphate glasses, the “coacervate route” for glass production, was proposed in several works [7,8,9,10,11]. It was mainly suggested the use of the coacervate route for glass preparation as a promising method for coating and joining processes. The coacervate route for glass formation has the advantage of allowing control of the glasses properties through coacervate formulation, since different metallic ions and counter-ions can be used, such as chloride, sulfide and nitride, besides the possibility of mixing different coacervates before melting. Additionaly, the glass formation through coacervate route occurs at room temperature, leading to a low-cost synthesis route, avoiding unnecessary energy consumption in comparison with the melt-quenching method. On the other hand, little is known about the structural differences between the conventional polyphosphate glasses (those obtained from the melt-quenching process of the precursors salts) and the polyphosphate glasses obtained via the coacervate route.
In this work we compare the thermal and spectroscopic properties of glasses obtained from the melting/quench of manganese polyphosphate coacervates and glasses obtained from the “traditional route”, i.e. melt/quench of the precursors salts NaPO3 and MnCl2. Differential Thermal Analysis (DTA), Differential Scanning Calorimetry (DSC) and Raman Spectroscopy were used in the determination of the structural characteristics of these materials and important differences were found, as described below.
2 Experimental
Manganese (II) polyphosphate coacervates were prepared by mixing MnCl2 solutions of different concentrations and NaPO3 (4 mol L−1) solutions, under constant stirring at room temperature. The phosphorus-to-manganese molar ratio, P/Mn+2, was set to different values by using adequate metal chloride solution volumes and ranged from 2 to 4, as described in Table 1. This composition range is in the coacervation region of other transition metals, such as Ni2+ and Co2+ [10, 12]. The coacervates, obtained after separation of the dense viscous phase from the mother solution, were kept in vacuum desiccators with silica gel during several days, in order to obtain transparent glassy materials. The Mn+2 polyphosphate coacervates are pale pink colored transparent solids. Samples of manganese (II) polyphosphate coacervates were also prepared with the addition of methanol to the mixture, following the compositions listed in Table 1.
After drying, the coacervates were melted in platinum crucibles at 1000 °C for 30 min and then quenched between two stainless steel plates. The glasses were pink colored and showed no devitrification evidence under optical microscopic. The samples were labeled according to the corresponding composition, as shown in Table 1. The term “coacervate-derived glass” will be used for these samples.
As a comparison between the coacervate route for glass preparation and the conventional method of glass making by quenching of the melt, (100-x)NaPO3-xMnCl2 glasses, with x ranging from 10 to 50 mol%, where prepared by weighting the calculated masses of the precursor salts NaPO3 (99% Merck) and MnCl2 (99% Merck), mixing and homogenization in an agate mortar, melting in platinum crucibles at 900 °C, followed by quenching of the melt between two stainless steel plates maintained at room temperature. Homogeneous transparent glasses were obtained up to 40 mol% MnCl2 and the term “conventional glass” will be used for these samples. Sample names, glass compositions and P/Mn2+ ratios are listed in Table 2.
Fourier-Transform Raman spectroscopy measurements were carried out using a Bruker RFS 100 instrument, an Nd3+ /YAG laser operating at 1064 nm in the near infrared and a CCD detector cooled with liquid N2. For the samples a good signal-to-noise ratio was obtained from 512 scans accumulated over about 30 min and 20 mW of laser power, with 1 cm−1 as operating spectral resolution. All spectra were recorded at least two times to demonstrate reproducibility and no changes in band positions and relative intensities were observed. Differential Thermal Analysis and Differential Scanning Calorimetry measurements were performed in a Shimadzu DTG-60 calorimeter with a nitrogen atmosphere and heat rate of 10 K × min−1.
3 Results
Figure 2a–e shows the DSC curves for the coacervate-derived glasses and Table 3 gathers the characteristic temperatures extracted from these curves.
DSC curves for the coacervate-derived NaPO3–MnCl2 glasses. Samples obtained without the addition of methanol: (a) PMn2, (b) PMn2.7, (c) PMn4. Samples obtained with the addition of methanol: (d) PMn4Me5; (e) PMn4Me35. See Table 1 for composition details
For the glasses obtained from the coacervates without methanol, the glass transition temperature increases with MnCl2 content, as seen in the inset of Fig. 2 and Table 3. Compared to the PMn4 sample, glassy samples PMn4Me5 and PMn4Me35, obtained from the coacervates with methanol in its synthesis, present very close values for glass transition temperature Tg and crystallization peak temperature Tp. The main thermal difference between these samples is in the onset crystallization temperature, Tx, which gives different thermal stability parameter (Tx − Tg) values. Due to temperature limitations of the DSC technique, the complete devitrification process is not shown in Fig. 2 and Differential Thermal Analysis (DTA) was performed in samples PMn4 and PMn4Me35 up to 900 °C. The resulting curves are displayed in Fig. 3a and b, respectively.
