Abstract
The fluoride pollution in the groundwater is a significant human health risk around the world. However, many diseases including dental, crippling and skeletal fluorosis cause due to high fluoride contamination in the drinking water. The objectives of this study were to investigate the occurrence of higher F− in the groundwater, geochemical composition, water type mechanism for minerals enrichment and groundwater quality for drinking and irrigation purposes. Therefore, fifty-seven groundwater samples were collected for the appraisal. The mean concentrations of physical parameters like depth, Temperature, electrical conductivity, total dissolved solid, power of hydrogen ion (pH) and turbidity were observed as 28 m, 19.78 °C, 2283 uS/cm, 1456 mg/L, 7.4 and 21.80 NTU respectively. Whereas, the mean concentrations of chemical parameters such as cations (Ca+2, Mg+2, Na+ and K+) were observed to be (92.12, 83.63, 251 and 12.40) mg/L and anions (Cl−, SO42−, HCO3−, NO3− and F−) were (375, 300, 285, 4.9 and 1.9) mg/L respectively. While the mean concentration of total Fe in the drinking water samples of study are were reported as 0.28 mg/L respectively. The range concentration of F− were 0.23–6.8 mg/L shows that 47% of drinking water samples exceeded the WHO limit (1.5 mg/L). The piper plot resulted in the dominance of (NaCl), and Gibbs plot represents rock and precipitation dominance ratios; while SAR and Sodium percentage (Na %) validates the salinity and sodium risk to the use of groundwater for irrigation purposes. Saturation indices expressed; the saturation of the fluorite mineral found within the range of (− 2.3 to 1.24). PCA results reveals four significant factors with total percentage contribution were 70.73%. Whereas, the water quality index resulted with mean and standard deviation as (2502.62 ± 2164). The overall quality of the groundwater is unfit for human consumption and irrigation purposes to the local population of the study area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Groundwater quality is affected by different substances which include different ions and trace elements [1,2,3]. Also some processes such as geochemical compositions, and bedrock geology of groundwater aquifer deteriorate the properties of water [3, 4]. About one third of the world’s community uses water for domestic, agriculture and industrial purposes. Thus with the appliance of groundwater for different purposes results in deterioration of groundwater both in terms of quality and quality. Therefore, groundwater contamination is obvious due to aforementioned processes and developmental sectors. The most significant among the anions that has the potential to cause global health concern is the fluoride (F−). Higher F− in groundwater causes significant environmental problem. It not only leads the deterioration of groundwater quality, but also agriculture soils in cases where F− concentrations exceed the self-purgation capacity of the soil [5]. It has shown that low calcium and higher alkalinity favor F− enrichment [6,7,8]. Katsanou et al. Reported that the concentration of F− in freshwater is always less than (1.0 mg/L) compared to seawater, which contains 1.3 mg/L [9]. The maximum allowable limit of F− concentration in potable water set by the WHO (2011) is 1.5 mg/L [10]. Higher levels of F− in drinking water cause dental fluorosis, and concentration higher than (4 mg/L) can lead to skeletal and crippling fluorosis [7, 8, 11]. Moreover, regular exposure of F− contamination may have an adverse effect on the reproductive, neurological, musculoskeletal, developmental, endocrine, and renal systems, in some cases leading to genotoxicity [8, 12].
A higher F− concentrations in groundwater have become a global concern [13]. Over 200 million people are suffering from fluorosis notably in South Korea, China, Pakistan, Bangladesh, India, Morocco, Algeria, Argentina [7, 14,15,16,17,18,19,20,21]. F− contaminated water accounts for about 65% of all waterborne diseases [22]. F− contamination may be of anthropogenic or geogenic origin [23, 24]. Precipitation of F− in groundwater mainly caused by the natural weathering of F− containing minerals and anthropogenic activities [25]. Significant anthropogenic point-sources include industries such as brick manufacturing, iron forging, electroplating, ceramic and glass production, beryllium extraction, aluminum smelting, and semiconductor manufacturing [26].
F− is an electronegative and chemically reactive element which belong to halogen group [27]. F− ion is a robust aqueous ligand, which reacts with cations Mg2+, Ca2+, Al3+, and Fe3+ to form a series of soluble complexes depending on pH and strong complexes formed by the F− with silica, boron, uranium, vanadium, beryllium [28]. The level of F− in groundwater depends on factors governing the dissolution of F− minerals and the formation of complexes, e.g. pH, availability of suitable complexing cations, the porosity of rocks and soil. F− in groundwater is derived mainly by the dissolution of the minerals fluorspar (CaF2), fluorapatite (3Ca3 (PO4)2 Ca (FCl)2).
In the above context we study F− concentrations in the groundwater of Tando Bago sub–district of Badin District, Sindh Province, Pakistan. The local residents of the area are using the groundwater for domestic and irrigation practices; however, the groundwater was found with elevated F− contamination. This study aimed at (1) to identify the physical and chemical characteristics of the groundwater; (2) The special distribution pattern and risk level of fluoride to the local population of the study area; (3) The association of fluoride with other groundwater parameter and contaminants source identification; (4) Evaluation of the geochemical triggers of fluoride enrichment in the aquifers system; and (5) And to examine the groundwater suitability for human consumption and irrigation purposes.
2 Study area
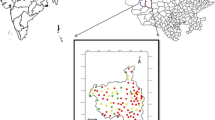
The Tando Bago sub–district located at latitude 24.7887°N, longitude 68.9655°E, in Badin District, Southern Sindh Province, in the southern Indus Plain of Pakistan (Fig. 1). This area is a prominent agrarian zone in which sunflower, rice, sugarcane, and cotton are the principal crops. According to district census report of 2017, the population of sub-district was 398,585 [29]. Since 1986, Badin district has been considered as an area of higher potential for oil and gas exploration [30]. The area has a desert climate, with an average annual rainfall of 125 mm and an average temperature of 27 °C. From March to October, temperatures are moderated by landward winds from the Arabian Sea [31].
