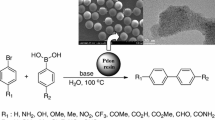
Abstract
Macroporous ion-exchange resins were used to immobilize palladium (Pd) metal in order to produce nanoparticle catalysts. The complexation of palladium acetate and the following reduction with NaBH4 resulting Pd nanoparticles are loaded on to DIANION™ PK228 macroporous cation exchange resins. Palladium loading was characterized by inductively coupled plasma-atomic emission spectroscopy, field emission scanning electron microscope/energy-dispersive X-ray spectrometry, BET (Brunauer–Emmett–Teller) and HR-TEM. Palladium nanoparticles are loaded on to DIANION™ PK228 macroporous cation exchange resin which shows and excellent catalytic activity for Suzuki–Miyaura cross coupling reaction. Furthermore, the catalyst can be recycled without significant loss of its catalytic activity up to ten times.
Similar content being viewed by others
1 Introduction
Homogenous Pd nanoparticle catalysts are widely used in many coupling and cross coupling reactions, such as Heck, Stile, Suzuki–Miyaura, Sonogashira, and Buckwald-Hartwig reactions. These catalysts trigger high reaction rates and great selectivity [1]. The field of catalysis has evolved to focus not only on altering the rate of a certain reaction, but also making the process environment-friendly, more efficient and more economical. On the other hand, homogenous catalysts are difficult to recycle and difficult to separate them easily, which leads to the loss of the expensive metals and hence it is essential to remove the impurities from the products [2]. This problem poses a big challenge to the industry as to how to make application of homogenous Pd nanoparticles catalysts in coupling reactions.
In order to solve this problem, heterogeneous Pd nanoparticles catalysts are better alternatives for the coupling reactions. Various solid supports, such as activated carbon [3,4,5], zeolite [6,7,8,9,10] and silica [11,12,13] and poly vinyl pyrrolidine (PVP) [14] have been used as supports, which can sometimes allow reaction under unfavourable conditions [10].
After studying recent publications, it is found that various modified catalysts have been developed to improve the efficiency of the reaction and to make the process environment-friendly. Other than the report on the immobilization and the characterization of Pd0 nanoclusters on gel–type functional polymers [15] and the use of amberlyst-supported systems [16], ion exchange resins [17] were somewhat less probed. However, the industrial process developed in recent years on Pd nanoparticles on ion exchange resin indicates that they are effective catalysts. Compared with the other solid supports, ion exchange indicates several inherent advantages. Firstly, most of these are commercially available at low cost. Secondly, they can stabilize metal nanoparticles due to the use of functional groups which give electrostatic stabilization and porosity gives steric stabilization. Also, they are easy to handle and they integrate into reactor equipment. Palladium has long been one of the most efficient metals in catalysis [18, 19], and its application in the Suzuki–Miyaura cross coupling is one of the most powerful and useful tools for the formation of carbon–carbon bonds for the synthesis of pharmaceuticals, drugs, agrochemicals, polymers, natural products and specialized engineering materials. The Suzuki–Miyaura coupling reaction has indeed come a long way. There are numerous publications related to its development and improvement in terms of various parameters such as catalytic systems and solvents. Overall, most of these catalysts gave promising results for Suzuki–Miyaura cross coupling reaction. But expensive reagents have been utilized by some and it is found that the reactions take longer times, whereas others have used reagents that are not easy to handle.
In the present work, we have used DIANION™ PK228 which is a porous type strongly acidic cation exchange resin. It has 14% cross-linked and excellent properties. It has a functional group sulphonic acid and ionic form is Na+. The catalyst that we synthesized is an alternative catalyst for the Suzuki–Miyaura cross coupling reaction in which we used macroporous cation exchange resin as a solid support. In this technique, water solvent is used and inert conditions are not required. This synthesized catalyst is of heterogeneous nature and it can be recovered and reused several times without any substantial loss in its catalytic efficiency. The catalyst shows a good reusability. The yields obtained by this method are excellent.
2 Materials and methods
The purchased cation exchange resin (procured from DIANION™ PK228, Mitsubhihi Chemical, Japan), contains styrene-DVB that has a strong cation exchange resin. All the chemicals were purchased from Hi-Media, Ratnagiri, Maharashtra, India and used without purification. Solvents were dried by standard methods to use for reactions. TLC was carried out with Merck silica gel 60-F254 plates and the technique column chromatography was used. Silica gel (60–120 mesh) obtained from commercial suppliers inserted in the column for purification of coupling products. 1H NMR spectra were taken on a Bruker AdvanceIII, Switzerland spectrometer (400 MHz) spectrometer using CDCl3 as solvent and tetramethylsilane as an internal standard.