The differences in the exothermic devitrification peaks around 500 °C and the endothermic melting peaks, around 700–800 °C for the two glasses are noteworthy. While the sample PMn4Me35 presents single symmetrical phase transition peaks, the devitrification and melting processes of sample PMn4 consist of a superposition of at least two phase transitions.
Figure 4 shows the DSC curves for the (100 − x)NaPO3 − xMnCl2 conventional glasses, with x ranging from 10 to 40 mol% (samples 9P1Mn, 8P2Mn, 7P3Mn and 6P4Mn). The glass transition temperature dependence on composition shows different behavior compared to the coacervate-derived glasses (see Fig. 2 with the decreasing Tg values with x. The glass composition with x = 20 and (P/Mn = 4) possesses the maximum value of glass transition temperature.
DSC curves for the NaPO3–MnCl2 glasses obtained directly from the melt of the precursor salts: (a) 9P1Mn, (b) 8P2Mn, (c) 7P3Mn, (d) 6P4MnSee Table 2 for composition details
Figure 5 shows a plot relating glass transition temperature and P/Mn ratio in the coacervate-derived and conventional glasses. Whereas coacervate-derived glasses show Tg values withing 360 < Tg < 390 °C range, the conventional glasses present lower Tg values, 305 < Tg < 330 °C. The higher values of Tg in coacervate-derivad glasses indicate that the coacervation process might contribute to the structural rigidity of the polyphosphatic chains, i.e., the chemical structure of the glassy matrix hardly changes, since the preparation method, in addition to the Mn2 + incorporation possibily connect the adjacent polyphosphatic chains, reflecting in the higher Tg values. This is an interesting result, showing that, even under melting, the coacervation process is useful in preserving the glassy chains interconnectivity. After melting, the coacervate-derived glasses can be regarded as oxide-based glasses, while the conventional glasses probably contains chloride ions embedded in the glassy matrix. The presence of chloride ions influences in the decrease of the glassy chains interconnectivity and lower Tg values are observed. It is noteworthy to highlight that future studies must be performed in order to elucidate the possible oxychloride nature of the glassy chains in the conventional glasses.
Figure 6 shows the Raman spectra for the precursor coacervates (a-d) and their derived glasses (e–h). The spectra profile is characteristic for polyphosphate glasses and bands at around 690 and 1160 cm−1 are attributed to the symmetric stretching mode in P–O–P bonds, between two Q2 species and symmetric PO2 stretching of terminal P–O bonds, in Q2 species, νs(P–Ob) and νs(P–Ot), respectively [13, 14].
Table 4 lists the wavenumber values of these vibrational modes for each sample. The vibrational characteristics of the different coacervates and resulting glasses are very close. But an inspection of the wavenumber values for the stretching modes gathered in Table 4 reveals a main difference in the νs(P–Ot) vibrational mode for the two systems. In glasses, it appears at larger wavelength values than in the coacervates. On the other hand, νs(P–Ob) vibrational mode appears nearly in the same wavenumber region both for coacervates and derived glasses.
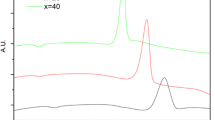
A comparison between the vibrational characteristics of the glasses obtained from the coacervate route (Fig. 6) and those obtained through the conventional method can be made by inspection of Fig. 7.
Raman spectra of Graham´s salt NaPO3 and glasses NaPO3–MnCl2 obtained directly from the melting of the precursor salts: (a) NaPO3; (b) 9P1Mn; (c) 8P2Mn; (d) 7P3Mn; (e) 6P4Mn. See text and Table 5 for detailed assignment of the bands labeled 1–9 in this figure
Figure 7 shows the Raman spectra of the conventional glasses with a composition (100 − x)NaPO3 − (x)MnCl2 (x = 10 to 40 mol%; samples 9P1Mn, 8P2Mn, 7P3Mn and 6P4Mn) as well as for the glassy NaPO3. The dependence of the vibrational profile with composition is noteworthy. Several features are observed with the increase of MnCl2 content in the glass composition. Compared to the NaPO3 spectrum, new bands are found at ca. 740 cm−1 and in the 900–1140 cm−1 region. This vibrational behavior is also observed for other phosphate glassy systems [14,15,16]. Deconvolution of the Raman spectrum of the 6P4Mn sample was performed and is seen in Fig. 7e. It shows the separate component for each vibrational mode in the glassy phosphate chain and can be used in the description of the structural evolution of the other glasses with varying composition. Although the attributions to these bands were based on references [14,15,16], in our deconvolution procedure we propose the existence of a vibrational mode not yet described in the literature, related to the peak 2 of the deconvolution of Fig. 7e. The vibrational attributions to these bands are shown in Table 5.