The bedrock beneath the Sindh Plain is of Tertiary and Cretaceous ages. Rock types include marl, chalky limestone, limestone, dolomite, and limestone. Three main physiographic divisions observed in the Sindh Plain. One is Khirthar mountain range in the east and west, chain of low-level peaks, under cover of Thar Desert Sindh. And other two ranges as Laki mountain range and Suleiman mountain range, covering by Arabian Sea in the southern part. The sub-surface rocks belong to the Pre-Cretaceous and Cretaceous periods with a thickness of about 20,000 ft, while non-clastic and clastic sedimentary rocks belong to partly marine and fluvial deposited environments. In the Lower Indus region such as southern Sindh, the aquifer is a plenteous resource of freshwater within a depth of 50 m, and below this depth, the water is saline. However, the primary groundwater recharge source is from the Indus River irrigation system, that originates from the Himalayas [32]. In 1980 an extensive drainage system known as the left bank outfall drain (LBOD) was constructed which directly drains into the Arabian Sea. Currently, this system highly contaminated due to municipal, industrial, and agricultural runoff effluent [30].
3 Methodology
3.1 Sampling strategy and collection
A systematic water sampling approach was used to collect the representative water samples around the 20 square km radius of Tando Bago. The four villages, namely: Malook Unar (M.U) Village in the central region, Bahadur Khan Kaloi (B.K.K) Village in the north-east, Ghulam Muhammad Memon (G.M.M) Village in the east, and Amb Khan Khoso (A.K.K) Village in the south-east were selected as sampling sites. In June 2018, fifty-seven drinking water samples were collected from two different sources like dug wells, and boreholes (Fig. 1). Water depths range from 15 to 40 m; pre-sterilized 200 mL bottles rinsed with deionized water and dried were used to store the groundwater samples. The samples were preserved with sufficient high grade pure HNO3 to maintain a pH < 2, and for the anion’s analysis unacidified samples were used. Water samples were properly managed with sampling label, code, sampling date, and GPS position and immediately transported to the Pakistan Council of Research in Water Resources (PCRWR) laboratory in Badin district for chemical analysis.
3.2 Sample analysis
Field parameters were measured as follows: pH an Info Lab pH 720 WTW Series meter, and electric conductivity (EC), total dissolved solids and temperature using an EC/TDS/Temp/RS232C/ Meter CON 110 m. Whereas, Physicochemical analyses were performed in the PCRWR laboratory Badin district, Sindh, as following American Public Health Association (APHA) methods: F− ion was measured using a Fluoride Ion Meter ExStik FL700. Titration methods were used for Cl− and HCO3−, while turbidity was analyzed using a turbidity meter, whereas SO42− determined by turbidimetric method and an ultraviolet spectroscopic screening method was used for the NO3−. Whereas, cations like Mg2+, Ca2+, K+, Na+ and Fe were determined by using atomic absorption spectrophotometer (Varian Spectra AA–240, manufacturing country Australia) in standard suitable conditions.
Water Quality Index (WQI) was calculated to evaluate the suitability of each sample for human consumption [33]. The calculation includes three steps, as follows:
First step—Assigning of weight (Wi) 1–5 to the selected water parameters (TDS, pH, turbidity, Ca2+, Mg2+, Na+, K+, Cl−, SO42−, HCO3− NO3−, F−, Fe,), according to their relative significance in the overall quality of water for the drinking purposes (Table 1).
Second step—Calculation of relative weight (Wi) for each chemical parameter using (Eq. 1), where, (Wi) is the weight of each parameter and 'n' indicates the number of parameters.
The third step—Assigning of a quality rating (qi) for each parameter using (Eq. 2), where Ci represents the concentration of chemical parameters in each water sample in mg/L, and Si is WHO guideline value (Table 1).
The sub-index (SIi) of each parameter is determined by (Eq. 3). Finally, the WQI is calculated as a summation of SIi for all parameters using (Eq. 4).
where SIi is the sub-index of the ith parameter, Wi is the relative weight of ith parameter, qi is the quality rating based on the concentration of ith parameter, and 'n' is the number of chemical parameters.
3.3 Reagent and standards
All the chemical used throughout the study for different parameters analysis were of analytical grade (AR). Different solution standards were prepared for each parameter by following successive dilution from certified standards (1000 mL) of E-Merck Company Germany. Double deionized water was utilized for standard and reagent preparation. All elements were analyzed three time and its mean were used for statistical analysis.
3.4 Quality control and assurance
Groundwater samples were analyzed three time and its average value were included in the statistical results. The ion balance error were measured to represent the analytical findings of groundwater [34]. The quality of the chemical analyses was checked by calculating Charge Balance Error (CBE) in the groundwater samples. Thus, the precision and accuracy of groundwater samples should be ensured after (CBE) calculation. The values of both cations and anions will occurs as positive of negative. Mostly cations acquired positive and anion adopt negative values [35]. The (CBE) values were calculated via (Eq. 1), which were expressed in meq/L. Overall values occurs within ± 5 meq/L.
3.5 Statistical analysis
Groundwater results were tested with different software such as XLSTAT 2014, IBMSPSS Statistic 20, Grapher and ArcGIS version 10.6 were used for descriptive statistic, Pearson correlation, source distribution (PCA), water type formation and study area mapping. Additionally, MS Excel was also used for statistical analysis, and saturation indices (SI) were calculated with PHREEQC Interactive 3.4.0.
