Abstract
In this present research work two different organic compounds are applied for the modification of Indian origin bentonite. One is with n-hexadecyltrimethylammonium bromide (CTAB), intercalated with an interlayer of bentonite via cation exchange mechanism. Whereas, another with 3-Aminopropyl trimethoxysilane (APTES) interlayer functionalization with bentonite –OH group. APTES and CTAB–intercalated bentonites samples were further cross modified with CTAB and APTES to obtain novel co-surfactant locked organo bentonite modeling (CLOM) like matrices. Original and modified bentonite samples were comparatively evaluated by advanced characterization techniques such as, powder X-ray diffraction, Fourier-transform infrared spectroscopy, thermal gravimetric analysis (TGA). Moreover, the applicability of the developed CLOM like materials were investigated in nylon 6 nanocomposite preparation by melt compounding method using a micro twin co-rotated extruder. Additionally, CLOM-nylon-6 polymer nanocomposites were characterized by wide angle X-ray diffraction, TGA, differential scanning calorimetry, atomic force microscopy and tensile strength measurement. The observed thermograph results confirmed no significant difference in the thermal properties of the developed composites. Whereas, the significant variation observed in the tensile strength results particularly for developed composite 5% of N6-OAMSB and N6 AMOSB 5 showed 111.5 and 76.6% respective enhancement in tensile strength results when compared with a bare nylon-6 polymer.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The composting of pristine polymer with different additives material is a high interest area of research to obtain reinforcement, such as thermal and mechanical strength than original polymer. Still now many different filler materials are applied for the improvement of mechanical and thermal properties of polymers such as silica, carbon, graphene, carbon nanotubes, graphite, calcium carbonate, including different types of natural clay minerals [1, 2]. Since 1990 onwards natural and modified clay based polymer composite materials have been attracting research fields due to clay material as a naturally abundant low cost material with anomalous structural and textural properties features. The clay-polymer composites applications are dramatically increased after the first time development by Toyota automotive industry in their manufacturing process for the polyamide-6-clay nanocomposite application in timing belt to improve the thermal and mechanical properties than unmodified nylon 6 [3, 4].
Among different types of clay minerals, Bentonite is one of the highly abundant low cost material with high swelling, cation exchange, excellent plasticity and lubricity these important properties make bentonite commercially viable for different industry applications, such as, foundry sand binding, drilling mud, iron ore palletization and as a additives in polymer composite industries. After the USA and China, India is the number third country in the largest production of bentonite. The Indian bentonite chemical structure composition was already reported by our research group as well as several researchers [5,6,7]. Basically bentonite is 2:1 smectite phyllosilicate clay minerals in which two tetrahedral sheets of silicon tetrahedron sandwiched with an edge-shared octahedral sheet which made up aluminium or iron hydroxide in several layers [8]. Though still now India exports high quality raw grade bentonite but there is still need to find out the bentonite modification processes because the modified form of bentonite has higher demand in several industries than the crude or natural bentonite.
Therefore, almost from the last one decade, numerous efforts have been made for the enhancement of physical and mechanical properties of natural bentonite clay minerals by several modifications which subsequently useful to improve the quality of clay based polymer composites. In this regard, most of the researchers concentrated on achieving a high degree of exfoliation and a better dispersion of clay minerals with different polymer moieties. The increased interlayer gallery distance of the bentonite/montmorillonite allows easier transportation of polymer chains between the bentonite layers, so that bentonite platelets easily exfoliate and interfere with polymer molecules. Therefore to increase the interlayer gallery spacing, different types of organic surfactants are used for the modification of bentonite or other clay minerals. Mostly in the modification long carbon chain surfactants are found more efficient than the short-chain length surfactant [9]. Similarly silane moiety surface functionalization of bentonite also found effective for increasing the interlayer distance [10,11,12,13]. Recently several attempts have been reported for the improvement of mechanical and thermal stability of polymer composites by using thermally or structurally stable organo-bentonite in unmodified polymer such as nylon 6, nylon 66, polyvinyl alcohol, polypropylene, polyvinylchloride, butadiene, styrene polymer, polymethyl methacrylate etc., [14,15,16,17,18,19,20]. Recently novel approaches are reported for the 2:1 types of expandable clay minerals where modifications are reported using two organic modifiers in which already modified clay minerals again undergoes modification with surfactant or silane functional moieties to obtained co-surfactant locker system (CLOM) [21]. Recently Katia and coworkers functionalized saponite clay with the surfactant n-hexadecyltrimethylammonium bromide (CTAB) and/or with the alkoxysilane 3-aminopropyltriethoxysilane (APTS) and investigated how clay functionalization affected on the capacity of saponite to adsorb caffeine [22]. Also, Linna Su and coworkers reported Montmorillonite (Mt) modification by grafting of APTES onto the interlayer surfaces of Mt, and then swelling property of the silylated product was evaluated by intercalating of cetyltrimethylammonium bromide (CTAB). They also concluded that success of the intercalation of surfactant into the silylated Mt suggests that some bulk size target matters can be loaded into the silylated clays [23].