The peak 1, situated at 1254 cm−1, is attributed to the asymmetric PO2 stretching mode of terminal P–O bonds, νas (P–Ot), in Q2 species. The vibrational mode related to peak 2, centered at 1191 cm−1, not yet described in the literature, must be related to symmetrical and/or asymmetric stretchings of P–Ot species. Despite having a very low relative intensity in the vibrational structure of NaPO3 and glasses with x ≤ 2, it offers a considerable contribution to the vibrational Raman profile of glasses with higher contents (x ≥ 3) of manganese chloride, mainly due to de decrease of the intensity of the band related to Peak 3. This band, centered at 1142 cm−1, is assigned to the symmetric PO2 stretching mode of terminal P–O bonds, νs (P–Ot), in Q2 species. Peak 4, located at 1103 cm−1, is associated both to the symmetric PO2 stretching mode, νs (P–Ot), in Q2 species in polyphosphate groups and asymmetric PO3 stretching mode, νas (P–Ot), in Q1 species in the polyphosphate and pyrophosphate groups. Peaks 5 and 6, at 1041 and 999 cm−1 are assigned to the symmetric PO3 stretching modes, νs (P–Ot), in Q1 species in the pyrophosphate and polyphosphate groups. Peak 7, situated at 919 cm−1, is composed of the symmetric PO4 stretching mode, νs (P–Ot) and by the asymmetric P–O–P vibration νas (P–Ob) in Q0 species in the orthophosphate groups. Finally, the peak 8, located at 742 cm−1, is attributed to the symmetric stretching mode νs (P–Ob) in P–O–P bonds, between two Q1 species or between one Q1 and one Q2 species, while peak 9, at 695 cm−1, is also associated to the symmetric stretching mode νs (P–Ob) in P–O–P bonds, but between two Q2 species.
A comparison between the energy of the main bands found on the Raman spectra of the coacervates and coacervate-derived glasses, and those obtained for the conventional glasses (Tables 4 and 5) is of special interest. The νs(P–Ob) stretching mode appears in conventional glasses, coacervates and coacervate-derived glasses at ca. 690 cm−1, but the νs(P–Ot) stretching mode, located at ca. 1165 cm−1 for coacervates and ca. 1170 cm−1 in the coacervate-derived glasses, is found at considerably lower wavenumber values, being around 1140 cm−1 in the 6P4Mn conventional glass.
4 Discussion
The melting process of both manganese polyphosphate coacervates and precursor salts NaPO3-MnCl2 produces good glass-forming melts. Stable, pink colored glasses were obtained in all tested compositions except for the 50NaPO3—50MnCl2 system. The sample 5P5Mn presented opaque crystalline regions in the glassy matrix, indicating that in the (100 − x)NaPO3 − (x)MnCl2 system, compositions with x ≥ 50 have a poor glass forming ability in the experimental conditions detailed above.
Figures 2 and 4 compare the thermal properties of the glasses obtained by the two proposed methods of glass preparation. The inspection of these figures and their insets show that the coacervate-derived glasses have higher glass transition temperatures than the conventional glasses. This fact is noteworthy, as higher values for Tg are related to the higher connectivity of the glassy network. This result indicates that the aggregation of the polyphosphate chains, in the coacervation process, via coordination with the Mn2+ cations, as described in reference [6], is maintained to some extent in the melt, generating largely polymerized phosphate chains of excellent glass-forming properties. Indeed, this important result shows that the coacervate route for phosphate glass preparation generates a highly connected glass network, relative to the conventional method of using the precursor crystalline materials.
