Abstract
The effect of ozone and ozone plus a biological catalyst (catalase) on the degradation processes of Reactive Black 5 was investigated. The ozonation process was carried out in a laboratory-scale batch reactor with a volume of 500 ml. Chemical oxygen demand (COD), pH, color removal and the BOD5/COD ratio were measured during the process. At the first stage, only ozonation was performed, at the second stage, ozonation plus NaCl and Na2CO3, and at the third stage, ozonation plus catalase enzyme were used. As a result of the oxidation of Reactive Black 5 with only ozone and ozone plus textile auxiliaries, chemical oxygen demand (COD) removal was 34% and 19%, respectively. COD removal efficiency was 43% when 10 ml of catalase was added to the ozone reactor. Carbonate and salt have a negative effect on the oxidation of reactive Black 5 with ozone. Reactive Black 5 was degraded into sub-components, and the biodegradability of the wastewater increased. The pH value decreased from 3.3 down to 1.6, which showed that organic acids were formed as a result of degradation. The use of enzymes in combination with ozonation shows that Reactive Black5 can rapidly convert into the intermediate within the 270-min ozonation time (BOD5/COD = 0.46). COD removal was not as high as color removal efficiency. This may have resulted from the formation of colorless oxidation products. The significant increase in biodegradability after ozonation in the presence of the catalyst may also be seen as a reduction of toxic intermediates.
Similar content being viewed by others
1 Introduction
In the textile industry, wastewater is generally released from dyeing processes and preparation steps. The wastewater produced (about 100,000 synthetic dyes) contains variable components such as strong color, turbidity and inorganic salts [3]. Chemical oxidation is a capable method to easily convert contaminants into biodegradable compounds. However, biodegradable compounds that are not chemically oxidized may slow down the purification process [30]. Ozone is increasingly being used to remove low-biodegradability compounds. There have not been enough studies to determine the biodegradability of intermediates formed during ozone treatment. Textile wastewaters are wastewaters that are colored and biologically difficult to degrade which have various compositions of colorful dyes, surfactants and toxic chemicals [22]. Reactive azo dyes are characterized by the presence of one or more azo bonds (–N=N–) in relation to one or more aromatic systems [24]. In some studies, the UV/Vis spectroscopy method was used in the determination of the intermediate and final products formed at the end of the reaction [21].
Most ozone processes are performed in batch or semi-batch bubble columns [15]. The process of ozonation follows two different paths based on the pH. In acidic conditions, ozone directly reacts with organic compounds as an electrophile. As a result, aldehydes, carboxylic acids and other by-products are formed [4]. At alkaline pH, ozone disintegrates rapidly to form hydroxyl radicals and other radical species. In dyes, ozone usually attacks the conjugated double bonds that are a part of chromophores [1, 13, 16, 31]. In general, ozone oxidation involves direct ozone oxidation or free radical reaction. Since the oxidation potential of hydroxyl radicals is much higher than that of ozone molecules, direct oxidation is slower than radical oxidation [6].
As a result of the process of ozonation, the dye composition is broken up into aromatic amines (NH2), and organic acids are formed as a result of increased reaction time. By ozonation, the amines in the composition of the dye (NH2) are converted into nitrate. Sulfonic groups are turned into sulfate [35]. It has been shown in the literature that biodegradation of wastewater may be maximized with pretreatment doses in the range of 0.23–1.04 mg O3/mgCOD [2].
Some researchers have recommended the use of microbial enzymes (peroxidases, laccases and azo reductase) in decolorization [12, 20, 26]. Intermediate products resulting from removal of some dyes may be more toxic. As a result of color removal of Reactive Blue 19 and Reactive Black dye, D. Magna (16 toxicity factor) and V. Fischeri (32 toxicity factor) bacteria have the highest toxicity [5]. Catalase is an enzyme that catalyzes decomposition of hydrogen peroxide into oxygen and water, and it is present in all aerobic cells [32]. The catalase enzyme has been used as an important enzyme in many biotechnological fields including bioremediation. The pH range suitable for the catalase enzyme is from 4 to 11 [14].