4 Results and discussion
4.1 Geochemistry of groundwater
The geochemical results of drinking water were shown in (Table 2). However, the overall groundwater was compared with the guideline set by world health organization (WHO, 2011). Whereas, blow the results with range, mean and standard deviation of physical and chemical parameters. The finding of physical parameter as fellow: Depth (15–40, and 27.61 ± 6.3) m, Temperature, (17–23 and 19.8 ± 1.7) °C, EC (984–4600 and 2283 ± 751) uS/cm, TDS (630–2944 and 1456 ± 485) mg/L, pH (6.9–8.5 and 7.4 ± 0.4) and Turbidity (0.75–361 and 22 ± 54.3) NTU, respectively. The chemical parameters were revealed including cations and anions. The cations were resulted as Ca2+ (56–188 and 92.1 ± 34.7) mg/L, Mg2+ (29–230 and 83.6 ± 36) mg/L, Na+ (52–453 and 251 ± 77.3) mg/L, K+ (6–30 and 12.4 ± 7.8) mg/L, respectively. However, the anions were signified as Cl− (136–797 and 375 ± 174) mg/L, SO42− (86–790 and 300 ± 136) mg/L, HCO3− (200–380 and 285.4 ± 38.4) mg/L, NO3− (0.92–13.5 and 4.9 ± 3.1) mg/L, F– (0.23–6.8 and 1.9 ± 1.5) mg/L and Fe (0–2.1 and 0.28 ± 0.45) mg/L respectively. The charge balance error for cation and anions were calculated to be within ± 5 meq/L.
The drinking groundwater of the area were slightly alkaline in nature [36]. The pH variation in groundwater mostly causes modification in geochemistry of groundwater. Most of the groundwater samples found with satisfactory results, however, the pH values were found within the WHO limits. The spatial variability of electrical conductivity (EC) and Total dissolved solid (TDS) values suggests that the groundwater geochemical profile was not homogenous which were controlled by various processes and mechanism. The dominant anions in the study area were reported as Cl ion. Whereas, SO42− indicate the next coming dominant anion in the study area. While F− is an important element for human health under the permissible limit; however, it becomes a health risk if taken up beyond the allowable concentration [7, 37, 38]. The weathering of granitic gneissic rock contains minerals like mica muscovite, biotite and amphibole which could releases F− in the water aquifer of study area. Whereas, Na+ was the dominant cations in the study area. The halite mineral prospect, dissolution of plagioclase mineral and weathering of silicate minerals are responsible for triggering of Na+ in the surrounding water aquifer [1].
4.2 Correlation matrix of drinking water
Pearson correlation ship in drinking water samples shows the nearest degree which represent linearity among water matrix both dependent and independent parameters [39]. This relationship further indicates that how water samples can be easily arranged in a true liner line. Table 3 represents the findings of Pearson's correlation coefficient values of drinking water variables. The correlation coefficient values mostly support the findings of PCA. Therefore the significant correlation pairs obtained for drinking water at the level 0.05 were observed as: EC and Ca (r = 0.75), EC and Mg (r = 0.76), EC and Cl (r = 0.77), EC and SO4 (r = 0.75), TDS and Ca (r = 0.73), TDS and Mg (r = 0.74), TDS and Cl (r = 0.78), TDS and SO4 (r = 0.76), pH and F (r = 0.50), Ca and Mg (r = 0.75), Ca and Na (r = − 0.78), Ca and Cl (r = 0.60), Ca and SO4 (r = 0.65), Ca and HCO3 (r = −0.51), Mg and Na (r = − 0.68), Mg and Cl (r = 0.58), Mg and SO4 (r = 0.62), Mg and HCO3 (r = − 0.50), Na and K (r = 0.55), Na and Cl (r = 0.81), Na and SO4 (r = 0.65), Na and HCO3 (r = 0.53), Na and NO3 (− 0.51), Na and F (r = 0.57), K and Cl (r = 0.57), Cl and SO4 (0.75) and F and HCO3 (r = 0.54) respectively. F− contains strong electronegativity; therefore, it is highly reactive with Ca2+ and responsible for the unnatural changes in human body parts, like teeth and bones [40]. The relationship (r = − 0.47) between Ca2+ and F− shows a negative association. The behavior of Mg2+ is the same as Ca2+ toward F− [41]. Gizaw, 1996, proposed that higher pH water becomes alkaline and favors the presence of HCO3− to enhance the enrichment and stability of F− ion in the groundwater [42]. It's worth noticing that significant positive higher concentrations for pH, Na+, K+, Cl−, HCO3− and significant negative concentrations for TDS, EC, NO3−, Ca2+ and Mg2+ have been recorded in most of the groundwater samples of study areas.
4.3 The spatial distribution, risk level and factor affecting F– concentration
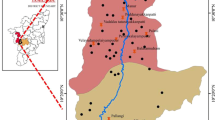
The spatial distribution of F− concentration in the study area is shown in (Fig. 2), whereas (Table 4) demonstrates the associated risk level of F−. Among the four sampling sites, B.K.K Village, located north-east of Tando Bago, showed the highest range of fluoride (0.72–6.8 mg/L). Conversely, M.U Village situated in the central part of Tando Bago exhibited the lowest F− concentration ranging from (0.39–1.73 mg/L). Moreover, G.M.M Village east of Tando Bago displayed F− concentration ranges (1.95–3.95 mg/L). However, A.K.K Village located south-east of Tando Bago presented F− range (0.23–4.23 mg/L).
Spatial distribution mapping showed five different classes, from lower to higher F− concentration in the study area (Fig. 2). Therefore, the results of F risk prevailed in the study were described in (Table 4). It reveals that 30 sample which shows 52.6% contribution were considered safe for drinking purposes regarding their F concentration. While 9 samples contributing 15.8% causes lower F risk and were responsible for dental fluorosis. Whereas, 17 samples show 29.8% contribution indicate high F which causes dental and mild fluorosis in the study area. However, only one sample contributing 1.75% indicate severe skeletal fluorosis in the study area. Overall, results demonstrated that 47% of the water samples showed that F− concentration has exceeded the permissible limit 1.5 mg/L set by (WHO, 2004). Notably, the Northern and Southern parts of Tando Bago area are at higher risk of F− exposure as comparing to the other parts. The presence of the higher F− in the groundwater, maybe because of the geogenic inputs via mineral dissolution and weathering of granitic gneissic rocks and variable soil texture [7, 38, 43]. In contrast, groundwater in the central part of Tando Bago has shown F− level within the permissible limit.