Moreover, the inset of Fig. 2 clearly shows that the increase in the Mn2+ content in the glass composition continuously increases the connectivity of the polyphosphate chains, leading to higher Tg values. On the other hand, the inset of Fig. 4 indicates that the 80NaPO3–20MnCl2 composition (sample 8P2Mn, P/Mn = 4) is the one which gives the more polymerized glassy network (higher Tg value). In contrast, compositions with more than 20 mol% on MnCl2 (P/Mn < 4) produces glassy networks with lower connectivity (lower Tg glasses). For P/Mn < 4, due to the high amounts of Mn2+ in the melt, these ions coordinate in terminal positions of the polyphosphate chains, causing a decreasein the chain sizes and connectivity, leading to the observed Tg decrease.
For the glasses obtained via coacervate route (Fig. 2 and inset), the Tg dependence on composition can also be compared to the changes in the structural disorder around the transition metal in the coacervation range, as obtained by EXAFS analysis in our previous work [6]. In fact, it is proposed that the metallic ions are responsible for the linkages between adjacent colloidal phosphate vesicles, leading to the coacervation process. It was found that the P/M2+ = 2 (M2+ = Co and Ni) ratio is the one with maximum structural disorder around the transition metal ions and that this ratio lies in the higher M2+ concentration limit for the coacervation range. The increase in the disorder around the metallic ions in the coacervates seems to be directly connected to the increase in the degree of polymerization of the polyphosphate chains on the melt, leading to higher Tg values for the derived glasses.
On the other hand, the coacervation process itself can be controlled to obtain different glassy materials. The glasses PMn4Me5 and PMn4Me35, obtained via coacervate route, but with 5.0 and 35.0 mL of methanol added to the precursor coacervates, respectively, can be compared to the PMn4 glass, obtained via coacervate route without methanol.
Figures 2 and 4, as well as Table 3, show that, while the coacervation process made with or without methanol do not considerably influence in the glass transition temperature of the corresponding glasses, the devitrification processes and, consequently, the melting processes are considerably affected. The use of methanol in the coacervate preparation leads to glasses with narrow and symmetrical devitrification peaks in DSC and DTA scans. Narrow devitrification peaks in non-isothermal DSC/DTA analysis are results of high crystallization rates, indicating that the glasses prepared via coacervate route with methanol present faster devitrification kinetics. The narrowing of the peaks also implies higher Tx values, leading to an increase of thermal stability parameters (Tx − Tg) values with increasing the methanol content (see Table 3). Also, while the sample PMn4Me35 presents a single symmetrical devitrification and melting peaks in DSC and DTA scans, the devitrification and melting processes of sample PMn4 consist of a superposition of several phase transitions. These observations can be considered as a consequence of the decrease of the dielectric constant caused by the methanol present in the precursor coacervate. In fact, it is known [8] that coacervates are formed in pure sodium polyphosphate solutions, even without the presence of electrolytes, if the dielectric constant is reduced by the addition of methanol, for instance. In this way, methanol can promote the coacervation process more efficiently by increasing the proximity of the charges inside the polyphosphate chains, leading to a more efficient agglomeration of the colloidal vesicles, but not strongly acting in the network connectivity. Since the metallic ions are the main responsible for the coacervate network connectivity [6] and the presence of methanol do not change the glassy polyphosphate network, samples PMn4 and PMn4Me35 present the same Tg values. On the other hand, as proposed, the structural properties of the coacervates are to some extent maintained in the melt and the glassy structure of the sample prepared with methanol is more compact, which accelerates the crystal growth kinetics and lead to a single narrow devitrification peak and the melting of a single crystalline phase. Being the structure of the coacervate prepared without methanol less compact, but yet highly polymerized, the devitrification process of the derived glass is more complicated, involving two, and possibly more, crystalline phases.
The main vibrational modes observed in the Raman spectra of sodium polyphosphate NaPO3 and derived glasses are the symmetric PO2 stretching mode of terminal P–O bonds, νs (P–Ot), in Q2 species and the symmetric stretching mode νs (P–Ob) in P–O–P bonds, between two Q2 species, located at ca. 1165 cm−1 and 690 cm−1, respectively, as seen in Fig. 6.
