Abstract
TiO2 has a wide range of applications since it has the best photocatalytic activity. Its photocatalytic activity can be enhanced by doping with suitable elements. Hydrothermal method and sol–gel synthesis are mainly considered for the preparation of doped TiO2 nanoparticles. Modern characterization techniques are used to study the doped material. The main contribution of doping is enhanced photocatalytic activity by shifting the absorption edge towards the visible region. Here we had made a review on the synthesis procedure, characterization and photocatalytic activity of the Sc, Hf doped, and co-doped TiO2 nanocrystals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Titanium(IV)oxide, semiconductor photocatalyst, also known as Titania, is the oxide of titanium, which is naturally occurring. It has a vast range of applications, and mainly includes photocatalysis, sensors, solar cells, and memory devices. Better chemical stability, low expenditure, undemanding availability, and best photocatalytic activity make TiO2 the finest one [1]. When comparison is done, the dye sensitized solar cells have low cost and they have large photon to electron conversion efficiency than normal cells [2]. TiO2 has three main polymorphs; rutile (tetragonal arrangement having density = 4.25 × 10−6 g/cm3), anatase (tetragonal arrangement having density 3.89 × 10−6 g/cm3) and brookite (orthorhombic arrangement having density 4.12 × 10−6 g/cm3) [3]. Rutile is mainly available as single crystals more than anatase, and also rutile is thermally stable. Hence at higher temperatures, anatase is transformed into rutile. This property of anatase is considered in the photocatalytic activity and sensors. Among the three phases, anatase has the highest photocatalytic activity, and it has a very important role in the decomposition of organic compounds when they are illuminated [1]. Even though TiO2 is the best photocatalyst, it has certain drawbacks, which hinder the photocatalytic activity. It mainly includes the large band gap (about 3.2 eV) the characteristic absorption of TiO2 in the U.V region of the spectrum, and it is ineffective for photocatalysis and fast rejoining of photo-induced electron–hole pairs which are produced on the surface of the crystal [4].
Numerous attempts have been made to reduce these drawbacks and improve the photocatalytic activity under visible light irradiation. The essential method is introducing foreign particles to the TiO2 system, called doping. Normally metals or non-metals are used for this purpose. The metal doping is proved as a very useful method because it introduces a new energy level within the band gap. Due to this, the excitation of electrons from the valence band to the conduction band becomes effortless. The average lifetime of the produced electron–hole pairs can be increased with metal doping and also leads to the shifting of absorption band towards the visible region. The consequences of Sc and Hf doping on TiO2 nanoparticles is considering here. Sc doped TiO2 is better than undoped TiO2 for the degradation of organic compounds because Sc doping increases the absorption of organic compounds, separation of electron–hole pairs, and the thermal stability of TiO2. Hf doped TiO2 improves the photocatalytic activity of TiO2 by the enhanced absorption and formation of the electron–hole pairs. Doping with Hf also shifts the absorption maxima to the visible region, which increases the photocatalytic activity [5].
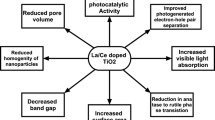
Transformation of anatase to rutile can be prevented by adding different types of dopants. Scandium is a major rare earth metal used for the purpose because it enhances the stability in terms of thermally in case of anatase phase whose photocatalytic activity is better than that of rutile phase [1]. The absorption of TiO2 nanoparticle can be shifted to the visible region by doping with non-metals, and the band gap become shorter, even though doping with metal ion will reduce the electron–hole pair rejoining by locking them temporarily. Therefore TiO2 can be modified by co-doping. The metal ion present in the TiO2 may alter its properties and enhances the photocatalytic activity based on the metal [6]. When Sc or Hf is incorporated into TiO2, the variations in the properties are summarized in the Fig. 1. This article reviews the synthetic process, various characters and photocatalytic efficiency of Sc, Hf doped and co-doped Titanium dioxide.
2 Synthesis
The common methods used for the synthetic process of Sc/Hf doped TiO2 are (1) sol–gel method and (2) hydrothermal method.
2.1 Sol–gel method
The sol–gel method involves the formation of a colloidal suspension or sol and followed by a three-dimensional network structure in a continuous liquid phase called gel. The method mainly includes hydrolysis, condensation, and drying. The product thus formed is converted into Xerogel (aerogel) on the basis of drying. The sol–gel method can be divided into two types on the basis of solvent used, which are aqueous and non-aqueous sol–gel. Figure 2.
