Abstract
We report a 4-t-butylbenzyl derivatization for the analysis of carboxylic acids by GC–MS (EI). Carboxylic acids were analyzed as a 4-t-butylbenzyl ester after the derivatization with 4-t-butylbenzyl bromide. On the mass spectra of 4-t-butylbenzyl ester, [M-15]+ ions were observed with high intensity. These ions were tertiary benzyl cations generated by the elimination of a methyl radical from molecular ions. After optimization of the 4-t-butylbenzyl derivatization conditions using microwave reactor, we established a method for the determination of the carboxylic acids in water samples. The method showed good sensitivity and good reproducibility, and was successfully applied to the analysis of rain water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Carboxylic acids are used in applications that involve polymer materials and synthetic intermediates, which makes them indispensable in chemical industries. Many organic compounds can be oxidized to form carboxylic acids. The detection of carboxylic acids in materials and industrial processes could make it possible to track when, where and how they were oxidized or contaminated. In addition, some short-chain carboxylic acids have been designated as specified offensive odor substances by the offensive odor control law in Japan. Therefore, it is very important to determine the presence of carboxylic acids in materials, wastewater and environmental water.
GC is an analytical method with high resolution, but derivatizations [1] such as silylation and alkylation are required for the analysis of carboxylic acids due to their high polarity. Typical methods for silylations performed by various reagents include trimethylsilylation, [2, 3] and t-butyldimethylsilylation [4]. Alkylation mainly includes methylation and pentafluorobenzylation. Methylation is carried out using methyl iodide [5], diazomethane [6], BF3/methanol [7], trimethylanilinium hydroxide [8] or trimethylsilyldiazomethane [9, 10]. For pentafluorobenzylation, pentafluorobenzyl (PFB) bromide [11, 12] and pentafluorophenyldiazomethane [13] are used as derivatizing reagents.
GC–MS is a highly sensitive analytical method. Generally, quantification by GC–MS after derivatization is advantageous in terms of sensitivity and reproducibility of analysis wherein an ion derived from the analyte is generated with high intensity in the high mass region of its mass spectrum. Silylation produces highly sensitive derivatives but has the disadvantage that silyl derivatives are unstable to moisture. Although methyl derivatives are stable, the base peak in EI spectrum becomes a relatively low mass (m/z 74) due to the McLafferty rearrangement [14]. In the case of pentafluorobenzyl (PFB) derivatives, it is difficult to identify a compound from its mass spectrum, since the PFB cations (m/z 181) formed becomes the exclusive base peak.
We hypothesized that 4-t-butylbenzylation of carboxylic acids as a derivatization method could overcome these disadvantages. The introduction of a 4-t-butylbenzyl group would enhance the sensitivity because electron ionization of the derivatized ester preferentially generates more stable tertiary benzyl cations than primary benzyl cations (Fig. 1, route b). The formed cations would include information about the mother carboxylic acids. Here, we report a 4-t-butylbenzyl derivatization for the determination of carboxylic acids by GC–MS (EI).
2 Experiment
2.1 Materials and apparatus
For this study, 4-t-butylbenzyl bromide and acetic acid-d4 were obtained from Sigma-Aldrich. Pyrene-d10 and dichloromethane 5000 were purchased from FUJIFILM Wako pure chemical. Carboxylic acids were purchased from FUJIFILM Wako, TCI, Kanto, or SIGMA-ALDRICH. Phase-transfer reagents and other reagents were obtained from FUJIFILM Wako or TCI. Water was processed using a Milli-Q VOC water purification system (Millipore, Bedford, MA, USA). Microwave irradiation was performed using Discover (CEM corporation). Membrane filters (0.45 μm HA) were purchased from Millipore.
2.1.1 Standard solution of carboxylic acids
Standard solutions of individual carboxylic acids (1000 μg/mL) were prepared using pure water (VA, OA, EHA, DMHA, and PFOA were dissolved using acetone) in glass sample tubes. A mixed solution (10 μg/mL in water) of nine carboxylic acids was prepared from 1000 μg/mL solutions. A surrogate solution (acetic acid-d4, 10 μg/mL) was also prepared using acetic acid-d4 and pure water. Pyrene-d10 was diluted with hexane to 2.0 μg/mL.
2.2 Instrumentation and conditions
All analyses were performed using an Agilent Technologies 240 Ion trap mass spectrometer equipped with a 7890B GC system. The column was an ID-BPX5 (30 m × 0.25 mm i.d., 0.25 μm film thickness, SGE). The carrier gas was high-purity helium (99.9999%) with a constant flow of 1.2 mL/min. The GC oven temperature was set at 60 °C for 1 min, which then was increased to 300 °C at 10 °C/min and held for 5 min. Mass spectrum was measured in an internal ionization mode. The injection, transfer line, ion trap and manifold temperatures were set at 280, 300, 200, and 45 °C, respectively. All injections were performed in the splitless mode with the split vent closed for 1 min. Full-scan EI data were acquired under the following conditions: mass range, m/z 50 to 600; scan time, 1 s/scan; emission current, 20 μA.