Considering the past research findings in the present study we modified Indian origin bentonite with CTAB and APTES and further tried to intercalate modified bentonite with cross modification with APTES and CTAB to increase the interlayer distance. In addition improved interlayered distance bentonite materials were applied in fabrication with nylon 6 by melt intercalating method to obtain novel clay-polymer composite with enhanced composite properties. Additionally for the first time we prepared the CLOM organo clay–nylon 6 clay nanocomposite via melt blending method using co-rotated-twin micro extruder and characterized with different analytical tools like wide angle X-ray diffraction (WAXD), thermal gravimetric analysis (TGA), differential scanning calorimetry (DSC), atomic force microscopy (AFM) and tensile strength measurements.
2 Experimental
2.1 Chemicals and materials
Nylon 6 (N6), thermoplastic polymer purchased from Sigma Aldrich, USA. 3-Aminopropyl trimethoxysilane (APTES) and n-hexadecyltrimethylammonium bromide (CTAB) were purchased from Sigma Aldrich, USA. The Indian Bentonite raw lumps are collected from the Kutch region bentonite mines in India.
2.2 Purification of raw bentonite
The natural Bentonite was purified by sedimentation technique [5]. The 2 wt% of the raw bentonite lumps was soaked for overnight in 10L of deionized water then subsequently kept for vigorous stirring for 4 h, after that bentonite suspension allowed it to settle overnight. The supernatant dispersion of particles < 2 μm particles size, calculated time 10 h at 30 °C according to stocks law of sedimentation dispersed with fine bentonite slurry layer was separated from the bottom impurity layer. The purified bentonite slurry again separated by centrifugation method. The obtained solid residue in the centrifuge process was collected and dried overnight at 80 °C, ground and passed through 200 mesh size sieves. The obtained purified bentonite material is designated as BMT and stored in packed vessels for further use. The cation exchange capacity of purified Bentonite was estimated as 85 meq/100 g of clay by known ammonium acetate method.
2.3 Silylation of bentonite by APTES
The surface hydroxyl group sialylation of purified bentonite was carried out by using 3-aminopropyl tri-methoxysilane [23]. In detail, 5 g of purified bentonite powder was dispersed in 50 ml of deionized water in a separate beaker and calculated amount of APTES (equivalent to calculated amount of total CEC) solution was prepared separately in 150 ml of methanol. Then APTES-methanol solution was dropwise added into a 50 ml of clay suspension mixture. After completion of addition APTES-bentonite slurry reaction mixture was kept for reflux with slow stirring at 65 °C for 12 h. After cooling the reaction solution was separated by centrifugation method and dried at 70 °C around 12 h. The obtained fine product was designated as AMSB.
2.4 CTAB intercalation in bentonite
CTAB intercalation in purified bentonite was carried out by cation exchange methodology as reported earlier [24]. In details, exactly 5 g of bentonite powder was dispersed in 200 ml of Milli-Q water, and calculated amount of CTAB on the basis of 2 times of bentonite CEC, was subsequently added in the bentonite suspension for complete interaction for 24 h, at room temperature. After completion of interaction reaction the mixture was separated by centrifugation, and thoroughly washed with milli Q water till suspension free from bromide ion from the solid product, tested with AgNO3. Finally, the obtained residue was dried at 70 °C and the grinded, dried material was sieved using 200 BSS mesh and defined as CTAB-BMT.
2.5 Synthesis of co-surfactant locked like organo-bentonite
The co-surfactant locked in the interlayer of bentonite was synthesized using two organic moieties namely CTAB and APTES. Both organic moieties were incorporated by cross modification approach [21,22,23]. Exactly 5 g of the AMSB sample dispersed in 200 ml of Milli Q water, and the same ideal process was followed to cross interaction with CTAB, washing and drying of material. The final obtained powder was designated as AMOSB. Similarly, 5 g of the CTAB-BMT again undergoes silylation reaction with APTES under identical conditions as mentioned in the silylation section above, and the obtained final product was designated as OAMSB.
