Abstract
We investigated the effects of short-term exposure of bare TiO2NPs and polyelectrolyte-coated TiO2NPs in the 5–25 nm size range, at relatively high concentrations (of 500 and 1000 mg/L) under light or dark conditions, in D. rerio embryos. The biological endpoints investigated included embryo viability and mRNA transcript levels of antioxidant and membrane transport genes relative to control embryos. The presence of nanoparticles on the surface of embryos was assessed using TEM. The results confirm an accumulation of TiO2NPs on the outer surface (chorion) of the embryo, but not within the embryo. No significant difference in embryo viability was detected following each exposure regime. The expression of antioxidant biomarker, SOD2, was significantly impacted by the type of TiO2NP, with TiO2NPs/PSS/PAH coating exposure showing down regulation; the concentration of the nanoparticles, with down regulation at 500 mg/L; and dark/light condition with down regulation in the light. The expression levels of the hypoxia and membrane markers, HIF1 and Pxmp2, were not significantly impacted by any factor. The study indicates that SOD2 mRNA expression levels may be useful in the detection of apparent oxidative stress induced by the titania nanoparticle build up on the embryo chorion surface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One of the most widely used engineered nanoparticles (NPs) worldwide is nano-sized titanium dioxide NPs (TiO2NPs) that is used for its photocatalytic properties [1]. TiO2NPs are used in sunscreens and cosmetic creams [2] for their ability to block UV light [3]. Titania is also used as a pigment in toothpaste [4], in skin treatments [5], and paints, as well as food industry applications [6, 7]. They have further wide-ranging uses from photosensitizing agents for photodynamic therapy of endobronchial and esophageal cancers [8], as disinfection agents in wastewater treatment [9], as well as in the environmental decontamination of soil, air, and water [10, 11].
TiO2NPs may be toxic when released into the aquatic environment as they form superoxide and hydroxyl radicals on exposure to sunlight (UV) and oxygen, which could then lead to damage of the cell contents if taken up by organisms [12]. TiO2NPs have been categorised into different forms: rutile, anatase, and amorphous [13] whereby the anatase forms are generally found to be more photo-active than rutile TiO2NPs. Al-Awady et al. [14] reported a significant nanotoxicity of TiO2NPs against algae and yeast, which depends on the particle surface charge. Nanoparticles with cationic surfaces are generally found to be more toxic than those with anionic surfaces [15]. Several sunscreen products contain anatase TiO2NPs, which has been found to generate reactive oxygen species (ROS) upon illumination with UV light [16]; this potentially leads to biological damage [17]. Previous studies have shown that TiO2NPs (at an exposure level of 5 mg/L) induce oxidative stress in the liver of zebrafish [18] and in the brain tissue of rainbow trout (Oncorhynchus mykiss) [19]. In contrast, the potential of TiO2NPs to produce oxidative damage to DNA without photoactivation is still unclear [20].
TiO2NP toxicity has been shown to be dependent on both particle size and the degree of particle aggregation. Smaller nanoparticles have been found to have higher mobility between biological compartments. For example, the 4 nm TiO2NPs have been observed penetrating into the deeper layer of the epidermis (to the basal cell layer) in hairless mice [21]. In contrast though, TiO2NP aggregation, increasing the size factor, has been shown to have a larger effect on cell viability and gene expression of biomarkers focused on stress, inflammation, and cytotoxicity in human acute monocytic leukemia and bronchial epithelial cell lines when compared with smaller aggregates of 166 nm [22]. Encapsulating the TiO2NPs with coating agents (such as inert oxides of silica, alumina, or zirconium) also mediates the ROS associated impacts [23] by reducing or eliminating them. Coatings based on silicon dioxide have also been found to reduce the oxidative activity of TiO2NPs on pig skin [24].
Overall, the increased use of TiO2NPs, and their ultimate release into the environment, suggests an increasing need to evaluate their potential toxicity, including the effect of the nanoparticle coating and size range. In this study, D. rerio embryos were used to study the effect of TiO2NPs coated with different number of layers of anionic and cationic polyelectrolytes on embryo viability. Selected oxidative stress markers, which are conserved in all vertebrate species, were also measured including superoxide dismutase 2 (SOD2), hypoxia inducible factor 1 (HIF1) and peroxisomal membrane transporter protein (Pxmp2) gene expressions.