The aim of this study was to evaluate the change in biodegradability of low BOD5/COD containing dye and determine the relationship between biodegradability, color change and final mineralization. The biodegradability of the ozonated Reactive black 5 dye was monitored by increases in the biological oxygen (BOD5) and BOD5/COD ratios.
2 Material and method
In order to investigate the effects of ozone and enzyme on COD and color removal from wastewater, Reactive Black 5 (non-biodegradable) was selected as the model compound. A schematic diagram of the laboratory-scale reactor is shown in Fig. 1. The experiments were conducted by putting 400 ml of samples into a glass reactor which is known as a 500 ml ozone gas washing bottle. In the experimental setup, there were 3 other gas washing bottles next to the reactor (Fig. 1). The bottle numbered 1 in Fig. 1 was the reactor where the dye solution was contained as the wastewater specimen, while the bottles numbered 2, 3 and 4 were gas washing bottles that were serially connected to the reactor with the purpose of catching the unreacted ozone and contained a 2% KI (potassium iodide) solution. Reactive Black 5 (C26H21N5Na4O9S6; Molecular weight: 991.82 g/mol, CAS number 17095-24-8) was purchased from Sigma-Aldrich (Turkey). The chemical structure of Reactive Black 5 is shown in Fig. 2. The other chemicals (KI, H2SO4,NaCl, Na2CO3) that were used in the experiments were also purchased from Sigma-Aldrich (Turkey). The Catalase enzyme was obtained from DyStar (CAS number 9001-05-2, 1–5%, 1.15–1.19 g/cm3, pH:5.1–5.4 EC: 45372010).
Ozone (2 g/h) was produced from the air by a laboratory scale ozone generator Triogen Model (Degremont Technologies). Ozone production, its homogenous distribution in water and facilitation of diffusional transition in the wastewater were supported by a KNF (D-79112) air compressor. All experiments were carried out at room temperature (19 ± 1 °C). The flow gauge of the ozone generator was set at 10 L/min. The ozone experiments were carried out at a dye concentration of 0.5 g/L and a reaction time of 4.5 h. The COD equivalent of 0.5 g/L of the dye solution was 417 mg/L. In the other experiments, auxiliary chemicals (NaCl and Na2CO3) and the catalase enzyme were used with ozone. In parallel with the textile sector, COD and color removal efficiency were investigated by ozonation. The absorbance values were converted to indices of transparency (RES) values. Color measurements were made by using the RES method at 3 different wavelengths (436 nm, 525 nm and 620 nm) [8].
In the first series of experiments, only the dye solution was used. No intervention was made on the pH value of the solution throughout the ozonation process. In the second set of experiments, with the purpose of obtaining the prototype of the dye bath, 10 g/L sodium chloride (NaCl) and 10 g/L sodium carbonate (Na2CO3) were added to the 0.5 g/L Reactive Black 5 solution. The pH was above 9 due to the alkalinity (Na2CO3) of the medium. In third series of experiments, ozonation was performed by adding 1.0- and 10.0-ml catalase enzyme to the number one bottle (reactor), respectively. Ozone and enzyme experiments were performed at pH = 6–7. The COD, BOD5 and color changes were observed by the ozone oxidation process.
The samples were collected in 15, 45, 60, 90, 120, 150, 180, 210, 240 and 270 min, respectively. No centrifugation or filtration process was applied in the color measurements of the samples. The color measurements were made by using the RES method at 3 different wavelengths [8]. The COD and BOD5 measurements were made in compliance with the Standard Methods [27]. Five-day oxygen consumption by microorganisms is called BOD5. COD represents the amount of oxygen required to completely convert organic matter into final products. The BOD5/COD ratio is a measure of the biodegradability of organic matter. The pH was adjusted with H2SO4 and NaOH by using a WTW340 pH meter. The residual concentration of Reactive Black 5 was measured by using a Hach Lange DR2800 spectrophotometer. The absorbance values of the samples at 436 nm, 525 nm and 620 nm wavelengths were measured by the multiple absorbance method selected in the spectrophotometer. RES values were calculated with the formula given in Eq. (1). Color removal efficiency [E = RESo − RESe/RESo] was calculated from the influent (RESo) and effluent RESe values.