2.6 Nylon 6-CLOM composite by twin micro extruder
The co-surfactant locked organically modified bentonite–nylon 6 nanocomposite was prepared by the melt blending method using twin micro co-rotator extruder. Approximately 3, 5 wt% of the co-surfactant locked organo clay was used for the nanocomposite preparation. The engineered polymer functionalized bentonite nano-composites were prepared at 4.6 g scale of polymer using DSM-Micro 5 twin-screw extruder and characterized for structural, mechanical and elongation strength variations. Nylon 6 pellets and modified bentonite were dried separately at 80 °C for overnight. The modified bentonite and polymer mixture was collected and fed into the extruder hopper. Before collecting the sample the Twin micro extruder machine programmed as 230 °C, 100 RPM, 3 min as a rotating time. The work was carried out using co-rotating intermeshing twin-screw extruder (Model: ZE-25, Krauss Maffei Berstorff GmbH, Germany; abbreviation: TSE). The TSE was maintained from about 240 °C of the feed zone and dried with N2 atmosphere. Simultaneously the prepared composite injection molded with a melting temperature of 220 °C, moldings temperature of 80 °C, a holding pressure of 500 kPa, Type V dog bone mold compliant with ASTM standard machine D638-10 [25]. 3–5% modified bentonite was used as filler for the N6-bentonite composite and denoted as N6 OAMSB 3, N6 OAMSB 5, N6 AMOSB 3, N6 AMOSB 5. TSE co-rotation mixing time and speed (RPM) and temperature all are summarized in Table 1.
3 Results and discussion
3.1 Material characterization’s
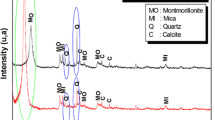
The PXRD analysis technique was utilized for the identification of natural and modified bentonite phases as shown in Fig. 1. The PXRD pattern of the natural bentonite showed characteristics 2:1 smectite bentonite phases at 2 theta values 6.51, 19.78, 35.35 and 61.90 respectively. The characteristics d (001) basal spacing of natural bentonite was observed around 13.87 Å. After CTAB intercalation in CTAB-BMT 2 theta value shifted towards a lower angle by increasing interlayer basal spacing around 19.87 Å. The obtained increased d spacing results were observed comparable with our previous report [26]. Similarly after APTES silylation basal spacing was also increased as 19.02 Å, results are also comparable with the previous report [22]. In case of the co-surfactant modified samples OAMSB basal spacing was increased up to 20.82 Å at 2θ 4.5°. Whereas, AMOSB basal spacing was increased to 21.04 Å at 2θ 4.20°. The increased d 001 basal spacing and reduced 2θ° valued for all modified products confirmed the successful intercalation of applied moieties in the interlayer as well interlayer surface hydroxyl groups of bentonite [22, 23, 27].
FT-IR spectroscopy measurement showed significant differences between the original and modified bentonite samples as shown in Fig. 2. The original Bentonite FT-IR spectra were found comparable with previous report [5]. In details purified bentonite showed a wide band at 3620 and 3698 cm−1 are due to –OH band stretching for Al–OH and Si–OH. The peak in the region of 1640 cm−1 is attributed to –OH bending mode of sorbed water. The peak at around 1115 cm−1 due to Si–O stretching (out of plane) and the peak at 1035 cm−1 attributed to the Si–O stretching plane (in plane). FT-IR peaks around 915, 875 and 836 cm−1 are confirming the presence of Al–Al–OH, Al–Fe–OH and Al–Mg–OH bending vibrations, respectively [5]. The APTES and CTAB modified bentonite samples showed characteristic’s additional spectra at 2937 and 3425 cm−1 and 1473 cm−1 due to presence of CH anti-symmetric and symmetric vibration of –C–H and –NH stretching vibrations caused by the CTAB/APTES. This spectrum intensity was observed to increase after intercalation with surfactant, due to the surfactant loaded on silylation and silylation grafting loaded on the surfactant modified BMT [22, 23].