The sol–gel method is an important method used for the synthesizing Sc/Hf doped nanoparticles. The reasons for the selection of this method are, it is simple, cost-effective, and the low temperature used, high purity of synthesized crystals, homogeneity, and controlled morphology. The steps involved in this method are shown in Fig. 3.
In the method, a sufficient amount of titanium precursor normally tetrabutoxytitanium (TBOT) was mixed and dissolved in absolute ethanol. To this, a solution of deionized water, ethanol, and concentrated HCl are added. Scandium nitrate or hafnium nitrate is added to the sol to obtain a colloidal suspension. The obtained solution is stirred well and aged to obtain a three-dimensional network, called a gel, and is dried in vacuum. It is then calcinated at a temperature 400–800 °C [7]. Various states involved in the sol–gel method of TiO2 nanocrystals are summarized in Table 1. The same method can be used for the synthesis of Sc and Hf co-doped TiO2 nanoparticles.
2.2 Hydrothermal method
It is another type of method which is used for the synthesis of nanoparticles. The reactants are taken in an autoclave and filled it with water. The reaction is taking place at high temperature and pressure conditions. Teflon coated autoclaves are generally used. It is resistant to the action of hydrochloric acid than the autoclaves made with glass and quartz. The hydrothermal method is also used to prepare the Sc, Hf doped, and co-doped nanocrystals [8]. The schematic diagram of the hydrothermal method is shown in Fig. 4.
3 Characterization of nanocrystals
UV–Visible, X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), Fourier transform infra red spectroscopy (FTIR), Tunnelling electron microscope(TEM), Brunaeur-Emmet-Teller surface area analysis(BET), and Photo-luminescence(PL) are used to explore the characteristic features nanocrystal of nanocrystals.
The crystalline dimension of each phase, as well as anatase to rutile ratio and crystal phase identification, all are done by X-ray diffraction spectroscopy (XRD). In the XRD of TiO2, the peaks observed at 2θ = 25.25° (101) and 480 shows the crystal is anatase form, and 2θ = 27.42° (110), and 54.5° peaks correspond to the rutile form. In XRD analysis, no peak observed corresponding to the generation of metal oxide Sc2O3. This is because Sc is well dispersed in the crystal, or it is in less amount in the sample, below the XRD detection limit [4]. At low calcination temperature, broad and weak peaks are obtained. As the calcination temperature is raised, the peaks became more sharp and intense. It shows that the crystalline feature is improved. The pure anatase phase exists up to 650 °C. Broad peaks at low calcination temperature are the sign of small TiO2 nanocrystalline particle size. Beyond this particular temperature, anatase to rutile transformation begins. As calcination temperature increases, the rutile form becomes more intense than the anatase phase, above 700 °C. As calcination temperature reaches 800C, anatase peak no longer exists, which shows that anatase is completely transformed into the rutile phase [7].
The dopant and co-dopant concentration and calcination temperature are the important factors that influence the concentration of the rutile phase and anatase phase present in the specimen. Anatase phase can be stabilized by increasing the dopant and co-dopant content. The ionic radius of Ti4+ is 0.68 Å and Sc3+ is 0.81 Å. Since the size of scandium is greater than titanium, it is very difficult to incorporate into the TiO2. It hinders the Ti–O–Ti network formation. As a result, the particle size increases [1, 9]. When the scandium content in the sample was increased, a moderate shift in the anatase TiO2 diffraction peak to a lower angle is identified. Variation in the optical band gap with respect to the composition of scandium is shown in Table 2 [8]. Similarly, in the case of hafnium doping, the ionic radius of Hf4+ is 0.79 Å, which is greater than that of titanium. Hence it also has the same effect as Sc; increases the particle size [10]. The decrease in the size of the crystal is mainly due to, the formation of Sc–Hf-TiO2 on the catalyst surface. There is no evidence for the formation of oxides of hafnium, which implies that hafnium is well dispersed on the surface of the TiO2.