2 Materials and methods
2.1 Preparation and characterisation of TiO2NPs
Three different batches of TiO2NPs were prepared in Milli-Q water: bare titania NPs (TiO2NPs), anionic NPs prepared with poly (sodium 4-styrene sulfonate) sodium salt (PSS) as TiO2NPs/PSS, and the cationic NPs prepared with poly (allylamine hydrochloride) (PAH) as TiO2NPs/PSS/PAH. The synthesis of TiO2NPs was conducted using the procedure described by Al-Awady et al. [14]—see Fig. 1a. Briefly, 1 M HNO3 was added drop-wise to 250 mL of Milli-Q water to adjust the pH to 2 followed by dropwise addition of a mixture consisting of 15 mL aliquot of isopropanol and 5.0 mL of titanium isopropoxide (TTIP) to the former solution with vigorous stirring, leading to the formation of a white turbid suspension as a result of the hydrolysis of TTIP. The suspension of Ti (OH)4 was heated to 70 °C for 20 h to form a yellow-white precipitate of titania that was filtered, washed with ethanol and further dried under vacuum (Gallenkamp vacuum oven) at 100 °C for 2 h. Aqueous dispersions of TiO2NPs was prepared by dispersing 4 mg of the titania sample in 10 mL aliquots of 20 mM aqueous solution of NaCl at pH 4 using a digital sonicator (Branson 450, 5 mm tip, 400 W maximal power) at 40% of the maximum power for 10 min at 1 s ON/1 s OFF pulse time and followed by filtration through a syringe filter of pore size 0.22 μm. The TiO2NPs were characterised in terms of size distribution and zeta potential in aqueous solutions using a Zetasizer Nano ZL (Malvern, U.K.). Transmission electron microscopy (TEM) images of the particle samples were obtained using JEM 2011 (JEOL, Japan) running at 200 kV.
a Schematic diagram of the coating of the bare TiO2NP with polyelectrolytes (PSS and PAH), b TEM image of the titania produced by annealing at 70 °C, c Size distribution of dispersed titanium dioxide NPs synthesised by hydrolysis and condensation of titanium isopropoxide at acidic medium for 20 h at 70 °C. d Zeta-potential of the bare TiO2NP, TiO2NP/PSS and TiO2NP/PSS/PAH at pH 5.5
2.2 Layer-by-layer polyelectrolyte-coated TiO2NPs
Polyelectrolyte-coated TiO2NPs were prepared using titania synthesised and annealed at 100 °C (anatase). 10 mL of 1500 μg mL−1 TiO2NPs dispersion in Milli-Q water was added drop-wise to an equal amount of 10 mg mL−1 of solution of PSS (M.W. ~ 70 kDa) dissolved in 1 mM NaCl solution. After shaking for 20 min, the particles were washed three times by centrifugation for 1 h at 8000 rpm to remove the excess of PSS and were finally re-dispersed in 10 mL of Milli-Q water. The PSS-coated TiO2NPs were then mixed drop-wise with 10 mL of 10 mg mL−1 PAH (M.W. 15 kDa) dissolved in 1 mM NaCl solution, shaken for 20 min and centrifuged again three times at 8000 rpm for 1 h to yield TiO2NPs/PSS/PAH. For further coating with PSS, the latter was mixed drop-wise with 10 mL of 10 mg mL−1 PSS whilst being sonicated. The mixture was shaken for 20 min, centrifuged and dispersed in Milli-Q water to produce TiO2NP/PSS/PAH/PSS. Furthermore, PSS and PAH of various molar masses (10 kDa and 70 kDa for PSS and 15 kDa and 56 kDa for PAH) were used to examine their effect on the size of the coated TiO2NPs. After each polyelectrolyte coating, the TiO2NPs were characterized by the Zetasizer Nano ZL to check their zeta potential and the particle aggregation.
2.3 Embryo exposure to TiO2NPs
Healthy D. rerio embryos (n = 10) at 0–72 hpf, with the chorion intact, were selected and exposed to a treatment dose of test media (bare TiO2NPs, TiO2NPs/PSS, or TiO2NPs/PSS/PAH) and incubated for 3 h in either dark conditions or illuminated with visible light, at particle concentrations of 0, 500 or 1000 mg/L based on published LC50 values to increase the likelihood of observable effects [25, 26] rather than environmentally-relevant levels. Healthy D. rerio embryos (n = 10) were used as a control group in parallel.