A: Absorbance of water sample at λ wavelength, d: Bathtub thickness (25.4 mm), f: Factor for obtaining Spectral Absorption in m−1, f = 1000, RES (λ): Spectral absorption coefficient (m−1).
3 Results and discussion
Color removal efficiencies (Figs. 3, 4 and 5) were calculated from the absorbance and RES values (not shown) at all three wavelengths (436 nm, 525 nm, 620 nm) depending on the ozonation time. The Reactive Black 5 molecule was oxidized by ozonation to a small organic molecular structure, based on high color removal efficiency and partial COD removal efficiency. In this study, organic matter was not defined depending on wavelengths. The final product conversion was defined based on the amount of organic matter (COD removal) removed from the medium. The reduction in chemical oxygen demand (417 to 285) showed a partial oxidation. As shown in Fig. 6, the COD removal efficiency obtained by ozonation of the Reactive Black 5 for 240 min was 32%. At the end of the reaction, the influent COD decreased from 417 to 285 mg/L. Additionally, Fig. 6 shows the effects of ozonation on biodegradability (BOD5/COD) and pH. With the ozone, the biodegradability (BOD5/COD) of the dye increased from 0.06 to 0.41. When this ratio is in the range 0.40–0.5, the wastewater can be biodegradable [18]. After four and a half hours of ozonation, the dye turns into intermediate products, which constitutes an important COD. As previously determined by several researchers, it was found that this increase (BOD5/COD) was caused by new organic substances (acetic acid, aldehydes, ketones) that could not be completely degraded as a result of the reaction of azo dyes with ozone [7, 9, 34]. Many researchers determined that the decreases in the pH values were caused by the outcome that the by-products that are released after ozonation were in the form of acid anions [19, 28, 36]. Nitrogen, carbon and oxygen and sulfur are present in the structure of the paint (Fig. 2). COD reduction (417–285 = 132 mg) proved that the final product was formed.
Without interfering with the pH of the solution, the initial pH:3.3 was reduced to 1.7 with the ozonation time. In a study conducted by Perez et al. [21] pH decreased due to the formation of organic acid as a result of oxidation (Ozone) of wastewater containing Reactive Black 5. In the light of all these results, it was determined that Reactive Black 5 was converted into by-products as a result of degradation by ozone, and its biodegradability increased. Considering the color removal value (> 95%) and BOD5/COD ratios (increase from 0.06 to 0.41) at low pH, it may be concluded that the C=C and N=N bonds in the structure of the dye were severed as a result of ozonation, and organic substances with fewer chains were formed. The COD concentration could not be further reduced by the formation of organic compounds which were not degraded by ozonation. Reactive Black 5 was first converted into some carboxylic acid intermediates. The main intermediates in the degradation of Reactive Black 5 are formic acid and oxalic acid. Oxidation of oxalic acid is much more difficult in comparison to other organic acids [10]. The highest increase in the BOD5/COD ratio occurred after 150 min. The BOD5/COD ratio was 0.2, 0.28, 0.34, 0.38 and 0.40 after 120, 150, 180, 210 and 240 min, respectively. After 240 min, the Reactive Black 5 was transformed from a non-degradable form into degradable products. The compounds formed by ozone oxidation are simpler in structure, and most are organic acids [21]. Similar results were reported by Suryawan et al. (2018) by using ozone oxidation in treating Reactive Black 5 [29].