It is necessary to evaluate the optical absorption spectra of pure TiO2 (101) surface and TiO2 doped system. The band width of all systems doped with the TiO2 increases to some extent and the photo-catalytic activity is improved to varying degrees. The absorption intensity as well as absorption region is maximum in the visible region. This is because of the hole state formation and the appearance of the impurity mid-level energy band. The impurity band is acceptor level, and hence it reduces the energy required for the transition of figure electrons and which leads to the effective separation of electron–hole pairs. On the other hand, some of the electrons in the valence shell jump to the conduction shell by absorbing small energy photons. This also impels the electron–hole pair generation [11].
Pure titanium has characteristic absorption in UV region (approx. 378 nm). This is due to the transition of electrons from 2p oxygen level to level of Ti 3d [4]. The charge transfer between the valence shell or the conduction shell of TiO2 and Sc 3d level is the reason for the redshift observed in the absorption spectra of the Sc doped TiO2. The proposed photo-catalytic activity of doped and undoped TiO2 is shown in Fig. 5. When a comparison is made between the commercial anatase sample of TiO2 and the scandium or hafnium modified titanium, it is possible to see a decrease in reflectance in the visible region(> 400 nm)is observed in case of modified powders; which increase with the increase in corresponding calcination temperature. It increases the anatase to rutile transformation. The transition depends on the crystalline size of the nanoparticles [12].
The XPS spectrum of titanium in titanium dioxide is single, well defined, spin–spin doublet, which is separated between 6 eV. This explained that Ti4+ is tetragonal in structure. The XPS spectrum of Sc and C codoped TiO2 is in the span of 397.6 eV and 408 eV. The first peak at 401.6 eV indicates Sc 2P3/2 spin-orbital, whereas other peaks at 401.6 eV indicate Sc 2P1/2 orbital of Sc2O3 [4].
In the FTIR spectrum, the intensity of each peak represents the amount of that particular functional group corresponding to the peak present in the specimen. The bands observed at 1620 cm−1 and 3427 cm−1 due to the bending and vibrational stretching of the O–H bond present in the exterior part of TiO2, which is from absorbed water molecules. The absorption below 900 cm−1 shows the vibrational stretching of Ti–O bonds. When scandium was introduced into the sample, a peak at 3427 cm−1 was observed. It indicates that when scandium was introduced, more hydroxyl groups were attracted to the surface of the specimen. Also, the variation in the band at 900 cm−1 was observed. It was due to the Sc in the sample shift the band towards higher wavenumber region [4].
Raman spectroscopy is very sensitive than XRD to explore nanomaterials. It is an important technique for the study of various phases existing in TiO2. The complex structures of photo-catalysts can be explored using the Raman spectrum. This is possible because each functional group and materials in the sample have their own characteristic frequency of vibration, which is easily observable and distinguishable. Anatase TiO2 shows the tetragonal arrangement, and it is in the D194h group [13, 2]. Also, it is observed that the Raman spectra of anatase TiO2 crystal show six peaks. They are at 144 cm−1, 197 cm−1, 399 cm−1, 513 cm−1, 519 cm−1 and 639 cm−1. Rutile phase show four peaks; 143 cm−1, 447 cm−1, 612 cm−1 and 826 cm−1. The observed bands changed into weak and broad due to the co-doping in TiO2. Broadening of Raman bands and lowering of intensity leads to the high dispersion of Sc in the crystal surface which indicates the decrease in the crystal size [4]. The characteristic band corresponding to the oxide of scandium (Sc2O3) occurs at 419 cm−1, and there was no such band in the spectrum. It means that no isolated scandium oxide was present in the surface of the crystal [14].
Well defined structure of a sample can be observed with TEM-HRTEM images of the prepared samples. The TEM images show that the nanoparticles formed have a spherical morphology which formed due to the high calcination temperature. The average particle size with respect to the significant number of the particle can be identified clearly. Well defined lattice fringes of TiO2 nanoparticles were clearly observed in HR-TEM. The crystalline domain corresponding to Sc-O- was not obtained in this method, which implies that no oxides of scandium exist in the TiO2 surface [14].
The specific area of the surface of the specimen is calculated by BET. When Sc is introduced into the TiO2 anatase, the surface area is increased and pore size decreased. The surface area is related to photocatalytic degradation. Contribution to the photocatalytic degradation of dyes is increased with increased surface area, and also the absorption increased [4].