All analytes were evaluated by comparing the peak area ratios of the derivatives to pyrene-d10 (m/z 212). Analytical data of carboxylic acids (nine analytes and one surrogate compound) and their 4-t-butylbenzyl derivatives are shown in Table 1.
2.3 Synthesis of 4-t-butylbenzyl ester
A solution of acetic acid (100 μg/mL, 100 μL), NaOH (5 M, 1 mL), 4-t-butylbenzyl bromide (50 μL), and TBA-HSO4 (500 mg/L dichloromethane solution, 10 mL) in 50 mL of water was heated and refluxed in a 100 mL three-necked flask for 2 h. After the reaction, the solution was transferred to a 100 mL separatory funnel. After the phase separation, the organic phase collected through a column of anhydrous sodium sulfate was concentrated to ca. 1 mL by nitrogen flow, and then measurement was performed by GC–MS (EI). 4-t-Butylbenzyl esters of the other acids were synthesized in a similar way.
2.4 Optimized microwave derivatization conditions
Tetraoctylammonium bromide (TOA-Br, 1.37 mg), dichloromethane (0.5 mL), carboxylic acid solution (1 μg/mL, 0.9 mL), surrogate solution (10 μg/mL, 0.1 mL), phosphate buffer (pH 7, 0.6 M, 0.1 mL), and 4-t-butylbenzyl bromide (10 μL) were added to a 10 mL microwave test tube. The reaction was performed with stirring in a microwave-focused chemical synthesizer (300 W, 150 °C, 15 min). After the reaction, the test tube was removed from the apparatus, and pyrene-d10 (2.0 μg/mL, 0.5 mL) was added as an internal standard. After shaking, the organic layer was collected and measured by GC–MS (EI).
2.5 Calibration curves
The diluted solutions (0, 0.005, 0.01, 0.03, 0.1, 0.3, 0.6, and 1.0 μg/mL) for the calibration curve were prepared by diluting the mixed solution (10 μg/mL) with pure water. The diluted solution was treated as described above.
2.6 Analysis of water sample
Sample water was taken from a pond located on the campus of Osaka University (December 2017). Rainwater was collected in Suita City (February 2018) and Hirakata City (July 2019). After the filtration of the water samples using a membrane filter, the samples were derivatized and analyzed under optimized conditions.
3 Results and discussion
In this study, 4-t-butylbenzyl bromide was employed as a derivatizing reagent for GC–MS.
3.1 Mass spectra of 4-t-butylbenzyl derivatives
First, 4-t-butylbenzyl derivatives of formic acid and acetic acid were synthesized under phase-transfer reaction conditions, and their EI-MS spectra were then measured (Figs. 2 and 3). The peaks at m/z 177 and 191 were detected as the base peaks, respectively. These ions corresponded to the cations [M-15]+ generated by the elimination of a methyl radical from molecular ions. The spectra showed that the generation of primary benzyl cations (m/z 147) was suppressed, and the peaks of m/z 177 and 191, which were tertiary benzyl cations, were obtained with high intensity. Although the intensity of the molecular ions was very small, the expected results were obtained.
Next, we investigated n-octanoic acid and its isomers. Similar to the previous results, the 4-t-butylbenzyl derivative of octanoic acids showed a peak that represented tertiary benzyl cations (m/z 275) with high sensitivity (Fig. 4). The formation of ions (m/z 206) generated by McLafferty rearrangement, which readily occurs with methyl derivatives, was suppressed.
Two structural isomers of n-octanoic acid, 2-ethylhexanoic acid and 2,2-dimethylhexanoic acid, had a branch at the α-position of the carbonyl group (Figs. 5 and 6). The mass spectra of these 4-t-butylbenzyl derivatives showed an increase in the peak intensity of the primary benzyl cations (m/z 147) with branching. On the other hand, the peak intensity of the tertiary benzyl cations showed only moderate intensity. The increased intensity of the primary benzyl cations was attributed to the stability of the secondary or tertiary alkyl radicals obtained during the subsequent decarboxylation reaction (Eq. 1).

These results indicate that 4-t-butylbenzyl derivatization is suitable for the sensitive analysis of carboxylic acids. Prior to the quantitative analysis, the reactivity of 4-t-butylbenzyl bromide was compared with that of pentafluorobenzyl bromide. The reaction of acetic acid with two benzylic bromides was performed by shaking at room temperature for 1 h under PTC conditions. Calculation of the peak area ratio with pyrene-d10, an internal standard substance, showed that 4-t-butylbenzyl bromide is less reactive than pentafluorobenzyl bromide (data not shown).