2.1 Preparation of PdNP@DIANION™ PK228 catalyst
The resin (2.7 g) and palladium acetate (Pd (OAc)2), (0.23 g) were placed inside a 100 mL round bottom flask. methnol solvent (30 mL, distilled) was added and the mixture was sonicated (Make Ultrasonic Cleaner) until the Pd (OAc)2 dissolved. The mixture was then shaken by using a mechanical overhead stirrer (Remi) at 400 rpm speed for 24 h. When the catalyst formed, the color of the resin changed from tan to blackish. Then the resin was washed with methanol several times to ensure that no unreacted Pd (OAc)2 remained in the resin. The resin was air-dried and 26 mL MeOH:dioxane (2:1) solvent was poured into the flask in addition to NaBH4 (0.12 g). The mixture was shaken for 24 h using a mechanical overhead stirrer (Remi) at 400 rpm. The resin was filtered, washed with methnol several times and finally with acetone. Finally, the resin, now silver in color, was air-dried. This catalyst was further used for Suzuki–Miyaura coupling reaction.
2.2 PdNP@DIANION™ PK228 catalyzed Suzuki–Miyaura coupling reaction
Aryl halide (1 eq.), Arylboronic acid (1 eq.), K2CO3 (2 eq.), PdNP@DIANION™ PK228 (0.025 g) and tetrabutyl ammonium bromide (TBAB) (0.25 eq.) were poured into a clean round bottom flask and then 10 mL water was added to the mixture. The reaction mixture was heated to reflux for 10–15 min. The reaction was monitored by TLC. After the completion of reaction, the reaction mixture was cooled to room temperature. Then ethyl acetate (30 mL) was added and the mixture was filtered through celitebed. 30 mL water was added to the filtrate. The organic layer was separated and washed with water (30 mL × 2). The organic layer was dried on sodium sulphate and was evaporated on rotary evaporator to get crude compound. Then the crude compound was purified by column chromatography using silica (60–120 mesh) as a stationary phase and 0–5% ethyl acetate: hexane as mobile phase. Purified compounds were characterized by 1H NMR.
2.3 Characterization of supports
Surface area analysis was carried out by BET (Brunauer–Emmett–Teller), SMART Instrument, India. Magnification and agglomeration of the catalyst were analyzed by field emission gun-scanning electron microscope (FE-SEM, SPECTRO Analytical Instruments GmbH, Germany). Adsorption of palladium was analyzed by energy-dispersive X-ray spectroscopy (EDS, SPECTRO Analytical Instruments GmbH, Germany). Element analysis was performed by inductively coupled plasma atomic emission spectroscopy (ICP-AES, SPECTRO Analytical Instruments GmbH, Germany). The synthesized catalyst was dissolved in the mixture of conc. HNO3 and conc. HCl, then the solution was suspended to the determination of the Pd content. Particle size analysis were recorded by emission gun-scanning electron microscope (HR-TEM, FEI Tecnai F30) and the specimens were prepared by diffusing samples in isopropyl alcohol and placing this on carbon coated copper grid.
3 Result and discussion
3.1 Macroporous resin support
To develop better solid supports, we considered macroporous ion exchange resins. Their characteristic rigid beads provide an advantage in easy separation after completion of reaction from reaction mixture and were investigated reusability in organic synthesis reaction. Macroporous cation resin were used, namely DIANION™ PK228 cation exchange styene DVB matrix. The starting material bead was moist, and tan in color. Beads were dried to remove water to get maximum loading. Complexation was evident by change in color of the beads. Color changed from colloidal brown to silver. The reactivity of catalyst was investigated using Suzuki–Miyaura coupling reaction and it was showed an excellent reactivity on preliminary observation. The specific surface area determined by N2 absorption decreases from 14.19 to 9.80 m2/g. This catalyst was recycled up to ten times without significant loss of reactivity. Inductively coupled plasma-atomic emission spectrometry (ICP-AES) analysis showed that the 44.05 mg Pd loaded on resin/500 mg of PdNP@DIANION™ PK228. The catalyst was further characterized using FE-SEM/EDS and HR-TEM.
The FE-SEM data of prepared catalyst is shown in Fig. 1. The FE-SEM pictures, taken at lower magnification, show very nice spherical resin particles. No separate agglomerates of palladium are seen at even higher magnification on resin structures. Small spheres of palladium nanoparticles were homogenously dispersed on the DIANION™ PK228 resin. Also catalyst reduction was carried out at room temperature and which prevent the particle aggregation on the resin surface.
The EDS data of catalyst prepared is as shown in Fig. 2. Energy dispersive spectroscopy (EDS) was used to map the element present in the sample. The EDS data confirms that the present of palladium in the given sample. Pd exchange resin sample the EDS data confirms the presence of element C, Pd, D and Na. The concentration of carbon prominent in the resin and the resin is made up of polymeric organic compounds. The S and O content in the sample is due to sulphonic acid (SOзH) group responsible for cation exchange. The lower traces of sodium in the EDS data indicates the higher degree of palladium (counter cation exchange). The higher exchange of palladium is reflected in the EDS data (9.88%). The apparent higher content of palladium in this sample might be due to the fact that most of the palladium is present at the sulphonic group (SOзH) this surface saturation of palladium is reflected in the repulsion of the resin beads in the sample container when be observed these beads the appears like metallic (color) beads (Table 1).