The variations in the surface conditions, the effectiveness of the charge carrier locking, and electron–hole pair conditions of semiconductor nanoparticle is investigated using photoluminescence spectrum. In TiO2 nanoparticle, the band gap excitation may be liberal or trapped excitation-emission due to rejoining of electron–hole pairs or visible region emission can be assigned to photoluminescence. In the spectrum, the peaks indicate the lock sites present in the specimen. The spectra provided information about the variation in the intensity of the spectra peak with the largest intensity. Hence the reduction in the intensity of the peak shows that Sc is doped in the titanium sample, which indicates the recombination of the electron–hole pair is reduced [15].
EDX spectra are mainly used to detect the presence of scandium in the TiO2 matrix. The atomic percentage of scandium and titanium can be measured, which matches the initial concentration of the reaction [14].
4 Photocatalytic activity
Photocatalysts have been widely used for the treatment of various hazardous materials like industrial effluents containing organic elements and dyes. The electronic structure of TiO2 plays an important role in the photocatalytic activity. The band gap difference between the valence shell and the conduction shell is approximately 3.2 eV [16]. Both holes and electrons are present in the valence and conduction band. When the material is allowed for light exposure, electrons are transferred to the conduction band, leaving holes behind. These holes migrate to the surface of the crystal and reduce or oxidize the reactants, which are adsorbed on the surface. These electron–hole pairs act as a good redox system [17]. The catalytic performance of a catalyst mainly depend upon the surface area. It increases the probability of degradation by increasing the pore size and pore volume [18].
As scandium or hafnium was introduced into the sample, absorption is shifted towards a longer wavelength region. Photocatalysis is predominant in the visible region. Hence doping helps in redshift. It enhanced the photocatalytic activity. Photocatalytic activity also depends on the calcination temperature for pure TiO2. The best degradation takes place at 450 °C. The photocatalytic activity of scandium doped titanium dioxide on decolorisation of rhodamine B was compared with commercial TiO2 under U V irradiation. At the highest calcination temperature, around 600 °C, the decolorisation is was highest. Lower decolorisation was observed at lower calcination temperature [12] [19].
The activity of Sc doped TiO2 as a photocatalyst is mainly due to reduced particle size, large surface area, reduced rejoining of electron–hole pair generated by absorbing photons, increased surface area roughness and photonic efficiency. Degradation of dyes under visible light irradiation can be used to evaluate photocatalytic activity [4].
5 Lithium storage batteries
Recently, rechargeable Li-ion batteries (LIBs) are used as a power source for electronic devices and operating tools. This is due to the excellent energy density, cycle life, and reliability. The operating voltage of LIBs is very high [20]. TiO2anatase is a more suitable material for high safety and for high power density. The insertion potential of Li+ is from 1.5 to 1.8 eV. Anatase has greater stability during the cyclic reaction because of the smaller volume change. The Hf doped TiO2-LIBs are low cost and environmentally friendly. Therefore Hf can be successfully doped into titanium source for improved power supply [21].
6 Conclusion
TiO2 is the best semiconductor photocatalyst. Good chemical stability, low-cost non-toxicity oxidative power high photodegradation power, and easy availability make TiO2 the best one. The photocatalytic property has been increased when suitable dopants are added into it. XRD studies show that when dopants such as scandium are introduced into the lattice of TiO2 anatase, the recombination of photogenerated electron–hole pair is suppressed, and the transformation of anatase to rutile is also reduced. In Sc, Hf doped and co-doped TiO2, the band gap energy is reduced, and redshift is observed towards the visible region, which led to increased photocatalytic activity. This article gives an outline related to the synthetic procedure, various characters and photocatalytic activity of Sc, Hf doped, and co-doped TiO2 nanocrystals.