2.4 Embryo viability following exposure to TiO2NPs
Embryos (at 48–72 hpf, n = 5) from each exposure regime (control, bare TiO2NPs, TiO2NPs/PSS, and TiO2NPs/PSS/PAH) were isolated after the exposure and washed with commercially supplied (nuclease free) molecular-biology grade water (Fisher Scientific, U.K.) three times, and re-dispersed with 1 mL molecular grade water and incubated with a drop of 98% fluorescein diacetate (FDA) (Honeywell Fluka, U.K.) in acetone (0.5 mg/L) for 15 min. This assay is based on accumulation of the fluorescent by-product (fluorescein) inside the viable embryos as a result of the hydrolysis of the diffused FDA by intracellular enzymes (esterases). The embryos were then washed again with deionised water and the cell viability examined using an Olympus BX51 fluorescence microscope attached to a DP70 digital camera and FITC fluorescence filter set. Living cells were identified as having taken up FDA and fluorescent green [27].
2.5 TEM imaging of embryos after exposure to TiO2NPs
The morphology of D. rerio embryos (n = 5) after a 3 h incubation with 0, 500 or 1000 mg/L of bare TiO2NPs TiO2NPs/PSS, or TiO2NPs/PSS/PAH was examined with TEM using the following protocol. The embryos were washed with deionised water and fixed in 2.5% glutaraldehyde (0.5 ml 25% glutaraldehyde stock solution, 4.5 ml 0.1 M cacodylate buffer and glucose (20 mL 0.2 M cacodylate stock, 10 mL Milli-Q water, 0.216 g glucose, pH 7.3, and final volume made up to 40 mL) for 1 h at room temperature. Next, cacodylate buffer was removed and embryos were fixed by 1% osmium tetra-oxide in cacodylate buffer (2.5 mL 2% Osmium tetroxide, 2.5 mL 0.1 M cacodylate buffer and glucose 0.03 M) at 4 °C overnight. After the cacodylate buffer was removed, embryos were stained for 30 min with 1% uranyl acetate (2 ml 2.5% uranyl acetate stock, final volume 3 mL) and washed with solutions of ethanol of increasing concentration (30%, 50%, and 70% overnight). The embryos were washed again the next day with ethanol solutions of 90% and 100%. After standard dehydration, the embryos were embedded in fresh epoxy/araldite at 60 °C for 48 h. The embedded embryo samples were removed from the oven and allowed to stand at room temperature for 48 h, then sectioned using an ultramicrotome. The Oxford Instruments INCA Energy Dispersive Spectroscopy (EDS) was attached to the TEM and run at 120 kV to identify and semi-quantitatively characterize the TiO2NPs on the surface, or within, of the D. rerio embryo samples. The sectioned samples were imaged using a JEOL 2010 TEM (Japan) operating at 80 kV and images were captured (from one randomly selected embryo per treatment group) with a Gatan Ultrascan 4000 digital camera (Gatan, Pleasanton, U.S.A.) and the corresponding software for imaging was the Digital Micrograph.
2.6 Target gene isolation and characterization
Total RNA was extracted from pooled samples of embryos (0–72 hpf with chorion intact, n = 10) from each treatment group, using the manufacturer’s protocol (Roche Diagnostics Ltd., Burgess Hill, U.K.). The embryo pooled sample exposures consisted of bare TiO2NPs, TiO2NPs/PSS, or TiO2NPs/PSS/PAH at 500 or 1000 mg/L particle concentration for 3 h exposure duration, in dark or illuminated with visible light as well as the corresponding control treatment group (n = 10). To assess the integrity of total RNA, samples were analysed on a denaturing agarose gel stained with ethidium bromide (Life Technologies, Paisley, U.K.). 100 ng of pooled RNA was used to generate cDNA using SuperScript VILO cDNA Synthesis reagents and protocol (Life Technologies, Paisley, U.K.) with 14 μL (~ 100 ng) of total RNA. In a 0.2 mL tube, the following reagents were added: 4 μL of 5x VILO Reaction Mix (includes random primers, MgCl2, and dNTPs in a buffer formulation) 2 μL of 10x Superscript enzyme mix. Each reaction was incubated at 25 °C for 10 min, and then 60 min at 42 °C followed by 5 min at 85 °C and a holding step at 4 °C. To degrade any remaining RNA, the following reagents were added: 0.5 μL (5 units) of RNase H (supplied in 100 mM KCl, 20 mM Tris–HCl (pH 7.5), 10 mM MgCl2, 0.1 mM EDTA, 0.1 mM dithiothreitol and 50% glycerol) and 2 μL of 10x RNase H Reaction Buffer (includes 75 mM KCl, 50 mM Tris–HCl, 3 mM MgCl2, 10 mM MgCl2 in pH 8.3 at 25 °C). All reagents were mixed, incubated at 37 °C for 45 min and then stored at − 20 °C.