Furthermore, high resolution transmission electron microscopy (HR-TEM) data of catalyst is as shown in Fig. 3. The HR-TEM image of the catalyst was also obtained, showing nanoparticles having a narrow size distribution of 20 nm.
3.2 Suzuki–Miyaura coupling reaction over PdNP@DIANION PK228
Reaction scheme

3.2.1 Biphenyl (Table 2, entry 1)
Iodobenzene (0.22 ml), phenylboronic acid (0.242 g), K2CO3 (0.276 g) and catalyst (0.025 g), tetrabutyl ammonium bromide (TBAB) (0.06 g) and water (10 mL) were used. Biphenyl was obtained as a white solid M.P = 69 °C (Rf = 0.72, Hexane: Ethyl acetate). 1H NMR was obtained to confirm the product and solution NMR (1H). 1H NMR δ 7.19–7.24 (m, 6H), δ 7.61 (dd, 4H).
3.2.2 4-Acetylbiphenyl (Table 2, entry 2)
4-Bromoacetophenone (0.398 g), phenylboronic acid (0.242 g), K2CO3 (0.276 g) and catalyst (0.025 g), tetrabutyl ammonium bromide (TBAB) (0.06 g) and water (10 mL) were used. 4-Acetylbiphenyl was obtained as a white solid M.P = 117 °C (Rf = 0.68, Hexane: Ethyl acetate). 1H NMR was obtained to confirm the product and solution NMR (1H). 1H NMR δ 8.07 (dd, 2H), δ 7.68 (dd, 2H), δ 7.52 (d, 2H), δ 7.42 (m, 2H), δ 2.60 (s, 3H), δ 10.08 (s, 1H).
3.2.3 4-Chlorobiphenyl (Table 2, entry 3)
Iodobenzene (0.22 ml), 4-chlorophenylboronic acid (0.312 g), K2CO3 (0.276 g) and catalyst (0.025 g), tetrabutyl ammonium bromide (TBAB) (0.06 g) and water (10 mL) were used. 4-chlorobiphenyl was obtained as a white solid B.P = 77 °C (Rf = 0.70, Hexane: Ethyl acetate). 1H NMR δ 7.54 (m, 4H), δ 7.42 (m, 5H).
3.2.4 4-Tertbutylbiphenyl (Table 2, entry 4)
Iodobenzene (0.22 ml), 4-tertbutylphenylboronic acid (0.356 g), K2CO3 (0.276 g) and catalyst (0.025 g), tetrabutyl ammonium bromide (TBAB) (0.06 g) and water (10 mL) were used. 4-tertbutylbiphenyl was obtained as a white solid M.P = 53 °C (Rf = 0.50, Hexane: Ethyl acetate). 1H NMR was obtained to confirm the product and solution NMR (1H). 1H NMR δ 7.80 (m, 5H), δ 7.42 (m, 4H), δ 1.41 (s, 9H).
3.3 Recyclability of PdNP@DIANION PK228
The catalyst recyclability was investigated for reaction with iodobenzene with phenylboronic acid at 90 °C. The catalyst was separated from the reaction mixture after every reaction by simple filtration and then washed with ethyl acetate, followed by drying in desiccators and reused in fresh reaction and the process of recycling was repeated up to ten times. It was observed that used catalyst remained active without any significant loss in catalytic efficiency Fig. 4. The better reusability of the catalyst, which can be used ten times and the yield was more than 90%.
4 Conclusion
The result of ICP-AES confirmed that palladium was successfully loaded on cation exchange resin under mild conditions. It was characterized using BET, ICP-AES, FE-SEM–EDS, HR-TEM. The capacity showed efficient activity for Suzuki–Miyaura Coupling reaction in water under base with 100% selectivity and maximum conversion > 93%. The catalyst was recovered by simple filtration and the used catalyst was washed with ethyl acetate several times and was air-dried. Then the dried catalyst was reused up to ten times without any significant loss of efficiency. The simple environment-friendly process, easy recovery and operational simplicity are the advantages of the synthesized catalyst. The reusability of the catalyst after ten times gave more than 90% yield.