The hydrochemical trend explained that continuous chemical variations in the groundwater might be due to leaching and weathering in the aquifer system. The observed pH range (6.9–8.5) shows that the groundwater is slightly alkaline with higher HCO3− and Na+. This condition causes a chemical interaction of groundwater with minerals in the aquifer, resulting in higher F−. At an alkaline pH, F− might be released by F− present in the mineral matrix which replaces OH− ion in water system as a result of similar ionic radii composition. However, at reducing condition pH decreases and H+ may replace F−. Different processes and mechanism are responsible for fluoride enrichment in water system. The most likely processes for the F− prevalence in the study area is resulted from the following processes. These processes were described as follow.
Evaporation is another process represents the second discharged mechanism for higher F− content in groundwater of district Badin. Thus evaporation and evapotranspiration processes exceeds the level of F− in water system [44]. Evaporation mechanism have the tendency to raise the concentration of F−, Na+ and Cl+. However, other water variables help in conversion of water into vapor. Thus, a significant increase in Ca+2 content of water are caused by evaporation which resulted significant precipitation of calcite and enrichment of F− [45]. Typical arid and semiarid climate condition, the rate of evaporation exceed precipitation ratio in district Badin, Pakistan. Therefore, evaporation and evapotranspiration influences the releasing of F in groundwater system [46].
In meanwhile, ion exchange, processes play important role in the triggering of F− in water sources of the study area. The exchange mechanism involves different reactions within the aquifer matrix. The existence of F− ion in the groundwater aquifers come up from mixing of water rock interaction in the vadose region. Additionally, weathering of rocks and evaporation processes can favour the dissociation of F− in project area. Different groundwater sources contain enriched F− minerals, like fluorite, muscovite and biotite indicate geochemistry of water aquifer. Thus, the continuous water rock interaction formed F− contaminated water. Overall, pH, HCO3−, OH−, Na+, and Ca+2 variables showed a remarkable role in creation of F− contaminated groundwater [1, 7, 47, 48]. The dissolution of mica minerals such as muscovite and biotite encompass F− at OH− positions (see reactions 4, 7 and 8). Both OH− and F− have similar ionic radius, as a result both can exchange their position with each other within mineral matrix. Therefore, F− ion are released into the surrounding water body which causes the deterioration of water aquifer [49, 50]. Mostly, groundwater TDS and Na+ ion represent the geogenic influence [51]. Study conducted by Rafique [41], shows that different rocks which mostly contain granite gneissic, quartz, hematite, quartzite, albite, pegmatites, hornblende, sericite, fluorite, apatite, biotite and muscovite continually interacted within aquifer to form F enriched water. The fluorite minerals interaction with Na2CO3− in water resulted the release of F− and Na+ which were shown in the following reactions.
The higher concentration of Ca+2 precipitated F− ion as CaF2 (see reaction 8):
Reactions 6–9 indicated the mechanism through which Na+ and F− are released and Ca+2 are precipitated in water aquifer of the study area. According to [7], lower Ca+2 and alkaline pH, higher Na+ and HCO3− concentration will favour the triggering of F in water aquifer. The dissolution of fluoride minerals such as fluorite and mica containing muscovite and biotite in water aquifer can be describe below:
As a result of these reaction F− and Na+ are discharge into the water system. Thus F− and Na+ were dissolved and Ca+2 precipated.
The reactions of fluorite minerals with comparatively high HCO3− content were thermodynamically favoured by Eqs. 11, 12, 13 (Sect. 4.4). These findings were supported by the geochemical investigation studies conducted by Chinese, Pakistani and Indian environmental scientist like [7, 52,53,54]. Comparatively higher concentrations of HCO3− and Na+ in water aquifer can easily supported the ionization and mobilization of F− in alkaline medium. The water rock interaction in aquifer favours the discharge of F− [55].
4.4 Hydrochemical facies and Gibbs plotting
Ions plays a unique role in the chemistry and characteristics of groundwater. The groundwater type of the area was designed through Piper plot (Fig. 3). It observed that higher-density of Na+ and K+ is inclined toward the cation side of the triangle and Cl− skewed toward the anion side of the triangle. Moreover, water types, as shown on the central diamond plot (Ca2+–Mg2+–Cl−) type and (Na+–Cl−) type, with the dominance of (Na+–Cl−). This mechanism indicates that chemical characteristics are responsible for the variable nature of groundwater in the study area. Generally, the concentration of higher F− in the groundwater is typically associated with the level of bicarbonate, higher Na+, lower Ca2+ and alkaline pH [7, 14, 37]. At alkaline pH (7.6 to 8.6), the existing condition is favorable for the precipitation of F− containing minerals in the groundwater [56], and a higher concentration of the Ca2+ in the groundwater resist the saturation of F− ion [57].
To more elaborate the groundwater chemistry with their key factors, and comprehend the association of groundwater characteristics to the particular aquifer such as rock dominance, precipitation dominance and evaporation dominance [58, 59]. The concept of Gibbs Plot proposed by Gibbs in (1970), to understand the mechanism of aquifer chemistry. An illustration (Fig. 4a, b), the association of TDS with the weight ratio of anions and cations calculated by the (Eqs. 6, 7). Gibbs plotting represent that groundwater aquifer of the area are influenced by weathering of rock, evaporation and precipitation dominance. Thus, rock evaporation, precipitation and weathering processes influence the existence of F− in water of the study area.
