Abstract
Wet flue gas desulfurization is widely used in power plants because of its high desulfurization efficiency. The reason why it is difficult to predict sulfur dioxide removal efficiency in flue gas is that it is related to many factors. In this paper, the SO2 was removed by absorbing with ammonia solution in a packed tower. Experimental studies have shown that the operating conditions affecting the removal of sulfur dioxide include pH, liquid-to-gas, the concentration of SO2 and inlet velocity. Based on the two-film theory, the mass transfer rate equation of SO2 in the ammonia desulphurization process was studied, and the prediction of SO2 removal efficiency based on certain assumptions was developed. In order to prove the responsibility of the model, significant factors such as pH, liquid-to-gas, the concentration of SO2, and inlet velocity were studied in a packed tower. The experimental results show that it is possible to improve desulphurization efficiency by increasing the liquid-to-gas ratio and pH value, lowering the concentration of SO2 and inlet velocity. Experimental results show that the experimental value is consistent with the predicted value.
Similar content being viewed by others
1 Introduction
Sulfur dioxide (SO2) discharged from thermal power plants is a significant pollutant in the atmosphere, which has a severe harm to human health and the environment [1,2,3]. Many countries have set strict emission standards to reduce sulfur dioxide emissions and various desulfurization methods came into being [4,5,6]. Flue gas desulfurization (FGD) is the most effective desulfurization method at present. According to whether water is added in the process and desulfurization behavior can be divided into wet method, semi-dry method and dry method. Among the different wet flue gas desulfurization processes, ammonia desulfurization technology has the advantages of simple equipment, low consumption, high flexibility of operation, and has the by-products of valuable ammonium sulfate which can be used as fertilizer [7, 8].
When ammonia solution contacts with SO2 in flue gas and the following reactions occur [9, 10].
In recent years, some studies have conducted experiments to describe the process of SO2 absorption with ammonia solution. Johnstone revealed the mechanism of SO2 absorption with the ammonia solution through the study of the gas–liquid equilibrium of the SO2–NH3–H2O system [11].
Wu used ammonium sulfite as adsorbent and the relationships of affecting factors and desulphurization efficiency were analyzed, and suitable operating conditions were achieved as well [12]. Gao performed experiments with a stirred tank reactor to study the characteristics of gas–liquid reaction between ammonium sulfite solution and SO2 [9].
The mass transfer and chemical reaction process of wet ammonia desulfurization process are complex and there are few studies on the prediction of wet ammonia desulfurization efficiency. Jia et al. have developed a mathematical model to simulate the absorption of SO2 in a spray scrubber [13]. Liu et al. established a mathematical model of ammonia desulfurization in spray tower by analyzing the mass transfer rate equation of SO2 in the process of ammonia desulfurization, and the experimental data agreed with the calculated values well [14]. In the process of deducing the mass transfer rate equation of ammonia desulfurization, most of the ammonia desulfurization processes are considered as a first-order irreversible reaction, and most of the absorption processes occur in a spray tower.
Sulfur dioxide emission standards are getting stricter in the world. How to improve desulfurization efficiency and reduce energy consumption is an important problem. In this study, the SO2 removal with ammonia solution in packed power was tested under different operating conditions, including liquid–gas ratio, the pH, the concentration of SO2, and inlet velocity. The goal is to estimate the model of mass-transfer in liquid sides with enhancement factors and sulfite concentration in the liquid phase based on the film theory to estimate the SO2 removal efficiency.
2 Process fundamentals and prediction
2.1 Assumptions
The following assumptions were made for the ammonia desulfurization process to simplify the calculation:
-
a.
The flow rate of mixed gas and liquid flowing through the whole tower is considered as constant.
-
b.
Temperature is constant inside the absorption tower.
-
c.
The concentration and velocity distribution of flue gas and absorption liquid on the same section of the absorber are uniforms.
-
d.
The oxidation of sulfites is negligible
2.2 Mass transfer rate equations
In the process of wet ammonia desulfurization, the absorption rate is determined as follows:
Sulfur dioxide is easily soluble in water, and the liquid film resistance is very small. So KG = kG.