Abbreviations
- ICP-AES:
-
Inductively coupled plasma-atomic emission spectroscopy
- FE-SEM/EDX:
-
Field emission scanning electron microscope/energy-dispersive X-ray spectrometry
- BET:
-
Brunauer–Emmett–Teller
- CH2Cl2 :
-
Dichloromethane
- PdNP@DIANION™ PK228:
-
Palladium loaded on the surface of resin
- EDS:
-
Electron diffraction spectroscopy
References
Sreedhar B, Surendra Reddy P, Keerthi Devi D (2009) Direct one-pot reductive amination of aldehydes with nitroarenes in a domino fashion: Catalysis by gum-acacia-stabilized palladium nanoparticles. J Org Chem 74(22):8806–8809
Zhang A et al (2014) Homogeneous Pd nanoparticles produced in direct reactions: Green synthesis, formation mechanism and catalysis properties. J Mater Chem A 2(5):1369–1374
Scheuermann GM, Rumi L, Steurer P, Bannwarth W, Mülhaupt R (2009) Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki-Miyaura coupling reaction. J Am Chem Soc 131(23):8262–8270
Wang P, Wang GY, Qiao WL, Feng YS (2016) Mesoporous carbon supporting Pd (0) as a highly efficient and stable catalyst for Sonogashira reaction in aqueous media. Catal Lett 146(9):1792–1799
Jana S, Haldar S, Koner S (2009) Heterogeneous Suzuki and Stille coupling reactions using highly efficient palladium(0) immobilized MCM-41 catalyst. Tetrahedron Lett 50(34):4820–4823
Zhu P et al (2017) A hollow Mo/HZSM-5 zeolite capsule catalyst: preparation and enhanced catalytic properties in methane dehydroaromatization. J Mater Chem A 5(18):8599–8607
Hamedi M, Wigenius J, Tai F, Björk P, Aili D (2010) Polypeptide-guided assembly of conducting polymer nanocomposites. Nanoscale 2:2058–2061
Zheng S, Jentys A, Lercher JA (2006) Xylene isomerization with surface-modified HZSM-5 zeolite catalysts: an in situ IR study. J Catal 241(2):304–311
Aboul-Gheit AK, Aboul-Fotouh SM, Abdel-Hamid SM, Aboul-Gheit NAK (2006) Hydroconversion of cyclohexene using H-ZSM-5 zeolite catalysts promoted via hydrochlorination and/or platinum incorporation. J Mol Catal A Chem 245(1–2):167–177
Navlani-García M, Martis M, Lozano-Castelló D, Cazorla-Amorós D, Mori K, Yamashita H (2015) Investigation of Pd nanoparticles supported on zeolites for hydrogen production from formic acid dehydrogenation. Catal Sci Technol 5(1):364–371
Hajipour AR, Shirdashtzade Z, Azizi G (2014) Silica-acac-supported palladium nanoparticles as an efficient and reusable heterogeneous catalyst in the Suzuki–Miyaura cross-coupling reaction in water. J Chem Sci 126(1):85–93
Ncube P, Hlabathe T, Meijboom R (2015) Palladium nanoparticles supported on mesoporous silica as efficient and recyclable heterogenous nanocatalysts for the Suzuki C–C coupling reaction. J Clust Sci 26(5):1873–1888
Ivashchenko NA et al (2012) Preparation, characterization and catalytic activity of palladium nanoparticles embedded in the mesoporous silica matrices. World J Nano Sci Eng 02(03):117–125
De Luna D, Alvarez HM, Aguiar LCS (2010) Microwave-assisted Suzuki reaction catalyzed by Pd(0)–PVP nanoparticles. Tetrahedron Lett 51(52):6814–6817
Nemamcha A, Moumeni H, Rehspringer JL (2009) PVP Protective mechanism of palladium nanoparticles obtained by sonochemical process. Phys Procedia 2(3):713–717
De Castro KA, Rhee H (2015) Resin-immobilized palladium nanoparticle catalysts for Suzuki–Miyaura cross-coupling reaction in aqueous media. J Incl Phenom Macrocycl Chem 82(1):13–24
Ma L, Jia I, Guo X, Xiang L (2014) Cobalt-catalyzed redox-neutral synthesis of isoquinolines: C–H activation assisted by an oxidizing N–S bond. Chin J Catal 35(2):108–119
Khan M et al (2017) Miswak mediated green synthesized palladium nanoparticles as effective catalysts for the Suzuki coupling reactions in aqueous media. J Saudi Chem Soc 21(4):450–457
Nadagouda MN, Varma RS (2008) Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem 10:859–862
Acknowledgements
The authors are grateful to Dr. Bhange D. S, Assistant Professor in Inorganic Chemistry, Shivaji University, Kolhapur, Maharashtra, India for his kind help also authors are grateful to Principal, Ahemednagar College, Ahemednagar for his constant encouragement and provided laboratory facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manjare, S.B., Chaudhari, R.A., Thopate, S.R. et al. Resin loaded palladium nanoparticle catalyst, characterization and application in –C–C– coupling reaction. SN Appl. Sci. 2, 988 (2020). https://doi.org/10.1007/s42452-020-2795-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2795-z