Thermogravimetric analysis results of BMT, AMSB, CTAB-BMT, OAMSB, AMOSB are comparatively displayed in Fig. 3. In case of BMT gradual weight loss was observed below 120 °C, due to loss of physisorbed water [5]. Whereas in case of the modified bentonite samples the weight loss due physisorbed water was comparatively reduced than the BMT, confirms the increase in hydrophobic nature of organically modified bentonite samples. Whereas, in case of OAMSB and AMOSB characteristics, weight loss peaks observed in temperature range from 200 to 400 °C, and 400–600 °C due to elimination of intercalated organic moieties [22, 23]. In APTES samples distinct weight loss peaks observed at 350–600 °C respectively confirm the decomposition of chemically grafted moieties [22, 23].
3.2 Characterization of nylon 6-CLOM composites
3.2.1 WAXRD analysis
The exfoliated form of polymer nanocomposites has more strength due to the uniform distribution of modified bentonite into the whole polymer matrix. WAXRD of N6, N6 OAMSB 3, N6 OAMSB 5, N6 AMOSB 3, N6 AMOSB 5 nanocomposites are shown in Fig. 4. Organo bentonite polymer nanocomposite with exfoliated clay phases have better thermal and tensile behavior than pristine bentonite–polymer composites. The modified bentonite based co-surfactant locked bentonite was homogeneously distributed with nylon 6 via melt intercalating method. As observed results, in all the organo bentonite 001 plane was dissociated by the addition of N6 via melt blending method. The 001 plane of OAMSB and AMOSB were confirmed 20.01 and 21.04 Å and for the 001 plane of nanocomposite phase of N6 OAMSB 3 and N6 OAMSB 5 and N6 AMOSB 3 and N6 AMOSB 5 composite materials. The d 001 were completely disappeared confirmed the exfoliated of bentonite layers in polymer-organobentonite nanocomposites [11]. The PXRD results of all the nanocomposites are given in Table 2.
3.2.2 TGA and DTA analysis
The result of TGA and DTA of N6 with their 3 and 5% CLOM composite are presented in Fig. 5a, b. By the TGA result the N6 was decomposed at 440 °C. The N6 and all nanocomposite decomposition started at an above the temperature 250 °C and ending of whole residue of decomposition temperature is 550 °C. By the TGA result AMOSB only exhibited slightly higher stability temperature than N6, The thermal decomposition temperature of nylon 6 observed that Tmax = 440 °C. By the DTG curve N6 AMOSB-5 and N6 AMOSB-3 nanocomposite decomposition temperature found at Tmax = 442.85 °C and Tmax = 442.75 °C. Other N6 OAMSB 3 and N6 OAMSB 5 CLOM clay-N6 nano-composite exhibit slightly equal thermal stability with N6. The thermal stabilities of all the nanocomposites are given in Table 3.
3.2.3 DSC
The melting transition (Tm), Heat of fusion (ΔHm), crystallization temperature (Tc) can be measured. Each of the nylon 6 and nylon 6/modified bentonite nanocomposite samples was heated, cooled and reheated to obtain above mentioned analysis. An amorphous phase, semi-crystalline polyamide-6 has three main crystalline forms, the stable monoclinic α form, metastable pseudohexagonal β form, and the unstable monoclinic γ form. Here, non-isothermal studies of nylon 6 with nylon6/CLOM bentonite composites were performed. First, all the prepared samples were heated to 250 °C to melt and rapidly cooled to 30 °C [25]. Figure 6a, b shows the DSC curves of each nylon6/CLOM composite sample cooled from melt. Results show that the Tc of N6-CLOM composite was increased slightly compared to neat nylon 6. The melting temperature of CLOM and nylon 6 was nearly 196 °C. The crystallization temperature enthalpy was slightly increased in N6 AMOSB 5 compared to unmodified nylon 6. The crystallization temperature enthalpies of nylon 6 and N6 AMOSB 5 were observed as 57.55 and 59.99 J/g respectively. The enthalpy was increased compared with neat nylon 6 (N6) means that the CLOM clay intercalated with nylon 6 showed increases its crystallinity. All the Other CLOM bentonite nylon 6 nanocomposite enthalpies were decreased compared to nylon 6, which suggested the CLOM intercalated with nylon 6 and decreased its crystallinity [28].