The (Fig. 4a,b), demonstrates the mix of rocks and precipitation dominance in the respective aquifer. However, the rock dominance by the process of weathering, erosion, and transportation from the parent rock to the groundwater table [60, 61]. Argued that the parent rock is the primary source of minerals by the chemical weathering of rocks forming minerals. Water permeates under the subsurface and responsible for the enrichment of contaminants in the aquifer with longtime water–rock interaction; also, this is the leading source for the F− enrichment in groundwater. Whereas the precipitation dominance sample points relatively equal to rock dominance part. Precipitation plays a dynamic role as a transportation medium for the minerals and salts into the groundwater [62].
4.5 (SAR) Sodium absorption ratio and (Na %) Sodium percentage
Sodium hazard expressed by the calculation of (SAR) sodium absorption ratio as calculated through (Eq. 16), this is a crucial tool to determine the groundwater quality for the irrigation purpose. The higher alkaline situations cause an interruption in the osmotic activity and reduce the water uptake capacity for a plant that directly negative impacts on the plant growth rate [58, 63] Reports according to SAR, groundwater classified as low (SAR < 10), medium (10–18), higher (18–26) and very higher (SAR > 26) sodium hazard. The SAR values of this study contain a range (1.183 to 6.882), with mean and standard deviation (4.591 ± 0.999).
The higher concentration of Na+ shows ion exchange with Ca2+ through the lithogenic dissolution of Na+ with dissolved Ca2+ in the aquifer system [64]. Based on (USSL) diagram (Fig. 5), [65] reports the comparison plot between SAR and EC. In this study the salinity hazard indication in the plot area as C3-S1, C4-S1, and C4-S2, that shows high, higher and highest salinity hazard [58]. Suggested water in above categories is not suitable for agriculture purposes.
However, sodium percentage (Na+ %) is also a critical measurement parameter to check the suitability of groundwater for the agricultural practices [64, 66, 67]. Higher sodium concentration reasons to decrease of flocculation, weaken permeability, and tilth of soil [64]. The sodium percentage Na% in the groundwater samples computed by the (Eq. 17).
While, the concentration of (Na+, K+, Ca2+, Mg2+) expressed in (meq/L). The Wilcox diagram (Fig. 6), showing the only 3.5% of samples lie in the Good to permissible zone while remaining 78.96% to (Permissible to doubtful and doubtful to unsuitable) zone and 17.54% in the (unsuitable) zone.
4.6 Source identification and mineral phases geochemical processes for F− enrichment
Principal component analysis (PCA) resulted in four PC factors that explain 70.73% of the total variance (Table 5). PCA is a useful technique to illustrate the minerals interaction in the aquifer by loading the hierarchy of PCs [68, 69]. PC1 is the leading factor with a variance of (42.88%); which points out the processes that may control the hydrogeochemistry of the study area. Furthermore, PC1 represents the positive relationship among the parameters (TDS, EC, HCO3−, Cl−, Ca2+, Na+, Mg2+, SO42−, K+, F− and NO3−). However, among these parameters, Na+, K+, F− and HCO3− have shown a strong positive correlation (Table 3). It can be predict that, Halite (NaCl), Sylvite (KCl) and Jarosite-k (KFe3+3(OH)6(SO4)2) minerals may be a stable source to the K+, Cl− and Na+ and origin for the F−, Ca2+, SO42− ions as a result by the precipitation of Fluorite (CaF2), Anhydrite (CaSO4), Gypsum (CaSO4·2H2O) and Melanterite (FeSO4·7H2O) in the aquifer system.
On the contrary, PC2 shows the dominance of Fe and Turbidity; this may lead by the dissolution of minerals such as Goethite (FeO(OH), Hematite (Fe2O3), Jarosite-k (KFe3+3(OH)6(SO4)2) and Melanterite (FeSO4·7H2O). The result of the saturation indices of these minerals suggests a possible source for Fe; however, turbidity is the result of the sand, silt, and clay mixture in the groundwater. PC3 reflects favorable loading for pH and depth. The rise of pH might be the result of proton reduction during the dissolution of the chemical reaction of minerals in the groundwater, and the process of the evapotranspiration may also increase the pH. PC4 indicates only loading for temperature.
Natural occurrence of groundwater F− is also associated with the geological processes, physical and chemical physiognomies of the aquifer, as well as the soil texture and properties, also the climatic condition of an area [70]. It has recorded that, a higher concentration of F− in the groundwater is due to water–rock interaction, with host rocks containing higher potential of F−, thus increasing concentration with time through the process of evapotranspiration. Depth factor also influences level of F−, while shallow groundwater contains higher F− than deep groundwater [71]. Saturation indices: is the ratio of different mineral addition that causes a disturbance in the equilibrium of water condition [72]. The nine phases of saturation indices obtained with the help of Phreeqc Interactive 3.4.0. The results showed mineral phases including Fluorite, Anhydrites, Goethite, Gypsum, Halite, Hematite, Jarosite-K, Melanterite, and Sylvite (Table 6).
Fluorite (CaF2) mineral has proven to be a potential source of F− in the groundwater [73]. The aquifer of the study area has shown fluorite (CaF2) mineral; 19% of the total groundwater samples were found supersaturated, and the remaining 81% were unsaturated [72]. Reports that fluorite mineral is the significant reason, among other minerals, that control the geochemistry of groundwater for the saturation of F− ion. In above situation [74] reports overall status of the study area may represent a thermodynamic equilibrium with low rate of mineral supersaturation might be occurring in the aquifer systems.
4.7 Water Quality Index (WQI)
The Water Quality Index (WQI) is an essential method to evaluate groundwater quality [75]. The groundwater quality influenced by the chemical parameters shown in (Figs. 5, 6) wholly represented WQI, according to the standard protocols for the drinking water by WHO [76]. The WQI results were categorized into the five water quality types as: excellent (< 50), good (50–100), poor (100–200), very poor (200–300) and water unsuitable for the drinking (> 300). (Table 7) express the WQI statistics of the study area, 100% of water samples lie in the range (926–15,263), WQI shows this groundwater is unsuitable for the drinking purpose.