For the generation of SOD2, HIF1, and Pxmp2 PCR products, 1 μL of cDNA was combined with 0.5 μl of 10 mM dNTPs, 5 μL amplification buffer, 0.5 μL of 0.5–4.5 mM MgCl2, 0.5 μL of 1.5 μM for each sense and antisense primers (Table S-1) and 0.25 μL (1.25 units) of Herculase II fusion DNA polymerase (Agilent Technologies, Wokingham, U.K.) for a total reaction volume of 25 μL. Elongation factor 1 (EF), 18S rRNA (18S) and β tubulin were evaluated as potential reference genes. Amplifications were carried out using the TC-4000 Thermal Cycler (Techne, Staffordshire, U.K.) equipped with a heated lid. All reactions were initially denatured at 94 °C for 30 s then cycled 35 times with 30 s at 94 °C denaturation, 30 s at 50/55/60 °C annealing and 30 s at 72 °C for the elongation step. A final extension step of 2 min at 72 °C was conducted. The PCR fragments were sequenced commercially by Macrogen (Amsterdam, Netherlands). Identities of PCR fragments were verified using a blastn search on the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and aligned using a multiple sequence alignment program, Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) to determine the correct isoform.
2.7 Quantitative qPCR analysis of mRNA expression
The qPCRs analyses for each pool of embryo cDNAs from each treatment group (n = 10) were carried out using 20 μL reaction volumes consisting of 10 μL of SYBR Green Master Mix (Roche, U.K.), 7 µL of sterilised water, 1 µL of the cDNA template, and 2 µL of optimised primer concentration (EF, HIF1: 200 nM; 18S, Pxmp2: 300 nM, SOD2: 400 nM). Two reference genes (EF and 18S) were determined as the most stable across treatment groups using geNorm software. Amplifications were carried out using a CFX96 Real-time PCR system, C1000 Thermal Cycler (Bio-Rad, Hemel Hempstead, U.K.), in triplicate and with negative controls. Reactions were started with denaturation at 50 °C for 2 min, 95 °C for 10 min, followed by a three-step protocol of 40 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 1 min, then 72 °C for 1 min. At the end, a melting/dissociation curve was conducted. A relative quantification method was used to determine changes in mRNA transcript levels of the targeted genes in the treatment group compared to untreated control samples using the geometric mean of the reference genes for normalization and the ΔΔCt method [28].
2.8 Statistical analysis
Each target gene was tested individually for significant differences among the controls and each treatment group. All data were tested for homogeneity of variances using Levene’s test in SPSS. A non-parametric test (Scheirer-Ray-Hare) was used to assess the effect of anatase TiO2NPs coating type (factor 1), TiO2NP concentration (factor 2) and the exposure condition (factor 3) and to determine the interactions among them. Significance for relative gene expression, between TiO2NP of different coatings, concentrations, or conditions was also tested individually using the Kruskal–Wallis non-parametric test. Differences were considered significant at P < 0.05.
3 Results
3.1 Characterization of the TiO2NPs size distribution and zeta potential
Aqueous dispersions of the titania samples were synthesized at different annealing temperatures and prepared by sonication as described by Al-Awady et al. [14]. Figure 1a illustrates schematically the process of coating of the bare TiO2NPs with consecutive layers of PSS and PAH respectively. Figure 1b shows a typical TEM image of the bare TiO2NPs used in these experiments. The titania produced was characterized as clusters of smaller crystallites of 5 nm domain size in solid state (Fig. 1b). Upon dispersing in Milli-Q water at pH 5.5, TiO2NPs of an average diameter 25 nm (Fig. 1c) were produced. The zeta potential of the anatase TiO2NPs in an aqueous solution decreased gradually from positive at low pH to negative at high pH with an isoelectric point at approximately 6.8 (see Fig. 1d).
3.2 TiO2NP uptake and impact on D. rerio embryo viability
Embryos displayed no significant impact on viability (Fig. 2) when exposed to all the TiO2NPs types (bare TiO2NPs, TiO2NPs/PSS, or TiO2NPs/PSS/PAH) at each exposure level (0, 500 and 1000 mg/L), in both dark and visible light conditions. D. rerio embryos from each of the treatment group were examined using EDS attached to TEM (Fig. 3, Table 1). EDS spectra confirmed the presence of TiO2NPs on the outer surface (chorion) of D. rerio embryo incubated with 500 mg/L of TiO2NPs/PSS under both dark and visible light conditions (Fig. 3b, c). In the samples examined using TEM, no TiO2NPs were detected inside of the embryos for any treatment or on the embryo outer surface (chorion) in the control group (Fig. 3a) or after incubation with 500 mg/L of TiO2NPs/PSS/PAH under both dark and visible light conditions (Fig. 3d, e). TiO2NPs were however detected on the outer surface (chorion) of embryos incubated with 1000 mg/L TiO2NPs/PSS/PAH incubated under dark conditions (Fig. 3f). No data was available (due to human error) for embryos incubated with 1000 mg/L TiO2NPs/PSS/PAH incubated under light conditions.