3.2 Optimization of 4-t-butylbenzylation using a microwave reactor
For practical analysis, the same reaction was performed under microwave irradiation. Table 2 shows the results of derivatization using a microwave reactor with valeric acid, chloroacetic acid, and octanoic acid as examples. The progress of the derivatization reaction was evaluated via the peak area ratio of [M-15]+ to pyrene-d10.
Tetraalkylammonium bromides, tetrabutylammonium (TBA), tetrahexylammonium (THA), tetraoctylammonium (TOA), and tetradecylammonium (TDA) were examined as PTC reagents (runs 1, 2, 3, and 5). With TBA-Br (run 1), the peak area of highly water-soluble acids such as FA, AA, and PA, was very small (data not shown). This is because short-chain tetraalkylammonium salt is highly water-soluble and difficult to transfer to the organic layer. No such trend was observed when using chloroacetic acid. Tetraoctylammonium bromide (TOA-Br) was the most effective PTC investigated for this study except for CLAA (run 3). Because reaction of CLAA was incomplete at 100 °C. Increasing the amount of TOA-Br did not improve the results (runs 3 and 4).
Solvents (toluene, dichloromethane (DCM), dichloroethane (DCE), and benzotrifluoride(BTF)) and phosphate buffer that ranged from pH 7 to 9 were examined (runs 3 and 6–8; and, 3, 9, and 10), but no significant difference was observed. Dichloromethane solvent and a buffer with a pH of 7 were selected as suitable conditions.
Next, the reaction temperature was examined within a range of from 50 to 150 °C (runs 10–14). When the reaction temperature was less than 150 °C, the peak area ratio of chloroacetic acid was extremely low due to the weak nucleophilicity of the chloroacetic acetate anions. The peak area ratio of the other carboxylic acids became almost constant above 100 °C.
The amount of derivatizing reagent was also tested (runs 14–16). At 20 μL, the peak area ratio of some carboxylic acids was lowered due to interference from the reagent. The optimal amount of reagent was 10 μL.

The peak area ratio remained constant for 10 min at 150 °C. The derivatization of chloroacetic acid was incomplete after 5 min. The optimal reaction time proved to be 15 min (run 18).
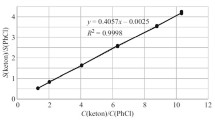
The optimized conditions that appear in Eq. 2 were confirmed when the derivatization proceeded almost quantitatively based on the peak area ratio of a synthesized standard product of an acetic acid derivative.
3.3 Calibration curves
Calibration curves were constructed under the optimized conditions using a standard solution with a concentration of 0–1.0 μg/mL. When reproducibility was confirmed with a 0.6 μg/mL aqueous solution, the relative standard deviation was within 7.3% for all compounds (Table 3). These results ensured good reproducibility.
Formic acid (FA) and acetic acid (AA) showed high blank values, which meant that the LOD and LOQ were also relatively high. Also, 2,2-dimethylhexanoic acid (DMHA) showed high values of LOD and LOQ due to the low intensity of the quantitative ions. With the exception of the carboxylic acids, the LOD was less than 3 ng/mL.
3.4 Recovery experiment from environmental water
A recovery test for the analysis of environmental water in a pond or in rainwater was conducted using our analytical method by adding nine carboxylic acids and deuterated acetic acid (AA-d4) as a surrogate compound. The recoveries of all carboxylic acids were almost quantitative and reproducible (Table 4). The recovery of AA-d4 was also very good. These results indicate that this type of derivatization is effective for an analysis of environmental water.
3.5 Analysis of rainwater
Rainwater was collected in Suita City and in Hirakata City, Osaka. Following filtration, AA-d4 was added to filtered rainwater for analysis. The rainwater was found to contain FA, AA and PA (Table 5). These values are reliable because recovery of the deuterated acetic acid was nearly quantitative with good reproducibility. No carboxylic acid was detected in the pond water.
3.6 Comparison of 4-t-butylbenzyl derivatives with methyl derivatives
Finally, 4-t-butylbenzyl derivatives were compared with conventional methyl derivatives for n-octanoic acid and 2-ethylhexanoic acid. Methylation was conducted using trimethylsilyldiazomethane [10]. The sensitivities were evaluated via the peak area ratios to pyrene-d10.