According to the above results of this study, low Ca2+ in contrast with Na+ and F− (Table 2), salinity hazard indication higher to very higher (Fig. 5), and Wilcox diagram (Fig. 6), presenting 96.5% of samples are permissible to doubtful to an unsuitable zone. These results are highly alarming because this study area lies on the coastal bank of Arabian Sea in Southern Sindh, Pakistan. Above results probably shows the intrusion of seawater into the aquifer system to increase the level of salts in the groundwater and become unsuitable for drinking purpose to the local community.
5 Conclusion
Groundwater profile of the study area shows fluoride contamination reflect the geogenic inputs which includes mica and fluorite minerals. Additionally, different multivariate statistical techniques such as Pearson correlation, PCA, Piper plot, USSL diagram, Wilcox plot, SAR, Na%, Saturation indices, and Water Quality Index were employed to trace the hydrogeochemical features responsible for higher F− enrichment in the groundwater. It observed that 47% groundwater samples found above the WHO guidelines value. Water Quality Index (WQI) indicated that groundwater extremely unfit for the human health and irrigation purposes. Piper plot revealed the dominancy of (NaCl) type water, and Gibbs plot shows a mix ratio of rock and precipitation dominance. Whereas, saturation indices figure out fluorite (CaF2) mineral under the saturation range (− 2.3 to 1.24) with a leading source for the F− dissolution in the aquifer. The groundwater recorded alkaline and brackish nature of groundwater. Pearson correlation showed that Na+, Cl−, SO42− and HCO3− positively correlated toward F−. While, Ca+2 and NO3− were negatively correlated. The northern and southern part of the study area found with higher range of F− in the groundwater. The groundwater suitability for the agriculture resulted by USSL and Wilcox plot, most of the groundwater samples found at higher risk because of Na% and salinity hazard. The higher concentration of F− in the groundwater of Tando Bago, leads many health risks to the local communities. It’s highly recommended that local government should takes action for the removal of fluoride by inserting water defluoridation filter.
References
Rashid A, Khattak SA, Ali L, Zaib M, Jehan S, Ayub M, Ullah S (2019) Geochemical profile and source identification of surface and groundwater pollution of District Chitral. Northern Pakistan. Microchem J 145:1058–1065
Ali L, Rashid A, Khattak SA, Zeb M, Jehan S (2019) Geochemical control of potential toxic elements (PTEs), associated risk exposure and source apportionment of agricultural soil in Southern Chitral. Pakistan. Microchem J 147:516–523
Rashid A, Khan S, Ayub M, Sardar T, Jehan S, Zahir S, Khan MS, Muhammad J, Khan R, Ali A (2019) Mapping human health risk from exposure to potential toxic metal contamination in groundwater of Lower Dir, Pakistan: application of multivariate and geographical information system. Chemosphere 225:785–795
Saby M, Larocque M, Pinti DL, Barbecot F, Sano Y, Castro MC (2016) Linking groundwater quality to residence times and regional geology in the St. Lawrence Lowlands, southern Quebec, Canada. Appl Geochem 65:1–13
Zhang H-M, Su B-Y, Liu P-H, Zhang W (2007) Experimental study of fluorine transport rules in unsaturated stratified soil. J China Univ Min Technol 17:382–386
Yang L, Mei K, Liu X, Wu L, Zhang M, Xu J, Wang F (2013) Spatial distribution and source apportionment of water pollution in different administrative zones of Wen-Rui-Tang (WRT) river watershed. China. Environ Sci Pollut Res 20:5341–5352
Rashid A, Guan D-X, Farooqi A, Khan S, Zahir S, Jehan S, Khattak SA, Khan MS, Khan R (2018) Fluoride prevalence in groundwater around a fluorite mining area in the flood plain of the River Swat. Pakistan. Sci Total Environ 635:203–215
Rashid A, Farooqi A, Gao X, Zahir S, Noor S, Khattak JA (2020) Geochemical modeling, source apportionment, health risk exposure and control of higher fluoride in groundwater of sub-district Dargai. Pakistan. Chemosphere 243:125409
Manousakas M, Papaefthymiou H, Eleftheriadis K, Katsanou K (2014) Determination of water-soluble and insoluble elements in PM2. 5 by ICP-MS. Sci Total Environ 493: 694–700.
Edition F (2011) Guidelines for drinking-water quality. WHO Chronicle 38:104–108
Msonda K, Masamba W, Fabiano E (2007) A study of fluoride groundwater occurrence in Nathenje, Lilongwe, Malawi. Phys Chem Earth Parts A/B/C 32:1178–1184
Petrone R, Zheng Z, Hissel D, Péra M-C, Pianese C, Sorrentino M, Becherif M, Yousfi-Steiner N (2013) A review on model-based diagnosis methodologies for PEMFCs. Int J Hydrogen Energy 38:7077–7091
W.H. Organization, Preventing disease through healthy environments: inadequate or excess fluoride: a major public health concern, in, World Health Organization, 2019.