For the different systems studied, i.e. conventional glasses, coacervates and coacervate-derived glasses, the νs(P–Ob) stretching mode (P–O–P bonds), peak 9 in the deconvolution of the Raman spectrum of Fig. 7, do not present relevant energy shift among the samples and appears at ca. 690 cm−1 for the different glasses and coacervates, with or without the use of methanol. This result indicates that the bond strengths responsible by the formation of the polyphosphate chain do not change considerably in the different amorphous systems studied. This feature is found to be independent of the chain sizes or connectivity: it was shown that the glasses have different Tg values depending on composition (see inset of Figs. 2 and 4). In other words, glasses with different network connectivity degree show the symmetric stretching mode νs (P–Ob) in P–O–P bonds between two Q2 species at the same energy range. On the other hand, it exists important differences between the stretching modes of terminal P–O bonds in Q2 species forming the polyphosphate network (peak 3 in the deconvolution of the Raman spectrum of Fig. 7). The value of the νs(P–Ot) stretching mode varies considerably, and their wavenumber are 1140, 1165 and 1170 cm−1 for the conventional glasses, coacervates and coacervate-derived glasses, respectively, with or without the use of methanol in the coacervate synthesis, as seen in Tables 4 and 5.
Considering that the conventional glasses have less polymerized glassy networks, with the occurrence of Q1 and even Q0 units, as indicated by thermal analysis and Raman spectra, one could infer that the terminal P–O bonds can symmetrically stretch with more ease. This is the reason why the νs(P–Ot) stretching mode appears in a lower energy region in the Raman spectra of the conventional glasses. In the coacervate, on the other hand, the coacervation process produces longer and highly branched polyphosphate chains, in a compact amorphous structure. In this way, the symmetrical P–Ot stretching mode requires more energy and appears in a higher energy region of the Raman spectrum. Since methanol use in the coacervates acts mainly in the chain agglomeration, and less on the chain connectivity, the feature described above does not depend on the coacervate synthesis method.
In the coacervate-derived glasses the high temperature used in the melting process (1000 °C) leads to a total loss of water from the structures described in [6]. Being the coordination water linked to the metal ions inside and outside the polyphosphate vesicles, its elimination must lead to a collapse in a yet more compact, amorphous structure. As a consequence, the symmetrical stretching of terminal P–O bonds is hampered and the νs(P–Ot) stretching mode appears in a higher energy region in Raman spectra of the coacervate-derived glasses.
While the vibrational characteristics of the coacervate-derived glasses appear to be almost identical, independent of the P/Mn ratio (see Fig. 6), for the conventional glasses a considerable profile evolution of the Raman curves is observed, as the P/Mn ratio changes (Fig. 7). From the bands attribution described in the text and gathered in Tables 4 and 5 it is clear that the main changes in the structural properties of the glasses obtained via conventional route are the increase of vibrational contribution of terminal P–O bonds of Q1 and Q0 species caused by the increase of Mn2+ content in the glass compositions. It is clear that the metallic ions act, in the conventional glass, as a glass network modifier and, for P/Mn < 4 ratios, lead to a decrease in network connectivity inducing the cleavage of P–O–P bonds, generating terminal PO3 and even isolated PO4 units. This is reflected in the decrease in Tg values, as described above and is in contrast with the data for the glasses obtained via coacervate route, in which no considerable changes are detected in the Raman spectra, as shown in Fig. 5. Besides, a gradual increase of Tg is observed with the transition metal concentration (Fig. 2).
The results above indicate that manganese polyphosphate glasses obtained via coacervate route are characterized by a more efficiently inclusion of metallic ions in the glassy network, acting as a glassy network former. On the other hand, the conventional method of melting of the crystalline precursors, in which for compositions with more than 20 mol% of MnCl2 the chain sizes and connectivity diminishes considerably, with the occurrence of Q1 and Q0 units. These results allow us to conclude that the coacervation process allows the maintenance of the polyphosphate network connectivity and chain lengths, as opposed to the traditional method of direct melting of the salts, leading to glasses of different structural properties.
5 Conclusion
The metal-polyphosphate chain coordination of the coacervation processes and the structural peculiarities preexisted in the coacervate glass precursors indeed play a significant role in the glass-forming properties of the melt, leading to the considerable differences between the glasses obtained from the coacervate route and those produced by the conventional method of melting of the crystalline precursors. The vibrational and thermal properties of the different glasses studied indicate that the coacervate-derived glasses have a more polymerized structure when compared to the traditional glasses. It is proposed here that the long interconnected polyphosphate chains of the coacervates, i.e. the main glassy network building units in this system, are maintained in a large extent after melting, characterizing the structure of the glasses produced via the coacervate route. At the same time, the direct melting of the precursor salts leads to a gradual increase of terminal Q1 and Q0 sites, leading to the diminution of the chain lengths with MnCl2 content.