The reduction in color with increasing ozone dosage is shown for Reactive Black 5 in Fig. 3. Reduction in absorbance and RES values (data not shown) at low and high wavelengths (RES 436, RES 525, RES 620) showed discoloration by cleavage of dye chromophores. Disruption of the parent compound aromatic rings was associated with reduced absorbance at UV wavelengths [2]. It was found that increasing ozone dose was associated with an increase in color removal. Color changes were low at ozonation times of 15, 45 and 60 min. At short ozonation times, color remains due to the complexity of the intermediates and the nature of the dye. The BOD5/COD measurements that were made in parallel to the color change showed that the BOD5 value also increased. In parallel to the findings in the literature [37], the decrease in the pH in this study at the end of the reaction time was caused by the formation of organic acids as a result of degradation of complex organic compounds by ozone. Simpler intermediates are formed in ozone reactions [21]. Most intermediate products obtained as a result of ozonation in the study conducted by Perez et al. [21] were composed of organic acids and less toxic substances. At all wavelengths, color removal efficiency was over 80% at the end of 150 min of ozonation. The BOD5/COD ratio within the same time interval was found as 0.28. The color removal efficiencies of Reactive Black 5 at the end of ozonation for 4.5 h were 95.21, 99.17 and 99.13% in RES-436 (Yellow), RES-525 (Red) and RES-620 (Blue), respectively. The color removal was higher starting with the 90th min. Although decolorization was sufficient with only ozonation, COD removal efficiency was limited. Ozone first reacts with the chromophore group of dyes. Therefore, color removal was fast [21, 29]. As a result of the ozonation process, the black dye solution turned into a light orange color. At the beginning of the ozonation process, dye removal efficiencies at the 436 and 525 nm wavelengths were quite low. Low and negative dye removal efficiencies are thought to result from the different absorption rates of intermediate products formed by oxidation of the dye. Association or dissociation of molecules also causes deviation in reading the correct absorbance value. The measurement values are within the limits of ± 5% deviation. The absorption and RES values (not shown in article) of all wavelengths decreased with ozonation time. At the beginning of ozonation at the 620 nm wavelength, the absorption values of the Reactive Black 5 could not be read on the instrument. Deviations in absorbance values were detected in the first minutes of oxidation. It was thought to be caused by the dye intermediate structure rather than instrument sensibility.
Salt and carbonate, which are some of the most commonly used auxiliary chemicals in the textile sector, were included in this study. Figure 7 shows the COD, BOD5/COD and color changes of the solution containing the dye and additional chemicals depending on the time of ozonation. The ozonation of Reactive Black 5 and auxiliary chemicals provided a limited rate of COD removal. When wastewater pH increases, the ozone stability in the wastewater decreases, and the amount of ozone present in the reaction decreases [23]. When the ozone time was increased to 270 min, the COD removal efficiency was found to be 19%. The ozone time increased the BOD5/COD ratio of Reactive Black 5. As a result of 60 min of reaction time, the BOD5/COD ratio was found as 0.22. High color removal and high BOD5/COD ratio indicated that Reactive Black 5 was converted to a limited extent to oxidation end products (CO2, H2O and NO3−). High color removal showed that the chromophores in the dye deteriorated. In this experiment, the COD removal efficiency of 18% indicated that the conversion of the dye into the final products was limited. The change in the BOD5/COD ratio from 0.056 to 0.38 indicated that this dye was converted into partially degradable substances. The presence of NaCl and Na2CO3 in the medium has a negative effect on the oxidation of Reactive Black 5. Ozonation of dyes usually results in COD by separating the dye into simple pieces [25]. In this series of experiments, the pH value was greater than 9.0 due to the alkaline environment (in the presence of carbonate). At alkaline pH, ozone was turned into.OH and other radical species by rapidly disintegrating [31]. The mineralization of Reactive Black 5 had a low rate (ECOD = 19%) due to the presence of salts and carbonates. The ozonation time of four and a half hours was not sufficient for the biodegradability of the dye. In the current literature, if the BOD5/COD ratio was between 0.2 and 0.4, the wastewater was slowly biodegradable [18]. Compared to Figs. 6 and 7, the BOD5/COD ratio decreased by 5.3% after 240 min of ozonation in the presence of salt and carbonate.