In the case of 2-ethylhexanoic acid, 4-t-butylbenzyl derivative was twice as sensitive as the methyl derivatives. Furthermore, 4-t-butylbenzyl octanoate was seven times more sensitive than the methyl derivatives (Table 6). Although the intensity of m/z 275 in 4-t-butylbenzyl EHA decreased with branching, it remained twice as sensitive as methylation. The high sensitivity of 4-t-benzyl derivatives is related to the generation of highly stable tertiary benzyl cations.
4 Conclusions
By derivatizing with 4-t-butylbenzyl bromide, we succeeded in performing a sensitive analysis of carboxylic acid. This derivatization suppressed the generation of the primary benzyl cations that were detected as a base peak during conventional benzyl derivatization. The peak of the tertiary benzyl cations was obtained with high intensity. McLafferty rearrangement was not observed with long-chain carboxylic acids. This derivatization is suitable for the analysis of low molecular weight carboxylic acids because it produces esters with a relatively high molecular weight.
With detection-oriented derivatization during the GC–MS (EI) analysis of carboxylic acids, it is well known that TMS [2, 3] and TBDMS [4] derivatives generate [M-15]+ and [M-57]+ ions, respectively, with strong intensity. This can be classified as an example of detection-oriented derivatization for GC–MS (EI), because the derivatization enhances the sensitivity of the carboxylic acids compared with other forms of derivatizations. Analytical methods for carboxylic acids with significantly lower detection limits have also been reported, [2] but present method has the advantages that the EI spectrum contains information about the mother carboxylic acid and the derivatives themselves are more sensitive. Further research into promoting an increase in sensitivity is now in progress.
References
Blau K, Halket J (1993) Handbook of derivatives for Chromatography. Wiley, New York
Jurado-Sanchez B, Ballesteros E, Gallego M (2012) Determination of carboxylic acids in water by gas chromatography-mass spectrometry after continuous extraction and derivatisation. Talanta 93:224–232
Guerin MR, Olerich G, Rainey WT (1974) Gas chromatograhic determination of nonvolatile fatty acids in cigarette smoke. Anal Chem 46:761–763
Mawhinney TP, Robin Robinett RS, Atalay A, Madson MA (1986) Gas-liquid chromatography and mass spectral analysis of mono-, di- and tricarboxylates as their tert.-butyldimethylsilyl derivatives. J Chromatogr 361:117–130
Ali SL (1973) Simulaneous determination of acetylsalicyclic acid and salicyclic acid by gas-liquid chromatography using a new methylation technique. Chromatographia 6:478–480
Kuhn R, Baer HA (1953) Methylglncosid-bildung von N-acyl-glucosaminen mit diazomethan. Chem Ber 86:724–730
Kleiman R, Spencer GF, Earle FR (1969) Boron trifluoride as catalyst to prepare methyl esters from oils containing unusual acyl groups. Lipids 4:118–122
Brondz I, Olsen I (1992) Intra-injector formation of methyl esters from phenoxy acid pesticides. J Chromatogr 598:309–312
Hashimoto N, Aoyama T, Shioiri T (1981) New methods and reagents in organic synthesis. 14. A simple efficient preparation of methyl esters with trimethylsilyldiazomethane (TMSCHN2) and its application to gas chromatographic analysis of fatty acids. Chem Pharm Bull 29:1475–1478
Hoai PM, Tsunoi S, Ike M, Sei K, Lu X, Tanaka M, Fujita M (2006) Dicarboxylic degradation products of nonylphenol polyethoxylates. Determination and structural elucidation in water samples by solid-phase extraction and gas chromatography-mass spectrometry after methylation. J Chromatogr A 1103:125–132
Kawahara FK (1968) Microdetermination of pentafluorobenzyl ester derivatives of organic acids by means of electron capture gas chromatography. Anal Chem 40:2073–2075
Kage S, Kudo K, Ikeda H, Ikeda N (2004) Simultaneous determination of formate and acetate in whole blood and urine from humans using gas chromatography-mass spectrometry. J Chromatogr B 805:113–117
Hofmann U, Holzer S, Meese CO (1990) Pentafluorophenyldiazoalkanes as novel derivatization reagents for the determination of sensitive carboxylic acids by gas chromatography-negative-ion mass spectrometry. J Chromatogr 508:349–356
McLafferty FW (1956) Mass spectrometric analysis. Broad applicability to chemical research. Anal Chem 28:306–316
Acknowledgements
We thank Mr. Tagawa for conducting the first experiment. We also thank the Instrumental Analysis Center, Faculty of Engineering, Osaka University, for assistance with collecting the spectral data.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsunoi, S., Yamamoto, N., Yasuhisa, T. et al. 4-t-Butylbenzylation of carboxylic acid for GC–MS analysis. SN Appl. Sci. 2, 856 (2020). https://doi.org/10.1007/s42452-020-2646-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2646-y