References
De Jager H, Prinsloo LC (2001) The dehydration of phospates monitored by DSC/TGA and in situ Raman spectroscopy. Thermochim Acta 376:187. https://doi.org/10.1016/S0040-6031(01)00582-2
Gomez F, Vast P, Barbieux F (1995) Simple coacervation of sodium polyphosphate obtention. Phosphorus Res Bull 5:143. https://doi.org/10.3363/prb1992.5.0_143
Umegaki T, Nayama Y, Kazanawa T (1976) Thermal change of magnesium highpolyphosphate coacervates. Bull Chem Soc Jpn 49:2105. https://doi.org/10.1246/bcsj.49.2105
Willot G, Gomez F, Vast P, Andries V, Martines M, Messaddeq Y, Poulain M (2002) Preparation of zinc sodium polyphosphates glasses from coacervates precursors. Characterization of the obtained glasses, and their applications. C Rend Chim 5:899. https://doi.org/10.1016/S1631-0748(02)01454-6
Franco DF, Barud HS, Santagneli S, Lamarca RS, Santos BF, Silva MAP, de Oliveira LFC, Ribeiro SJL, Nalin M (2016) Preparation and structural characterization of sodium polyphosphate coacervate as a precursor for optical materials. Mater Chem Phys 180:114. https://doi.org/10.1016/j.matchemphys.2016.05.049
Silva MAP, Franco DF, Oliveira LFC (2008) New insight on the structural trends of polyphosphate coacervation processes. J Phys Chem A 112:5385. https://doi.org/10.1021/jp801585v
Walbrou O, Aldon L, Vast P, Couturier D, Martines MAU, Jumas JC, Messaddeq Y (2001) Iron polyphosphate coacervate: a new route for the treatment of inorganics wastes? Phosphor Res Bull 12:211–218. https://doi.org/10.3363/prb1992.12.0_211
Gomez F, Vast P, Llewellyn PH, Rouquerol F (1997) Dehydroxylation mechanisms of polyphosphate glasses in relation to temperature and pressure. J Non-Cryst Solids 222:415. https://doi.org/10.1016/S0022-3093(97)90144-7
Franco DF (2011) Master thesis. Federal University of Juiz de Fora, Brazil, Department of Chemistry
Franco DF, Manzani D, Barud HS, Antonio SG, de Oliveira LFC, Silva MAP, Ribeiro SJL, Nalin M (2016) Structural investigation of nickel polyphosphate coacervate glass–ceramics. RSC Adv 6:91150. https://doi.org/10.1039/C6RA20696D
Dias Filho FA, Carlos LD, Messaddeq Y, Ribeiro SJL (2005) Spectroscopic study and local coordination of polyphosphate colloidal systems. Langmuir 21:1776. https://doi.org/10.1021/la0476837
Novita DI, Boolchand P (2007) Synthesis and structural characterization of dry AgPO3 glass by Raman scattering, infrared reflectance, and modulated differential scanning calorimetry. Phys Rev B 76:184205. https://doi.org/10.1103/PhysRevB.76.184205
Weil M, Puchberger M, Auf der Günne JS, Weber J (2007) Synthesis, crystal structure, and characterization (vibrational and solid-state.31P MAS NMR spectroscopy) of the high-temperature modification of calcium catena-polyphosphate(V). Chem Mater 19:5067. https://doi.org/10.1021/cm071239i
Massera J, Bourhis K, Petit L, Couzi M, Hupa L, Hupa M, Videau JJ, Cardinal T (2013) Effect of the glass composition on the chemical durability of zinc-phosphate-based glasses in aqueous solutions. J Phys Chem Solids 74:121. https://doi.org/10.1016/j.jpcs.2012.08.010
Mamedov S, Stachel D, Soltwisch M, Quitmann D (2005) Local environment and dynamics of PO4 tetrahedra in Na–Al–PO3 glasses and melts. J Chem Phys 123:124514. https://doi.org/10.1063/1.2038707
Fletcher LB, Witcher JJ, Troy N, Reis ST, Brow RK, Vazquez RM, Osellame R, Krol DM (2011) Femtosecond laser writing of waveguides in zinc phosphate glasses [Invited]. Opt Mater Express 1:845. https://doi.org/10.1364/OME.1.000845
Acknowledgements
The financial support of FAPEMIG, CNPq and CAPES/PROINFRA (Brazilian agencies) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santos, P.B., Franco, D.F., Machado, T.M. et al. The coacervate route for polyphosphate glasses preparation. SN Appl. Sci. 2, 1314 (2020). https://doi.org/10.1007/s42452-020-3142-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-3142-0