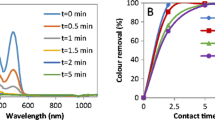
The effect of 10 g/L NaCl and 10 g/L Na2CO3 on the ozone reaction rate for decolorization was investigated, and the results are shown in Fig. 4. The time required for decolorization was associated with auxiliary chemicals and enzyme concentration along with ozone concentration. Hydroxyl and other radicals are effective instead of ozone at high pH values [21]. As a result of 45 min of reaction time at the wavelength of 525 nm, there was a 76% color removal rate. Within the same reaction time, the color removal rates at the wavelengths of 436 and 620 nm were 58.13% and 71.12%, respectively. As a result of 90 min of reaction time, it was observed that the pigments that formed the color in the dye were degraded by > 75% and converted into by-products (BOD5/COD = 0.26). The color removal efficiency values at the wavelengths of RES-436, RES-525 and RES-620 were found as 87.57%, 95.26% and 95.29%, respectively.
It was determined that the by-products of the reaction were not converted into final products, and therefore, the COD removal efficiency was low (19%). Perez et al. [21] found that, as a result of the reaction between RB5 and ozone, short-chain organic acid was formed as the final product. Due to their refractory characteristics, these dyes are resistant to biodegradation. However, the yield of color decomposition with salt and carbonate was found to be faster and higher than that of ozone alone. The presence of carbonate in the medium delayed the conversion of the dye into final oxidation products. Moreover, some researchers reported that oxidation processes affected the degradation and removal of dyes negatively in the presence of salts. High concentrations of salt (40 g/L) decrease the solubility of ozone [25]. In the oxidation of dye-containing wastewater with ozone, a high salt concentration decreases color removal, while a lower salt concentration results in faster color removal [17]. The results showed that an increase in BOD5/COD of the wastewater was in parallel to the color removal. The color of the solution containing the Reactive Black 5 with the ozonation time changed to yellow and orange tones. Orange and yellow color formation occurred at the end of the reaction. Because of the different absorbability values of the intermediate products and variability, it was thought that the color removal efficiency at the beginning of the ozonation process created a negative result.
The catalase enzyme (12, 14, 20) has been used in textile products to reduce the surface roughness. Its use varies depending on the textile product profile. Therefore, the catalase enzyme was selected in this study. Reactive Black 5’s ozonation by adding the catalase enzyme in different volumes (1 ml/400 ml and 10 ml/400 ml) was performed in the batch reactor with a constant ozone flow rate of 2 g/h. A constant ozone dose (2 g/h) was used to see the effect of the enzyme (Fig. 8). The COD concentration of Reactive Black 5 may be reduced to a certain degree, and it is very difficult to reduce further COD by only ozonation. Reactive Black 5 contains plain and unsaturated bonds which are separated by ozone plus the catalase enzyme. The oxidation of unsaturated bonds results in a decrease in the effluent COD concentration. When 1.0 and 10.0 ml of catalase were added to the reactor (influent COD = 417 mg/L), the effluent COD value decreased to 260 and 245 mg/L, respectively. When the enzyme concentration increased from 1.0 to 10 ml, the COD removal efficiency increased by 5%. When the enzyme was used as a catalyst, ozonation showed the highest efficiency as it provided more than 95% color removal in 270 min with 41% COD reduction. A combined treatment of dye-contaminated wastewater (ozone/white rot fungi) resulted in a reduction in toxicity by more than 70% [33]. As a result of the combination of ozone oxidation and enzyme processes, it was determined that the ratio of biodegradable material increased, and a high color removal occurred.
The pre-reaction biodegradability of the Reactive Black 5 was very low (0.056), so it was not biodegradable. The biodegradability of Reactive Black 5 was found to be above 0.46 as a result of the enzyme plus ozonation process. As this ratio (BOD5/COD) is close to 0.5, wastewater can be easily biodegradable [18]. In the study by Alvares et al., the oxidation of Reactive Black 5 with ozone increased the biodegradability of hydrolyzed Reactive Black 5. The optimal dose requirement for the maximum biodegradability increase was 1.8 g/l, which increased the BOD/TOC and BOD/COD ratios from 0 to 0.58 and 0.27, respectively [2]. The decrease of the effluent COD and the increase of the effluent BOD5 at the end of the reaction indicated that the dye was degraded to lower molecular weight compounds and final products. There was no significant difference in COD and color removal in the 1.0 ml and 10.0 ml enzyme applications. Biodegradability increased when ozone was used in combination with the enzyme. When the enzyme concentration was increased from 1 to 10 ml, a 4.4% increase in the BOD5/COD ratio was found after 270 min.