The equilibrium partial pressure at the gas–liquid interface directly affects the mass transfer force with the expression of mass transfer rate. The partial pressure of SO2 in the gas–liquid interface [11] can be obtained by:
where lgM = 5.865–2369/T.
It can be seen from the above correlation formula that there are various types of ions in the ammonia desulfurization process. The concentration of the main elements of N and S can be used to characterize the characteristics of the solution. The model assumes that the absorption solution does not contain sulfate ions.
The total reaction of ammonia flue gas desulfurization could be expressed as follows:
The study of Chen [15] shows the reaction of the ammonia flue gas desulfurization is an irreversible second-order reaction. For a second-order irreversible reaction A + bB → Q, The enhancement factor is determined by the following equation:
where E∞ is the instantaneous irreversible reaction enhancement factor, which could be expressed as follows:
When the reaction rate constant and absorption liquid component concentration is large, the reaction at this time could be considered as an instantaneous reaction. The enhancement factor E of the second-order reaction is equal to the instantaneous reaction enhancement factor E∞. The above equation can be written approximately as following:
Diffusion coefficients at different temperatures can be calculated by the Stokes–Einstein equation [16]:
According to Henry’s law, the concentration of sulfur dioxide at the interface can be expressed by the partial pressure of sulfur dioxide at the interface, as showed in Eq. (15).
The correlation formula proposed by Setchenow [17] is adopted to estimate the solubility constant of the absorbed solution.
Since the reaction is rapid, SO2 reacts completely on the membrane surface. The concentration in the bulk of the liquid phase is zero, cso2 = 0. Therefore, the mass transfer rate equation could be expressed as:
2.3 Modeling of the packed tower
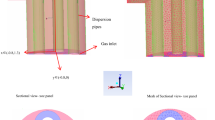
The packed column is divided into several micro elements from top to bottom, as showed in Fig. 1. For each micro element, the amount of concentration reduction of SO2 is equal to the amount absorbed by the absorbing solution, and the following equations are obtained [18]:
In the absorption process of the packed column, the absorption process accompanied by a chemical reaction, the specific surface area of effective mass transfer can be calculated according to the following equation [19]:
The mass transfer coefficient of gas–liquid absorption is calculated by the correlation Eq. (21) [20]:
The expression of desulfurization rate can be obtained by sorting out the above formula:
3 Experimental
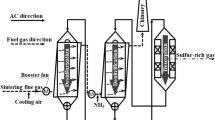
A schematic of the experimental setup is shown in Fig. 2. The experimental equipment consists of three parts: smoke simulation system, absorption system, and tail gas detection device. The desired amount of sulfur dioxide at inlet gas is prepared by mixing SO2 with air. The simulated smoke enters from the bottom of the packed tower, and countercurrent contacted with the descending liquid. The solution of ammonia (10%) added into the bottom of the tower depends on the pH of the solution. Gas flow rates are metered by rotameters and gas samples at the inlet and outlet of power are measured by a gas analyzer. The inner diameter of the tower is 0.05 m. The height of the packing layer is 0.3 m. The following equation calculates desulfurization efficiency:
pH was measured by PHS-25 digital pH meter. Concentrations of SO2 inlet and outlet were measured with a flue gas analyzer (KM940). The concentration of each component in the absorption solution was determined by chemical analysis. Experimental conditions are shown in Table 1.
The diagram of the calculation process is present in Fig. 3.
4 Results and discussion
4.1 The effect of pH
When gas flow was 1 m3/h, the liquid–gas ratio was 3 L/m3, the absorption temperature was 25 °C, and the SO2 inlet concentration was 1500 mg/m3, the effect of desulfurization efficiency with pH is shown in Fig. 4. The pH of the absorbent is an important factor affecting the desulfurization efficiency. As can be seen in Fig. 4, at a lower pH, there is a big difference between the removal rate calculated by the experiment and the model, and after the pH is higher than 6.4, the difference between the experimental value and the calculated value of the model is decreasing. This is because the absorption process of SO2 is complex, and there are many ions in the solution. Many influencing factors are not considered in the model, resulting in the difference between the calculated value of the model and the experimental value. On the whole, the trend of model calculated value and the experimental value is generally consistent.