FDA live/dead assay applied to D. rerio embryos (at 48–72 hpf, n = 5) exposed to different types of TiO2NPs samples at total concentrations of 1000 mg/L for each media and under visible light conditions: a Control, b bare TiO2NPs, c TiO2NPs/PSS, d TiO2NPs/PSS/PAH. Scale bars are 200 μm (a–d). The fluorescence signal indicates that the embryos are still viable after exposure
TEM images of D. rerio embryo outer surface (chorion) in a control treatment, b after incubation for 3 h with 500 mg/L of TiO2NPs/PSS in dark conditions, c after incubation for 3 h with 500 mg/L of TiO2NPs/PSS in visible light, d after incubation for 3 h with 500 mg/L of TiO2NPs/PSS/PAH in dark, e after incubation for 3 h with 500 mg/L of TiO2NPs/PSS/PAH under visible light, f after incubation for 3 h with 1000 mg/L of TiO2NPs/PSS/PAH in dark conditions. The circles represent the areas used for the EDS spectrum analysis
3.3 qPCR analysis of target gene expression in D. rerio embryos following exposure to TiO2NPs
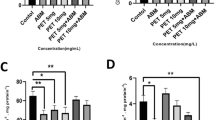
The expression levels of SOD2, HIF1, and Pxmp2 mRNA were analysed in control embryos and embryos pooled from each treatment group (n = 10) of TiO2NPs with concentrations of 0, 500 and 1000 mg/L in dark and visible light conditions using the optimised qPCR method. Firstly, an overall statistical analysis using the Scheirer-Ray-Hare test (Table 2) showed that for the nanoparticles the TiO2NP type, concentration, condition, the interaction between the types and concentration; and the interaction between the concentration and condition all significantly affected the relative gene expression levels of SOD2 mRNA (Table 2). The expression level of HIF1 was only affected by the condition (light or dark) used in the experiment (Table 2). Pxmp2 mRNA expression level was not significantly impacted by any of the types of TiO2NPs, concentration, nor the exposure regime (Table 2).
A Kruskal–Wallis test highlighted further significance within the dataset as follows. Significant difference in SOD2 expression level was detected as a result of TiO2NP type, concentration, and condition (Fig. 4). HIF1 expression was significantly affected by condition (Fig. 5). Pxmp2 expression was not affected by any condition (Fig. 6). Separate Kruskal–Wallis tests (Table 2) for one-way analysis of the individual data between TiO2NPs types, concentration, and condition, revealed that SOD2 mRNA expression was significantly affected by both types and condition. HIF1 mRNA didn’t show any significant differences in expression by any factors, and Pxmp2 mRNA expression shows significant difference only by condition as a result of light/dark effect.
4 Discussion
From this study, using two relatively high exposure levels of uncoated and coated titania NPs (500 and 1000 mg/L), it is evident that overall embryo viability is not affected (Fig. 2), even though titania can be detected on the surface (chorion) of embryos (Fig. 3b, c, f), and significant changes in oxidative stress gene expression, particularly SOD2 was detected in selected exposure treatment groups (Fig. 4). On the other hand, the markers adopted for hypoxia (HIF1, Fig. 5) and membrane function (Pxmp2, Fig. 6), suggest that such high exposure levels of these NPs are not having a significant impact on these specific endpoints under this exposure regime.
In terms of NPs availability and uptake, the anatase NPs employed in this study had an average diameter of 25 nm with a range of different coatings. Their impact on viability and their apparent absence within the embryos analysed, would suggest that they are not crossing the embryo and/or causing mortality under the exposure conditions used. This is consistent with studies using eggs and adult zebrafish that reported an LC50 value of > 1600 mg/L for uncoated (< 100 nm sized) TiO2NPs after a 48 h exposure period [29]. Studies using significantly longer exposure times (of 23 days) have shown decreased survival of D. rerio embryos exposed to TiO2NPs at lower concentrations from 10 μg/L to 10 mg/L [30]. Adding UV light has also been shown to increase mortality, in larvae rather than embryos, at concentrations of 1-100 mg/L [31, 32]. The D. rerio embryo is ~ 1.5 mm thick [33], is surrounded by a protective chorion, which, at 72 hpf, is considered open to the passage of materials through pores or via passive transport [34], yet these results suggest that no nanoparticle transfer across the chorion has occurred.