Ozonation by itself was not an effective method due to low COD removal and by-product formation. To increase ozone efficiency, adding a catalyst provides increased activity. BOD5 and discoloration increased because the non-degradable compound was converted into more biodegradable compounds after treatment with ozone plus enzyme. Increasing the BOD5/COD ratio of the Reactive Black 5 from 0.06 to 0.46 showed that the intermediate products were transformed into biodegradable products. Similar to this study, enzymes have been used for color removal in the current literature [12, 20, 26]. It was stated by Venkatesh et al. [35] that organic acids are formed as a result of degradation of dyes by ozone. A reaction of Reactive Black 5 and ozone results in formation of short chain organic acids [21]. As shown in Fig. 5, when the ozonation time was increased from 50 to 270 min, the color removal rate increased significantly. The color removal efficiency at the 10 ml enzyme concentration was greater than 95%. Addition of enzyme to the ozone reactor increased the color removal efficiency and speed. Color removal was fast for the first 150 min, and then, the rate of decolorization was greatly reduced. At all wavelengths, color removal efficiency was over 90% at the end of 200 min of ozonation. At the 50- and 270-min reaction times and at the 620 nm wavelength, decolorization efficiency was 57% and 99.32%, respectively. The efficiencies at the RES-436, RES-525 and RES-620 wavelengths were found as 96.99%, 99.0% and 99.32%, respectively. It was found that the BOD5/COD ratio of the Reactive Black 5 increased in parallel with color removal. The oxidation of dye molecules with ozone will oxidize the double bonds of the chromophore group [35]. Enzyme use increased color removal efficiency at all wavelengths. As a result of this study, color formations of orange and its tones were realized. Color readings could not be performed during the first 45 min of ozonation at 620 nm. Deviations in absorbance values were detected in the first minutes of oxidation.
4 Conclusion
The biodegradability of the dye examined before and after the ozone treatment was measured by the BOD5/COD ratio. The oxygen uptake rate, specific oxygen uptake rate and BOD5/COD ratio parameters were used in the biodegradability experiments. This study showed that ozone and the biological catalyst improved the color removal and biodegradability of Reactive Black 5. Oxidation of Reactive Black 5 with enzyme plus ozone was more effective in terms of the biodegradability of the Reactive dye. When the ozone plus enzyme was used, the efficiency of COD removal and decolorization were higher than the use of ozone and ozone plus auxiliary chemicals (NaCl and Na2CO3). As a result of ozonation of the Reactive Black 5 at low pH, the BOD5/COD ratio increased from 0.06 to 0.41. This value decreased down to 0.38 in the case that NaCl and Na2CO3 were added to the medium. The BOD5/COD ratio increased to 0.46 when the enzyme was used. A BOD5/COD ratio of less than 0.3 corresponds to the low biodegradability of organic material in wastewater [11]. The initial BOD/COD value of the dye was close to zero. With the use of ozone and enzyme, this value has increased above 0.4. This indicates that the paint was disintegrating. When 1 ml and 10 ml enzymes were used together with ozone, the BOD5/COD ratio increased by 13.6% and 17.4%, respectively. Reactive Black 5 has turned into a readily decomposable form after 150 min for enzyme plus ozonation conditions. In the case of ozone alone, after 270 min, it became easily decomposable. The combined use of ozone and the catalase enzyme increased the color removal. As a result, it was concluded that ozonation was an effective process for color removal, but it was not an effective method for removing the whole COD from wastewater. The combination of ozone plus enzyme disrupts the chemical structure of Reactive Black 5. Further purification was needed for the removal of intermediate products that were formed.