After monitoring the crystallization temperature, it raises the heat up to 250 °C. The Tm of nylon 6 and nylon 6-aminoclay nanocomposite samples also nearly 220 °C. The Tm was similar to that of neat nylon 6. The melting temperature enthalpy of nylon 6 N6-and AMOSB 3 was observed as 57.55 and 71.94 J/g respectively. The nanocomposite enthalpy slightly increased compared with nylon 6. The lower temperature of the α-form is due to lower crystalline density and increased entropy of melting point indicated that the reduction in trans conformation bonding compared to the ɑ-form [29]. The polymer and composite melting temperature and crystallization temperature are given in Table 4.
3.2.4 AFM analysis
Figure 7 shows the surface morphologies of the nylon 6 nanocomposite. Nylon 6 CLOM clay nanocomposite of (a) nylon 6 (N6), (b) N6 OAMSB 3, (C) N6 OAMSB 5, (d) N6 AMOSB 3 and N6 AMOSB 5. 3 and 5 wt% of the CLOM clay was incorporated with nylon 6 by melt blending method. Surface modification helps, to cation exchange carried out with long chain surfactant even grafting with silane molecules increases the gallery spacing between the silicate layers. The increase in gallery spacing, to separate the stacked nano clay layers allowing for easier transport of polymer chains between the clay layers, by the way clay platelets exfoliated and interfered with polymer molecules. The roughness was significantly increased in the polymer molecules addition of CLOM organo bentonite in the PCN material. Significant differences observed among the N6 and with N6-CLOM organically modified bentonite composite. The result showed that the organoclay change significant changes in the surface morphologies of the nylon 6. The AFM result shows the average surface roughness of nylon 6 is 14.3 nm (without incorporation of the CLOM organo clay) changed to 24.1 nm for OAMSB 3, 17 nm for OAMSB 5, 18 nm for AMOSB 3, and 22.2 nm for AMOSB 5 corresponding to double surfactant modified organo clay respectively. This result obeyed that CLOM clay with nylon 6 nanocomposite shows better roughness, because of clay particles excellently homogeneous with nylon 6 by melt blending method. The Roughness mean square Sq value gradually increased from N6 to N6 AMOSB 5 and the result shown in Table 5. N6 (14.02.7 nm) N6 OAMSB 3 (27.7 nm), N6 OAMSB 5 (19.8 nm), N6 AMOSB 3 (20.4 nm), N6 AMOSB 5 (25.1 nm). The roughness mean square Sq value also increased after organo clay incorporated with N6 as shown in Table 5 [30,31,32,33].
3.2.5 Analysis of tensile strength
The tensile strength of N6 and CLOM organoclay nanocomposite are shown in Fig. 8. The results are also shown in Table 6. The thickness of all the nanocomposite material was 2.7 ± 0.03 mm and the width was 3.3 ± 0.05 mm, the testing specimen gauge length was 3.3 cm and specimen height 6.3 cm. After experiments the tensile modulus of N6 was 681.7 MPa and the tensile stress yield was 68.1 MPa. The tensile modulus was increased more than 50% after adding the CLOM clay with nylon 6. The 5% of the CLOM organobentonite filler showed better modulus strength than 3% of CLOM organobentonite nylon 6 nanocomposite and neat nylon 6 (N6). The tensile modulus of the 5% N6-OAMSB 5 and N6-AMOSB 5 clay platelets were 1442 and 1204 MPa respectively. The addition of 3% N6-OAMSB 3 and N6-AMOSB 3 filler nanocomposite tensile modulus was 983 and 1083 MPa respectively. In percentage it has been increased to 111.52 and 76.61% with respect to nylon 6. The 5% CLOM bentonite filler showed significant improvement compared with 3% of the CLOM clay on PCN material compared with 3% of the CLOM bentonite filler on the tensile property progress. The percentage of tensile modulus increased as 44.19 and 58.86% of N6-OAMSB 3 and N6-AMOSB 3 nanocomposite with respect to N6.
This mechanical test shows that increasing the CLOM bentonite concentration in the nylon 6 matrix improves the mechanical character. This CLOM clay fully exfoliated and dispersed with nylon 6 matrixes, and improved the tensile modulus. Depending on the wt% the tensile modulus was significantly increased to the neat nylon 6 matrix formation of composite [3, 4, 27, 34].
4 Conclusions
The present work shows a promising and prospective improvement of N6–CLOM clay filler based nanocomposite with better mechanical strength. The CLOM–modeling clay was prepared by the conventional method and chemical grafting method. The WAXRD reveals that the clay was exfoliated and homogeneously mixed with nylon 6 nanocomposite formation. An AFM result indicates that the roughness morphology of nylon 6 nanocomposite was significantly increased by CLOM clay mineral. The 5% of the CLOM clay mineral based nanocomposite showed good mechanical strength than the 3% based CLOM clay mineral nanocomposite.