Two coatings were compared with each other in terms of relative uptake and toxicity. The TiO2NPs/PSS/PAH used are cationic and UV-photoactive, similarly to the uncoated TiO2NPs, i.e. their positive charge is anticipated to promote adhesion on the embryo surface. However these were only identified on the surface of the embryos exposed to the higher 1000 mg/L level (Table 1), with possible disruption of the negatively charged cell membrane [14]. Previous work using microalgae, Chlamydomonas reinhardtii, and yeast, Saccharomyces cerevisiae, has also confirmed the formation of a significant build-up of NPs on the cell surface for bare and cationic polyelectrolyte-coated TiO2NPs at pH 5.5 [14]. In contrast, titania was also detected on the outer surface of microtome-sectioned D. rerio embryos exposed to 500 mg/L anionic-coated TiO2NPs/PSS in both dark and visible light conditions (Table 1). Regarding possible mechanisms to understand how such particle build up may occur, the negative charge of the cell membrane has been suggested to facilitate internalization, and affect the toxicity of positively charged coated NPs in other studies, such as gold NPs (AuNPs), which are more toxic than negatively and/or neutrally charged AuNPs [35, 36]. In terms of additional coatings and nanohybrids, the toxicity of TiO2NP and TiO2-MWCNT nanohybrid has also been assessed with and without UV light exposure using zebrafish embryos, and neither presented acute toxicity [37]. The acute effects of TiO2NPs in zebrafish embryos thus depend on both the type of formulation and the illumination condition.
Three biological effects markers of sub-lethal impacts were examined. The expression of SOD2 mRNA in pooled zebrafish embryos (n = 10) was affected by the type of TiO2NPs, concentration, and condition (Table 2, Fig. 5) specifically indicating an oxidative stress response. This finding is consistent with those reported by Bar-Ilan et al. [30] whereby exposure to 10 μg/L–10 mg/L TiO2NPs, illuminated with a lamp, produced toxicity through cumulative reactive oxygen species. The expression levels of the hypoxia and membrane markers, HIF1 and Pxmp2, were not significantly impacted by any factor using the same exposure conditions. The SOD enzyme catalyses the conversion of the reactive superoxide ion (O2−) to yield hydrogen peroxide (H2O2) and oxygen molecule during oxidative oxygen processes [38]. Other markers of oxidative stress, increased catalase and glutathione S-transferase expression levels have also been reported in zebrafish embryos, exposed to TiO2NPs for 96 h, under either visible light or a combination of visible and ultraviolet (UV) light [32]. Felix et al. [39] examined sub-lethal biological effects impacts of 0.1, 1 or 10 mg/L of uncoated TiO2NPs, poly(acrylic acid)-coated TiO2NPs, and the polymer coating alone, in the presence or absence of UV light, reporting that uncoated TiO2NPs produced hydroxyl radicals, delayed hatching, induced lipid peroxidation, increased catalase activity and total glutathione levels, and up-regulated glutathione peroxidase 1a gene expression in the presence of UV light, while polymer-coated TiO2NP increased thiobarbituric acid reactive substances production and total glutathione levels under simulated sunlight illumination. Further experiments are needed, with an increased number of embryos in each treatment group, and a shorter defined embryo stage (from 0 to 72 hpf range), in order to reduce the variation of gene expression within treatment groups observed.
5 Conclusions
In summary, TiO2NP size, surface charge, concentration and the presence/absence of light have been shown to determine their potential toxicity measured in this study as specific gene expressions. Polyelectrolytes coatings were used in formulations to enhance dispersion stability [40]. The polyelectrolyte multilayer films (PAH/PSS) provide a stable nanocomposite thin film that interacts with the NPs [41]. The nanotoxicty of polyelectrolyte-coated TiO2NPs have been previously been studied in yeast and microalgae and the results showed that the toxicity of the coated TiO2NPs changes with their surface charge where cationic polyelectrolyte coating were more toxic than the anionic polyelectrolyte coating [14]. Here, we compare the toxicity of different TiO2NPs coatings on D. rerio embryos and find that D. rerio embryos remain viable after exposure to 500 and 1000 mg/L of TiO2NPs coated with anionic and cationic polyelectrolytes for 3 h. Also, embryos exposed to TiO2NPs coated with cationic polyelectrolytes showed no Ti on the embryo using EDS while the higher dose of 1000 mg/L of the same coating start to show NPs residues. Importantly, the biological sub-lethal effects marker, SOD2 expression, showed significant changes related to all factors, indicative of oxidative stress. Similarly, HIF1 expression showed a significant difference in response to condition. This study focused on a relatively short-term exposure with concentrations that are not environmentally relevant. It would be interesting to understand how NPs at environmentally realistic exposure levels affect D. rerio over longer exposure periods, at defined embryo stages, and also several generations.