References
Abidin A, Fahmi SA, Mohd Makhtar SNN, Rahmat NR, Ahmad R (2016) Decolourization and cod reduction of textile wastewater by ozonation in combination with biological treatment. Int J Automot Mech Eng (IJAME) 13(1):3141–3149
Alvares ABC, Diaper C, Parsons SA (2001) Partial oxidation of hydrolysed and unhdrolysed textile azo dyes by ozone and the effect on biodegradability. Trans I Chem E 79(2):103–108
Asgari G, Faradmal J, Nasab HZ, Ehsani H (2019) Catalytic ozonation of industrial textile wastewater using modified C-doped MgO eggshell membrane powder. Adv Powder Technol 30:1297–1311
Asghar A, Raman AAA, Daud WMAW (2015) Advanced oxidation processes for in situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: a review. J Clean Prod 87:826–838
Baumer JD, Valerio A, Selene MA, Souza GU, Erzinger GS, Furigo A, Souza AAU (2018) Toxicity of enzymatically decolored textile dyes solution by horseradish peroxidase. J Hazard Mater 360:82–88
Chu LB, Xing XH, Yu AF, Sun XL, Jurcik B (2008) Enhanced treatment of practical textile wastewater by microbubble ozonation. Process Saf Environ Prot 86(5):389–393
Constapel M, Schellentriager M, Marzinkowski JM, Gab S (2009) Degradation of reactive dyes in wastewater from the textile industry by ozone: analysis of the products by accurate masses. Water Res 43(3):733–743
DIN 38 404 (1991) German Methods fort he Examination of Water, Wastewater and sludge, physical and physical-chemical parameters, Determination of Colour, Deutsches Institut fur Normung e.v., Berlin, pp 1–7
Fahmi MR, Abidin CZA, Rahmat NR (2011) Characteristic of colour and COD removal of azo dye by advanced oxidation process and biological treatment. In: International conference on biotechnology and environment management. IPCBEE vol 18, pp 13–18
Huang YH, Huang YF, Chang PS, Chen CY (2008) Comparative study of oxidation of dye-Reactive Black B by different advanced oxidation processes: Fenton, electro-Fenton and photo-Fenton. J Hazard Mater 154(1–3):655–662
Jamil TS, Ghaly MY, El-Seesy IE, Souya ER, Nasr RA (2011) A comparative study among different photochemical oxidation processes to enhance the biodegradability of paper mill wastewater. J Hazard Mater 185:353–358
Kalyani DC, Phugare SS, Shedbalkar UU, Jadhav JP (2011) Purification and characterization of a bacterial peroxidase from the isolated strain Pseudomonas sp. SUK1 and its application for textile dye decolourization. Ann Microbiol 61(3):483–491
Kasprzyk-Hordern B, Ziolek M, Nawrocki J (2003) Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl Catal B Environ 46(4):639–669
Kaushal J, Mehandia S, Singh G, Raina A, Arya SK (2018) Catalase enzyme: application in bioremediation and food industry. Biocatal Agric Biotechnol 16:192–199
Khuntia S, Majumder SK, Ghosh P (2016) Catalytic ozonation of dye in a microbuble system: hydroxyl radical contrubition and effect of salt. J Environ Chem Eng 4:2250–2258
Lopez-Lopez A, Pic JS, Debellefontaine H (2007) Ozonation of azo dye in a semi-batch reactor: a determination of the molecular and radical contributions. Chemosphere 66:2120–2126
Muthukumar M, Sargunamani D, Selvakumar N, Venkata Rao J (2004) Optimisation of ozone treatment for colour and COD removal of acid dye effluent using central composite design experiment. Dyes Pigm 63(2):127–134
Nagwekar PR (2014) Removal of Organic Matter from Wastewater by Activated Sludge Process–Review. Int J Sci Eng Technol Res (IJSETR) 3(5):1260–1263
Neamtu M, Yediler A, Siminiceanu I, Macoveanu M, Kettrup A (2004) Decolorization of disperse red azo dye in water by several oxidation processes a comparative study. Dyes Pigm 60:61–68