References
Karamipour S, Ebadi-Dehaghani H, Ashouri D, Mousavian S (2011) Effect of nano-CaCO3 on rhelogical and dynamic mechanical properties of polypropylene: experiments and models. Polym Test 30:110–117
Fuad MYA, Hanim H, Zarina R, Mohd. Ishaka ZA, Hassan A (2010) Poly propylene/calcium carbonate nanocomposites—effects of processing techniques and maleated polypropylene compatibiliser. Polym Lett 4:611–620
Kojima Y, Usuki A, Kawasumi M, Okada A, Kurauchi J, Kamigaito O, Kaji K (1995) Novel preferred orientation in injection-molded nylon 6-clay hybrid. J Polym Sci 33:1039
Okada A, Fukushima Y, Kawasumi M, Inagaki S, Usuki A, Sugiyama S, Karauchi T, Kamigaito O (1988) Composite material and process for manufacturing same. US Patent, 4,739,007
Patel HA, Bajaj HC, Jasra RV (2007) Synthesis and characterization of organic bentonite using Gujarat and Rajasthan clays. Curr Sci 92:1004–1009
Pawar RR, Lalhmunsiama, Bajaj HC, Lee SM (2016) Activated bentonite as a low-cost adsorbent for the removal of Cu(II) and Pb(II) from aqueous solutions: batch and column studies. J Ind Eng Chem 34:213–223
Pawar RR, Gosai KA, Bhatt AS, Kumaresan S, Lee SM, Bajaj HC (2015) Clay catalysed rapid valorization of glycerol towards cyclic acetals and ketals. RSC Adv 5:83985–83996
Odom IE (1984) Smectite clay minerals properties and uses. R Soc Land 311:391–409
Pawar RR, Patel HA, Sethia G, Bajaj HC (2009) Selective adsorption of carbon dioxide over nitrogen on calcined synthetic hectorites with tailor-made porosity. Appl Clay Sci 46:109–113
Li S, Wu P, Li H, Zhu N, Li P, Wu J, Wang X, Dang Z (2010) Synthesis and characterization of organo-montmorillonite supported iron nanoparticles. Appl Clay Sci 50:330–336
Borpatra Gohain M, Pawar RR, Karki S, Hazarika A, Hazarika S, Ingole PG (2020) Development of thin film nanocomposite membrane incorporated with Mesoporous Synthetic Hectorite and MSH@UiO-66-NH2 nanoparticles for efficient targeted feeds separation, and antibacterial performance. J Membr Sci 609:118212
Asgari M, Abouelmagd A, Sundararaj U (2017) Silane functionalization of sodium montmorillonite nanoclay and its effect on rheological and mechanical properties of HDPE/clay nanocomposites. Appl Clay Sci 146:439–448
Ingole PG, Pawar RR, Baig MI, Jeon JD, Lee HK (2017) Thin film nanocomposite (TFN) hollow fiber membranes incorporated with functionalized acid-activated bentonite (ABn-NH) clay: towards enhancement of water vapor permeance and selectivity. J Mater Chem A 5:20947
Chen L, Phang IY, Wong SC, Lv PF, Liu T (2006) Embrittlement mechanisms of nylon 66/organoclay nanocomposites prepared by melt-compounding process. Mater Manuf Process 22:153–158
Chang JH, Jang TG, Ihn KJ, Lee WK, Sur GS (2003) Poly (Vinyl alcohol) nanocomposites with different clays: pristine clays and organo clays. J Appl Polym Sci 90:3208–3214
Normand G, Mija A, Pagnotta S, Peuvrel-Disdier E, Vergnes B (2017) Preparation of polypropylene nanocomposites by melt mixing comparison between three organ clays. J Appl Polym Sci 134:4503
Haiyan H, Mingwang P, Xiucuo L, Xudong S, Liucheng Z (2004) Preparation and characterization of poly (vinyl chloride)/organoclay nanocomposite by in situ, intercalation. Polym Int 53:225–231
Kim MS, Kim GH, Chowdhury SR (2007) Poly butadiene rubber/organoclay nanocomposites: effect of organoclay with various modifier concentrations on the vulcanization behavior and mechanical properties. Polym Eng Sci 47:308–313
Mauroy H, Plivelic TS, Suuronen JP, Hage FS, Fossum JO, Knudsen KD (2015) Anisotropic claypolystyrene nanocomposites: synthesis, characterization and mechanical properties. Appl Clay Sci 108:19–27