Chae G-T, Yun S-T, Mayer B, Kim K-H, Kim S-Y, Kwon J-S, Kim K, Koh Y-K (2007) Fluorine geochemistry in bedrock groundwater of South Korea. Sci Total Environ 385:272–283
Currell M, Cartwright I, Raveggi M, Han D (2011) Controls on elevated fluoride and arsenic concentrations in groundwater from the Yuncheng Basin. China. Appl Geochem 26:540–552
Luo W, Gao X, Zhang X (2018) Geochemical processes controlling the groundwater chemistry and fluoride contamination in the Yuncheng Basin, China—An area with complex hydrogeochemical conditions. PloS One 13
Hoque A, Khaliquzzaman M, Hossain M, Khan A (2003) Fluoride levels in different drinking water sources in Bangladesh. Fluoride 36:38–44
Narsimha A, Rajitha S (2018) Spatial distribution and seasonal variation in fluoride enrichment in groundwater and its associated human health risk assessment in Telangana State. South India. Hum Ecol Risk Assessm Int J 24:2119–2132
Karroum M, Elgettafi M, Elmandour A, Wilske C, Himi M, Casas A (2017) Geochemical processes controlling groundwater quality under semi arid environment: a case study in central Morocco. Sci Total Environ 609:1140–1151
Chaouch N, Khelfaoui A (2019) Defluoridation of groundwater in the south east of Algeria by adsorption. Mater Biomater Sci 2:014–017
Zabala ME, Manzano M, Vives L (2016) Assessment of processes controlling the regional distribution of fluoride and arsenic in groundwater of the Pampeano Aquifer in the Del Azul Creek basin (Argentina). J Hydrol 541:1067–1087
Narsimha A, Sudarshan V (2017) Contamination of fluoride in groundwater and its effect on human health: a case study in hard rock aquifers of Siddipet. Telangana State, India. Appl Water Sci 7:2501–2512
Amini M, Mueller K, Abbaspour KC, Rosenberg T, Afyuni M, Møller KN, Sarr M, Johnson CA (2008) Statistical modeling of global geogenic fluoride contamination in groundwaters. Environ Sci Technol 42:3662–3668
Brindha K, Rajesh R, Murugan R, Elango L (2010) Natural and anthropogenic influence on the fluoride and nitrate concentration of groundwater in parts of Nalgonda district. Andhra Pradesh, India. J Appl Geochem 12:231–241
Rao NS, Devadas DJ (2003) Fluoride incidence in groundwater in an area of Peninsular India. Environ Geol 45:243–251
A. Geiger, J. Cooper, Overview of airborne metals regulations, exposure limits, health effects, and contemporary research, US Environmental Protection Agency. Accessed Accessed on August, 25 (2010) 2015.
Chopra D (2012) Is organic fluorine really “not” polarizable? Cryst Growth Des 12:541–546
Deng Y, Nordstrom DK, McCleskey RB (2011) Fluoride geochemistry of thermal waters in Yellowstone National Park: I. Aqueous fluoride speciation. Geochimica et Cosmochimica Acta 75:4476–4489
G.o. Pakistan, Census, Pakistan Bureau of Statistics (2017).
Qureshi MA, Mastoi GM, Mastoi AA, Bhatti MA, Rind AM, Chandio S, Mastoi AW (2015) ARC. A Case Study of Coastal Area District Badin, Sindh, Pakistan, GIS Based Interpretation of Ground Water Pollution
G.o. Sindh, Disaster Risk Management Plan District Badin Government of Sindh, in, 2008.
Shahab A, Shihua Q, Rashid A, Hasan FU, Sohail MT (2016) Evaluation of water quality for drinking and agricultural suitability in the lower indus plain in Sindh Province, Pakistan, Polish Journal of Environmental Studies, vol 25
J.A.J.J.A.W.W.A. Cotruvo, 2017 WHO Guidelines for Drinking Water Quality: First Addendum to the Fourth Edition, 109 (2017) 44–51.
Lloyd JW, Heathcote J (1985) Natural inorganic hydrochemistry in relation to ground water
Bozdağ AJEES (2015) Combining AHP with GIS for assessment of irrigation water quality in Çumra irrigation district (Konya), Central Anatolia, Turkey, 73:8217–8236.
Jordana S, Batista E (2004) Natural groundwater quality and health. Geologica Acta 2:175–188
Rafique T, Naseem S, Usmani TH, Bashir E, Khan FA, Bhanger MI (2009) Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area. Sindh, Pakistan. J Hazard Mater 171:424–430
Sahu P (2019) Fluoride pollution in groundwater. Iin: Groundwater Development and Management, Springer, pp 329–350.
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—a case study. Water Res 38:3980–3992
Suthar S, Garg VK, Jangir S, Kaur S, Goswami N, Singh S (2008) Fluoride contamination in drinking water in rural habitations of Northern Rajasthan, India. Environ Monitor Assessm 145:1–6
Rafique T, Naseem S, Bhanger MI, Usmani TH (2008) Fluoride ion contamination in the groundwater of Mithi sub-district, the Thar Desert. Pakistan. Environ Geol 56:317–326
Gizaw B (1996) The origin of high bicarbonate and fluoride concentrations in waters of the Main Ethiopian Rift Valley, East African Rift system. J Afr Earth Sci 22:391–402
Fallahzadeh RA, Miri M, Taghavi M, Gholizadeh A, Anbarani R, Hosseini-Bandegharaei A, Ferrante M, Conti GO (2018) Spatial variation and probabilistic risk assessment of exposure to fluoride in drinking water. Food Chem Toxicol 113:314–321
Alarcón-Herrera MT, Bundschuh J, Nath B, Nicolli HB, Gutierrez M, Reyes-Gomez VM, Nunez D, Martín-Dominguez IR, Sracek O (2013) Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: Genesis, mobility and remediation. J Hazard Mater 262:960–969
Li C, Gao X, Wang Y (2015) Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci Total Environ 508:155–165
Hsieh JC, Chadwick OA, Kelly EF, Savin SM (1998) Oxygen isotopic composition of soil water: quantifying evaporation and transpiration. Geoderma 82:269–293
Guo Q, Wang Y, Guo Q (2010) Hydrogeochemical genesis of groundwaters with abnormal fluoride concentrations from Zhongxiang City. Hubei Province, central China. Environ Earth Sci 60:633–642
Young SM, Pitawala A, Ishiga H (2011) Factors controlling fluoride contents of groundwater in north-central and northwestern Sri Lanka. Environ Earth Sci 63:1333–1342