References
Rollerova E, Tulinska J, Liskova A, Kuricova M, Kovriznych J, Mlynarcikova A, Kiss A, Scsukova S (2015) Titanium dioxide nanoparticles: some aspects of toxicity/focus on the development. Endocrine Regulations 49:97–112
Gelis C, Girard S, Mavon A, Delverdier M, Paillous N, Vicendo P (2003) Assessment of the skin photoprotective capacities of an organo-mineral broad-spectrum sunblock on two ex vivo skin models. Photodermatol Photoimmunol Photomed 19:242–253
Tsuji JS, Maynard AD, Howard PC, James JT, Lam C, Warheit DB, Santamaria AB (2006) Reasearch strategies for safety evaluation of nanomaterials, part IV: risk assessment of nanoparticles. Toxicol Sci 89:42–50
Kaida T, Kobayashi K, Adachi M, Suzuki F (2004) Optical characteristics of titanium oxide interference film and the film laminated with oxides and their application for cosmetics. J Cosmet Sci 55:219–220
Wiesenthal A, Hunter L, Wang S, Wickliffe J, Wilkerson M (2011) Nanoparticles: small and mighty. Int J Dermatol 50:247–254
Chen J, Poon C-S (2009) Photocatalytic construction and building materials: from fundamentals to applications. Build Environ 44:1899–1906
Koivisto AJ, Lyyranen J, Auvinen A, Vanhala E, Hameri K, Tuomi T, Jokiniemi J (2012) Industrial worker exposure to airborne particles during the packing of pigment and nanoscale titanium dioxide. Inhal Toxicol 24:839–849
Ackroyd R, Kelty C, Brown N, Reed M (2001) The history of photodetection and photodynamic therapy. Photochem Photobiol 74:656–669
Cho M, Chung H, Choi W, Yoon J (2004) Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Resour 38:1069–1077
Esterkin CR, Negro AC, Alfano OM, Cassano AE (2005) Air pollution remediation in a fixed bed photocatalytic reactor coated with TiO2. AIChE J 51:2298–2310
Choi H, Stathatos E, Dionysiou DD (2006) So-lgel preparation of mesoporous photocatalytic TiO2 films and TiO2/Al2O3 composite membranes for environmental applications. Appl Catal B 63:60–67
Uchino T, Tokunaga H, Ando M, Utsumi H (2002) Quantitative determination of OH radical generation and its cytotoxicity induced by TiO(2)-UVA treatment. Toxicol In Vitro 16:629–635
Therapeutics Good Administration (TGA) (2013) Literature review on the safety of titanium dioxide and zinc oxide nanoparticles in sunscreens. TGA, Symonston, ACT
Al-Awady MJ, Greenway GM, Paunov VN (2015) Nanotoxicity of polyelectrolytefunctionalized titania nanoparticles towards microalgae and yeast: role of the particle concentration, size and surface charge. RSC Adv 5:37044–37059
Halbus AF, Horozov TS, Paunov VN (2019) Self-grafting copper oxide nanoparticles show a strong enhancement of their anti-algal and anti-yeast action. Nanoscale Adv 1:2323–2336
Barker PJ, Branch A (2008) The interaction of modern sunscreen formulations with surface coatings. Prog Org Coat 62:313–320
Dodd NJF, Jha AN (2011) Photoexcitation of aqueous suspensions of titanium dioxide nanoparticles: an electron spin resonance spin trapping study of potentially oxidative reactions. Photochem Photobiol 87:632–640
Xiong D, Fang T, Yu L, Sima X, Zhu W (2011) Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ 409:1444–1452
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430
Petersen EJ, Reipa V, Watson SS, Stanley DL, Rabb SA, Nelson BC (2014) DNA damaging potential of photoactivated P25 titanium dioxide nanoparticles. Chem Res Toxicol 27:1877–1884
Wu J, Liu W, Xue C, Zhou S, Lan F, Bi L, Xu H, Yang X, Zeng F-D (2009) Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol Lett 191:1–8
Okuda-Shimazaki J, Takaku S, Kanehira K, Sonezaki S, Taniguchi A (2010) Effects of titanium dioxide nanoparticle aggregate size on gene expression. Int J Mol Sci 11:2383–2392
Mills A, Le Hunte S (1997) An overview of semiconductor photocatalysis. J Photochem Photobiol, A 108:1–35