Realinho V, Velasco JI, Antunes M, Sánchez-Soto M, Maspoch ML (2010) Characterization of highly oriented organoclay/poly(methyl methacrylate) moulded nanocomposites. J Nanosci Nanotechnol 2:1304–1312
Khajehpour M, Gelves GA, Sundararaj U (2015) Modification of montmorillonite with alkl silanes and flurosurfactant for clay/fluorelstomer (FKM) nanocomposites. Clays Clay Miner 63:1–14
Marçal L, de Faria EH, Nassar EJ, Trujillano R, Martín N, Vicente MA, Rives V, Gil A, Korili SA, Ciuffi KJ (2015) Organically modified saponites: SAXS study of swelling and application in caffeine removal. ACS Appl Mater Interfaces 7:10853–10862
Su L, Tao Q, He H, Zhu J, Yuan P (2012) Locking effect: a novel insight in the silylation of montmorillonite surfaces. Mater Chem Phys 136:292–295
Alvi MU, Zulfiqar S, Yavuz CT, Kweon HS, Sarwar MI (2013) Influence of aminosilane coupling agent on aromatic polyamide/intercalated clay nanocomposites. Ind Eng Chem Res 52:6908–6915
Szabo A, Gournis D, Karakassides MA, Petridis D (1998) Clayaminopropyl siloxane compositions. Chem Mater 10:639–645
Bhatt AS, Sakaria PL, Vasudevan M, Pawar RR, Sudheesh N, Bajaj HC, Mody HM (2012) Adsorption of an anionic dye from aqueous medium by organo clays: equilibrium modeling, kinetic and thermodynamic. RSC Adv 2:8663–8671
González TV, Salazar CG, Rosa JRDL, Gonzalez VG (2008) Nylon 6/organoclay nanocomposites by extrusion. J Appl Polym Sci 108:2923–2933
Syed MA, Akhtar S, Syed AA, Siddaramaiah (2011) Studies on the physic-mechanical, thermal, and morphological behaviors of high density polyethylene/coleus spent green composites. J Appl Polym Sci 119:1889–1895
McAdam CP, Hudson NE, Liggat JJ, Pethrick RA (2008) Synthesis and characterization of nylon 6/clay nanocomposites prepared by ultrasonication and in situ polymerization. J Appl Polym Sci 108:2242–2251
de Sousa FDB, Scuracchio CH (2014) The use of atomic force microscopy as an important technique to analyze the dispersion of nanometric fillers and morphology in nanocomposites and polymer blends based on elastomers. Polimeros 24:661–672
Jose T, George SC, Maya MG, Maria HJ, Wilson R, Thomas S (2014) Effect of bentonite clay on the mechanical, thermal, and pervaporation performance of the poly (vinyl alcohol) nanocomposite membranes. Ind Eng Chem Res 53:16820–16831
Zhang J, Watson BA, Keown RW, Malone CP, Barteau MA (1996) Investigatin of sulfonated aromatic compound (SAC) modificatin to nylon film. 2. Study of SAC sorption isotherm and atomic force microscopic characterization of nylon surfaces. Langmuir 11:3018–3023
Li C, Xiang M, Zhao X, Ye L (2017) In situ synthesis of monomer casting nylon-6/graphene-polysiloxane nanocomposites: intercalation structure, synergistic reinforcing, and friction-reducing effect. ACS Appl Mater Interfaces 9:33176–33190
Rao YQ, Pochan JM (2007) Mechanics of polymer–clay nanocomposites. Macromolecules 40:290–296
Acknowledgements
This work was supported by the fund of CSIR–Delhi CSC-0135 Network project. SK is glad to thanks CSIR-CSMCRI and NCL Pune for supporting the analytical and instrumental facility to complete the work. PGI acknowledged the Department of Science and Technology under the Nano mission project DST/NM/NT/2018/143 (GPP-0357).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumaresan, S., Rokade, D.S., Marathe, Y.N. et al. Synthesis and characterization of nylon 6 polymer nanocomposite using organically modified Indian bentonite. SN Appl. Sci. 2, 1412 (2020). https://doi.org/10.1007/s42452-020-2579-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2579-5