4.2 The effect of liquid–gas ratio
The liquid–gas ratio is vital in the absorption process. When the gas flow was 1 m3/h, SO2 concentration in the flue gas was 2500 mg/m3. The pH of the absorption solution was 8. The relationship between the liquid–gas ratio and desulfurization efficiency is shown in Fig. 5. It can be seen that the calculated values of the model are consistent with the experimental values. When the liquid–gas ratio is higher than 3 L/m3, the experimental value is close to the theoretical value. When the liquid–gas ratio is lower than 3 L/m3, the experimental value is higher than the theoretical value. The reason may be with the increase of the liquid–gas ratio, the content of (NH4)2SO3 in the spray solution increases, and the mass transfer impetus increases, which is good for the absorption process. The flow of flue gas enhances the surface fluctuation of the liquid film and it increases the actual active contact area, the desulfurization rate measured by the experiment is higher than the theoretical value in the process of the experiment.
4.3 The effect of inlet concentrations of SO2
Experimental conditions: temperature was 25 °C, the pH was 8, the liquid–gas ratio was 3 L/m3, the gas flow rate was 1 m3/h.
Since SO2 concentration in industrial coal combustion flue gas varies greatly with the sulfur content of coal burning, the range can be from l000 to 3500 mg/m3, that is, SO2 concentration in flue gas is not a constant value, so it is of great significance to explore the influence of SO2 concentration on desulfurization rate. The experimental results are shown in the Fig. 6. The variation trend of the fitted curve is basically consistent with the experimental results. Desulfurization efficiency gradually decreases with the increase of the initial concentration of SO2. When the inlet concentration of SO2 is less than 2000 mg/m3, calculation value and experimental value fitted better. The reason may be the concentration of sulfite in the absorption solution exceeds the required for gas phase reactant when the inlet concentration is less than 2000 mg/m3. When the inlet concentration is greater than 2000 mg/m3, the calculated value of the model is higher than the experimental value. The reason may be the volatilization of ammonia in the experimental operation, which reduces the concentration of ammonium sulfite in the absorption solution and reduces the absorption rate.
4.4 The effect of flue gas velocities
Experimental conditions: absorption temperature was 25 °C, pH was 8, liquid–gas ratio was 3L/m3, the concentration of SO2 was 2000 mg/m3.
It can be seen from Fig. 7 that the variation trend of the fitted curve is basically consistent with the experimental results, and the desulfurization rate decreases with the increase of inlet speed. The absorption rate obtained by the simulation calculation is lower than the experimental value. The reason may be the absorption reaction is carried out under the countercurrent condition, and the reduction of absorption liquid will appear back mixing phenomenon which strengthens the mass transfer in the system. This also shows that the ratio of liquid to gas is not completely proportional to the desulfurization reaction rate. When the ratio of liquid to gas reaches a certain value, the enhancement effect of the ratio of liquid to gas on the desulfurization rate decreases accordingly.
4.5 Effect of temperature on desulfurization efficiency
Experimental conditions: pH was 8, liquid–gas ratio was 3 L/m3, the concentration of SO2 was 2000 mg/m3. The effect of temperature on absorption of SO2 is observed in Fig. 8. An examination of Fig. 8 shows that when the inlet temperature increases from 20 to 100 °C, the desulfurization efficiency decreases slightly. The change of absorption reaction temperature has an important effect on the equilibrium partial pressure of SO2 besides the basic data of diffusion coefficient, density, viscosity and surface tension in the mass transfer rate formula. With the increase of temperature, the equilibrium partial pressure of SO2 also increases, and the driving force of chemical absorption decreases, thus reducing the desulfurization rate. Therefore, in the actual wet desulfurization device, the high temperature original flue gas is first cooled down and then entered into the absorption tower, which is conducive to SO2 absorption.