Carlotti ME, Ugazio E, Sapino S, Fenoglio I, Greco G, Fubini B (2009) Role of particle coating in controlling skin damage photoinduced by titania nanoparticles. Free Radical Res 43:312–322
Ma H, Brennan A, Diamond SA (2012) Photoxicity of TiO2 nanoparticles under solar radiation to two aquatic species: Daphnia magna and Japanese medaka. Environ Toxicol Chem 31:1621–1629
Adams LK, Lyon DY, Alvarez PJJ (2006) Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res 40:3527–3532
Oparka KJ, Read ND (1994) The use of fluorescent probes for studies on living plant cells. In: Harris N, Oparka KJ (eds) Plant cell biology’ A Practical Approach, pp 27–50
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 25:402–408
Kovriznych JA, Sotnikova R, Zeljenkova D, Rollerrova E, Szabova E, Wimmerova S (2013) Acute toxicity of 31 different nanoparticles to zebrafish (Danio rerio) tested in adulthood and in early life stages—comparative study. Interdiscip Toxicol 6:67–73
Bar-Ilan O, Chuang CC, Schwahn DJ, Yang S, Joshi S, Pedersen JA, Hamers RJ, Peterson RE, Heideman W (2013) TiO2 Nanoparticle exposure and illumination during zebrafish development: mortality at parts per billion concentrations. Environ Sci Technol 47:4726–4733
Ma H, Diamond SA (2013) Phototoxicity of TiO2 nanoparticles to zebrafish (Danio rerio) is dependent on life stage. Environ Toxicol Chem 32:2139–2143. https://doi.org/10.1002/etc.2298
Clemente Z, Castro VLSS, Moura MAM, Jonsson CM, Fraceto LF (2014) Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions. Aquat Toxicol 147:129–139
Sun CK, Chu SW, Chen SY, Tsai TH, Liu TM, Lin CY, Tsai HJ (2004) Higher harmonic generation microscopy for developmental biology. J Struct Biol 147:19–30
Cunningham S, Brennan-Fournet ME, Ledwith D, Byrnes L, Joshi L (2013) Effect of nanoparticle stabilization and physicochemical properties on exposure outcome: acute toxicity of silver nanoparticle preparations in zebrafish (Danio rerio). Environ Sci Technol 47:3883–3892
Goodman CM, McCusker CD, Yilmaz T, Rotello VM (2004) Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem 15:897–900
Lin J, Zhang H, Chen Z, Zheng Y (2010) Penetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano 4:5421–5429
Silva GHD, Clemente Z, Khan LU, Coa F, Neto LLR, Carvalho HWPVL, Martinez DST, Monteiro RTR (2018) Toxicity assessment of TiO2-MWCNT nanohybrid material with enhanced photocatalytic activity on Danio rerio (Zebrafish) embryos. Ecotoxicol Environ Saf 165:136–143. https://doi.org/10.1016/j.ecoenv.2018.08.093
Velkova-Jordanoska L, Kostoski G, Jordanoska B (2008) Antioxidative enzymes in fish as biochemical indicators of aquatic pollution. Bulgarian J Agric Sci 14:235–237
Felix LC, Folkerts EJ, Yuhe He Y, Goss GG (2017) Poly(acrylic acid)-coated titanium dioxide nanoparticle and ultraviolet light co-exposure has minimal effect on developing zebrafish (Danio rerio). Environ Sci Nano 4:658–669. https://doi.org/10.1039/c6en00436a
Batley GE, Kirby JK, Mclaughlin MJ (2013) Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc Chem Res 46:854–862
Cho J, Caruso F (2005) Investigation of the interactions between ligand-stabilized gold nanoparticles and polyelectrolyte multilayer films. Chem Mater 17:4547–4553
Acknowledgements
This work was financially supported by the University of Jordan in funding to Zeinab Arabeyyat.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report that there are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arabeyyat, Z.H., Al-Awady, M.J., Greenway, G.M. et al. Toxicity of polyelectrolyte-functionalized titania nanoparticles in zebrafish (Danio rerio) embryos. SN Appl. Sci. 2, 1312 (2020). https://doi.org/10.1007/s42452-020-3137-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-3137-x