Guo Q, Wang Y, Ma T, Ma R (2007) Geochemical processes controlling the elevated fluoride concentrations in groundwaters of the Taiyuan Basin, Northern China. J Geochem Explor 93:1–12
Dar MA, Sankar K, Dar IA (2011) Fluorine contamination in groundwater: a major challenge. Environ Monit Assess 173:955–968
Naseem S, Rafique T, Bashir E, Bhanger MI, Laghari A, Usmani TH (2010) Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area. Thar Desert, Pakistan. Chemosphere 78:1313–1321
Farooqi A, Masuda H, Firdous N (2007) Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts. Punjab, Pakistan and possible contaminant sources. Environ Pollut 145:839–849
Gao X, Wang Y, Li Y, Guo Q (2007) Enrichment of fluoride in groundwater under the impact of saline water intrusion at the salt lake area of Yuncheng basin, northern China. Environ Geol 53:795–803
Xiao J, Jin Z, Zhang F (2015) Geochemical controls on fluoride concentrations in natural waters from the middle Loess Plateau. China., J Geochem Explor 159:252–261
Li X, Hou X, Zhou Z, Liu L (2011) Geochemical provenance and spatial distribution of fluoride in groundwater of Taiyuan basin. China. Environ Earth Sci 62:1635–1642
Saxena V, Ahmed S (2001) Dissolution of fluoride in groundwater: a water-rock interaction study. Environ Geol 40:1084–1087
Reddy A, Reddy D, Rao P, Prasad KM (2010) Hydrogeochemical characterization of fluoride rich groundwater of Wailpalli watershed. Nalgonda District, Andhra Pradesh, India. Environ Monitor Assessm 171:561–577
Talib MA, Tang Z, Shahab A, Siddique J, Faheem M, Fatima M (2019) Health, hydrogeochemical characterization and suitability assessment of groundwater: a case study in central Sindh, Pakistan. Int J Environ Res Public Helath 16:886
Jehan S, Khan S, Khattak SA, Muhammad S, Rashid A, Muhammad NJM (2019) Hydrochemical properties of drinking water and their sources apportionment of pollution in Bajaur Agency, Pakistan
Selvakumar S, Ramkumar K, Chandrasekar N, Magesh N, Kaliraj S (2017) Groundwater quality and its suitability for drinking and irrigational use in the Southern Tiruchirappalli district, Tamil Nadu, India. Appl Water Sci 7:411–420.
Adimalla N, Venkatayogi S, Das S (2019) Assessment of fluoride contamination and distribution: a case study from a rural part of Andhra Pradesh, India, 9:94
Qureshi MA, Mastoi GM, Mastoi AA, Bhatti MA, Rind AM, Chandio S, Mastoi AW. ARC. GIS based interpretation of ground water pollution: a case study of coastal area District Badin, Sindh, Pakistan
Marghade D, Malpe D, Zade A (2011) Geochemical characterization of groundwater from northeastern part of Nagpur urban, Central India. Environ Earth Sci 62:1419–1430.
Ali W, Aslam MW, Junaid M, Ali K, Guo Y, Rasoo Al, Zhang HJEP (2019) Elucidating various geochemical mechanisms drive fluoride contamination in unconfined aquifers along the major rivers in Sindh and Punjab, Pakistan
Nakhaei M, Dadgar MA, Amiri V (2016) Geochemical processes analysis and evaluation of groundwater quality in Hamadan Province, Western Iran. Arab J Geosci 9:384
Wilcox LVJJ (1958) Water quality from the standpoint of irrigation, vol 50, pp 650–654
Amiri V, Sohrabi N, Dadgar M (2015) Evaluation of groundwater chemistry and its suitability for drinking and agricultural uses in the Lenjanat plain, central Iran. Environ Earth Sci 74:6163–6176.
Praus P (2007) Urban water quality evaluation using multivariate analysis. Acta Montanistica Slovaca 12:150–158
Vlassopoulos D, Goin J, Zeliff M, Porcello J, Tolan T, Lindsey K (2009) Groundwater geochemistry of the Columbia River basalt group aquifer system: Columbia Basin groundwater management area of Adams, Franklin, Grant, and Lincoln Counties, Columbia River basalt group aquifer system: Columbia Basin groundwater management area of Adams, Franklin, Grant, and Lincoln Counties, Othello, Washington
Feenstra L, Vasak L, Griffioen J (2007) Fluoride in groundwater: Overview and evaluation of removal methods. International Groundwater Resources Assessment Centre, Report nr. SP 2007–1, in, UNESCO
Brindha K, Elango L (2011) Fluoride in groundwater: causes, implications and mitigation measures. Fluoride Propert Appl Environ Manage 1:111–136
Brahman KD, Kazi TG, Afridi HI, Naseem S, Arain SS, Wadhwa SK, Shah F (2013) Simultaneously evaluate the toxic levels of fluoride and arsenic species in underground water of Tharparkar and possible contaminant sources: a multivariate study. Ecotoxicol Environ Saf 89:95–107
He X, Ma T, Wang Y, Shan H, Deng Y (2013) Hydrogeochemistry of high fluoride groundwater in shallow aquifers. Hangjinhouqi, Hetao Plain. J Geochem Explor 135:63–70
Salifu A, Petrusevski B, Ghebremichael K, Buamah R, Amy G (2012) Multivariate statistical analysis for fluoride occurrence in groundwater in the Northern region of Ghana. J Contam Hydrol 140:34–44
Sadat-Noori S, Ebrahimi S, Liaghat AJEES (2014) Groundwater quality assessment using the Water Quality Index and GIS in Saveh-Nobaran aquifer, Iran. 71:3827–3843
Edition FJW (2011) Guidelines for drinking-water quality, vol 38, pp 104–108.
Acknowledgments
This research was supported by the Pakistan Council of Research in Water Resources (PCRWR), laboratory Badin district, Sindh, Pakistan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
On behalf of all authors, there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Talpur, S.A., Noonari, T.M., Rashid, A. et al. Hydrogeochemical signatures and suitability assessment of groundwater with elevated fluoride in unconfined aquifers Badin district, Sindh, Pakistan. SN Appl. Sci. 2, 1038 (2020). https://doi.org/10.1007/s42452-020-2821-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2821-1