References
Zhang DR, Kang YS (2007) Synthesis and characterization of nanocrystalline TiO2 doped with 2 at.% Sc3+ and V5+ ions. Solid State Phenom 121:41–44
Praveen P, Viruthagiri G, Mugundan S, Shanmugam N (2014) Sol–gel synthesis and characterization of pure and manganese doped TiO2 nanoparticles A new NLO active. Spectrochim Acta A 120:548–557
Ahmad A, Buzby S, Ni C, Shah SI (2008) Effect of Nb and Sc doping on the phase transformation of sol–gel processed TiO2 nanoparticles. J Nanosci Nanotechnol 8(5):2410–2418
Nasir M, Lei J, Iqbal W, Zhang J (2016) Study of synergistic effect of Sc and C co-doping on the enhancement of visible light photo-catalytic activity of TiO2. Appl Surf Sci 364:446–454
Lezhong L, Weiqing Y, Yingchun D, Xinghua Z (2012) First principle study of the electronic structure of hafnium-doped anatase TiO2. J Semicond 33(1):012002
Amritha A, Sundararajan M, Rejith RG, Mohammed-Aslam MA (2019) La-Ce doped TiO2 nanocrystals: a review on synthesis, characterization and photocatalytic activity. SN Appl Sci 1(11):1441
Ahmad A, Shah JA, Buzby S, Shah SI (2008) Structural effects of codoping of Nb and Sc in titanium dioxide nanoparticles. Eur J Inorg Chem 6:948–953
Hirano M, Date K (2005) Scandium-doped anatase (TiO2) nanoparticles directly formed by hydrothermal crystallization. J Am Ceram Soc 88(9):2604–2607
Zhang DR, Kim YH, Kang YS (2006) Synthesis and characterization of nanoparticle of TiO2 co-doped with Sc3+ and V5+ ions. Curr Appl Phys 6(4):801–804
Zheleznov VV, Voit EI, SushkovYV Sarin SA, Kuryavyi VG, Opra DP, Gnedenkov SV, Sinebryukhov SL, Sokolov AA (2016) Nanostructured microtubes based on TiO2 doped by Zr and Hf oxides with the anatase structure. Mater Sci Eng 112:012016
Li D, Li R, Qin X, Yan W (2019) First-principle studies of the magnetism and optical properties of (Sc, Y)-codopedanatase TiO2 (101) surface. Phy Lett A 383(23):2679–2684
da Silva DW, Manfroi DC, Teixeira GF, Perazolli LA, Zaghete MA, Cavalheiro AA (2014) Photocatalytic decomposition of rhodamine-B using scandium and silver-modified TiO2 powders. J Adv Mater Res 975:213–218
Ohsaka T, Izumi F, Fujiki Y (1978) Raman spectrum of anatase, TiO2. J Raman Spectrosc 7(6):321–324
Kang YS, Zhang DR (2006) Synthesis and characterization of titanium dioxide doped with Sc3+ ions. Int J Nanosci 5(02n03):351–357
Keller K, Khramenkova EV, Slabov V, Musin A, Kalashnikov A, Vinogradov AV, Pidko EA (2019) Inkjet printing of Sc-doped TiO2 with enhanced photoactivity. Coatings 9(2):78
Latini A, Cavallo C, Aldibaja FK, Gozzi D, Carta D, Corrias A, Salviati G (2013) Efficiency improvement of DSSC photoanode by scandium doping of mesoporous titania beads. J Phys Chem C 117(48):25276–25289
Jain A, Vaya D (2017) Photocatalytic activity of TiO2 nanomaterial. J Chil Chem Soc 62(4):3683–3690
Prasannalakshmi P, Shanmugam N, Kannadasan N, Sathishkumar K, Viruthagiri G, Poonguzhali R (2015) Influence of thermal annealing on the photo catalytic properties of TiO2 nanoparticles under solar irradiation. J Mater Sci: Mater Electron 26(10):7987–7996
Zhang DR, Liu HL, Han SY, Piao WX (2013) Synthesis of Sc and V-doped TiO2 nanoparticles and photodegradation of rhodamine-B. J Ind Eng Chem 19(6):1838–1844
Ellis LJA, Papadiamantis AG, Weigel S, Valsami-Jones E (2018) Synthesis and characterization of Zr-and Hf-doped nano-TiO2 as internal standards for analytical quantification of nanomaterials in complex matrices. R Soc Open Sci 5(6):171884
Gnedenkov SV, Sinebryukhov SL, Zheleznov VV, Opra DP, Voit EI, Modin EB, Sergienko VI (2018) Effect of Hf-doping on electrochemical performance of anatase TiO2 as an anode material for lithium storage. R Soc Open Sci 5(6):171811
Acknowledgements
The authors thank the Director, CSIR-National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Trivandrum, Kerala, India for extending the laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Athira, L., Sundararajan, M., Renjith, R.A. et al. A review of scandium–hafnium doped TiO2 nanocrystals. SN Appl. Sci. 2, 814 (2020). https://doi.org/10.1007/s42452-020-2508-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2508-7