Parshetti GK, Parshetti S, Kalyani DC, Doong R, Govindwar SP (2012) Industrial dye decolorizing lignin peroxidase from Kocuria rosea MTCC 1532. Ann Microbiol 62:217–223
Perez A, Rodriguez JL, Galicia A, Chairez I, Poznyak T (2019) Recycling strategy for water contaminated with Reactive Black 5 in the presence of additives treated by simple ozonation. Ozone Sci Eng 41(1):46–59
Polat D, Balcı I, Özbelge TA (2015) Catalytic ozonation of an industrial textile wastewater in a heterogeneous continuous reactor. J Environ Chem Eng 3:1860–1871
Shaikh IA, Ahmed F, Sahito AR, Pathan AA (2014) In-situ decolorization of residual dye effluent in textile jet dyeing machine by ozone. Pak J Anal Environ Chem 15(2):71–76
Shen Y, Han S, Xu Q, Wang Y, Xu Z, Zhaoa B, Zhang R (2016) Optimizing degradation of Reactive Yellow 176 by dielectric barrier discharge plasma combined with TiO2 nano-particles prepared using response surface methodology. J Taiwan Inst Chem Eng 60:302–312
Silva AC, Pic JS, Sant’Anna GL, Dezotti M (2009) Ozonation of azo dyes (Orange II and Acid Red 27) in saline media. J Hazard Mater 169(1–3):965–971
Singh RL, Singh PK, Singh RP (2015) Enzymatic decolorization and degradation of azo dyes—a review. Int Biodeterior Biodegrad 104:21–31
Standard Methods for the Examination of Water and Wastewater (1995) 18th Edition, Washington Dc, USA
Strickland AF, Perkins WS (1995) Decolorization of continious dyeing wastewater by ozonation. Text Chem Color 27(5):11–15
Suryawan IWK, Helmy Q, Notodarmojo S (2018). Textile wastewater treatment: colour and COD removal of reactive black 5 by ozonation. In: The 4th international seminar on sustainable urban development, IOP conference series: earth and environmental science, vol 106, pp 1–6
Tabrizi GB, Mehrvar M (2004) Integration of advanced oxidation technologies and biological processes: recent developments, trends, and advances. J Environ Sci Health Part A 39:3029–3081
Turhan K, Durukan I, Ozturkcan SA, Turgut Z (2012) Decolorization of textile basic dye in aqueous solution by ozone. Dyes Pigm 92(3):897–901
Vainshtein BK, Melik-Adamyan WR, Barynin VV, Vagin AA, Grebenko AI (1981) Three-dimensional structure of the enzyme catalase. Nature 293:411–412
Vanhulle S, Trovaslet M, Enaud E, Lucas M, Taghavi S, Vander Lelie D, VanAken B, Foret M, Onderwater RCA, Wesenberg D, Agathos SN, Schneider YJ, Corbisier AM (2008) Decolorization, cytotoxicity, and genotoxicity reduction during a combined ozonation/fungal treatment of dye-contaminated wastewater. Environ Sci Tecnol 42(2):584–589
Venkatesh S, Quaff AR, Pandey ND, Venkatesh K (2015) Impact of ozonation on decolourization and mineralization of azo dyes: biodegradability enhancement, by-products formation, required energy and cost. Ozone Sci Eng 37(5):420–430
Venkatesh S, Venkatesh K, Quaff AR (2017) Dye decomposition by combined ozonation and anaerobic treatment: cost effective technology. J Appl Res Technol 15(4):340–345
Zhang F, Yediler A, Liang X, Kettrup A (2004) Effects of Dye Additives on the ozonation process and oxidation by products: a comparative study using hydrolyzed CI Reactive Red 120. Dyes Pigm 60(1):1–7
Zhou H, Smith DW (1997) Process parameter development for ozonation of Kraft pulp mill effluents. Water Sci Technol 35(2–3):251–259
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dinçer, A.R. Increasing BOD5/COD ratio of non-biodegradable compound (reactive black 5) with ozone and catalase enzyme combination. SN Appl. Sci. 2, 736 (2020). https://doi.org/10.1007/s42452-020-2557-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2557-y