5 Conclusions
In this paper, experiments for SO2 absorbed by the ammonia solution in the packed power were carried out to get the removal efficiency of SO2. The results show that the reasonable values of the process parameters: pH value is 7–8, inlet concentration of SO2 is 1500–2000 mg/m3, gas–liquid ratio is 3–4 L/m3. Mass transfer equation of SO2 in flue gas removal by the ammonia method was developed for the prediction of the SO2 removal efficiency using the two-film theory. The results show that the desulfurization efficiency increased by increasing the liquid–gas ratio and pH of the absorbed liquid, and decreased by increasing the inlet concentration of flue gas flow. The desulfurization model is calculated by using the operating parameters of a laboratory-scale packed column, and the results show that the theoretical calculated values are basically consistent with the experimental data.
Abbreviations
- A :
-
The cross-sectional area of packed column, m2
- a :
-
Effective interfacial area, m2/m3
- a t :
-
Packed column specific surface area, m2/m3
- b :
-
Stoichiometric number of ammonium sulfite reactions
- c Ai :
-
Concentration of sulfur dioxide in liquid film, kmol/m3
- c BL :
-
Concentration of ammonium sulfite in the bulk of the liquid phase, kmol/m3
- D r :
-
Relative density
- c i :
-
Ion concentration, kmol/m3
- Dso2, D (NH4)2SO3 :
-
Diffusion coefficients of sulfur dioxide and ammonium sulfite in liquid film, m2/s
- E :
-
Enhancement factor
- G :
-
Volume flow of flue gas, m3/s
- H w, H :
-
The solubility coefficient of a solute in water and solution, kmol/(kPa m3)
- I :
-
Current strength of aqueous electrolyte solution, kmol/m3
- Ha:
-
Hatta number
- k L :
-
Mass transfer coefficient in liquid phase,m/s
- k s :
-
Salt effect coefficient, m3/kmol
- L :
-
Volume flow rate of spray solution, L/s
- L G :
-
Mass flow of liquid, kg/(m2 s)
- M :
-
Molar mass, kg/kmol
- N :
-
The total concentration of ammonia in the absorption solution N = [NH3(l)] + [NH4+], Mol/100molH2O
- N′:
-
The concentration of (NH4)2SO4 in the absorbing solution, mol/L
- N SO2 :
-
SO2 mass transfer rate in flue gas,kmol/(m2 s)
- P SO2,i :
-
Partial pressure of sulfur dioxide at the gas–liquid interface, kPa
- p BM :
-
Logarithmic average partial pressure of air, Pa
- R :
-
Gas constant, kJ/kmol K
- S :
-
[SO2(l)] + [HSO3−]+[SO32−] concentration of total sulfite, mol/100molH2O
- X :
-
Concentration of ammonium sulfite in the absorbed solution, mol/L
- Y :
-
Concentration of ammonium bisulfite in the absorption solution, mol/L
- η:
-
SO2 removal efficiency
- ρL :
-
Density of liquid, kg/m3
- σc :
-
Critical surface tension of packing material, N/m
- σL :
-
Surface tension of liquid, N/m
- in:
-
Flow into
- out:
-
Flow out
References
He F, Qian Y, Xu J (2019) Performance, mechanism, and kinetics of Fe(III)EDTA reduction by thiourea dioxide. Energy Fuels 33:3331–3338
Liu J, Cheng D-L (2016) Modeling of the flue gas condensation for the PM2.5 removal efficiency of an integrated technology. Arab J Sci Eng 41:2399–2408
Guo Y, Li Y, Zhu T, Ye M (2015) Investigation of SO2 and NO adsorption species on activated carbon and the mechanism of NO promotion effect on SO2. Fuel 143:536–542
He F, Zhu X, Chen X, Ding J (2019) Evaluation of FeIIEDTA-NO reduction by thiourea dioxide in NO removal with FeIIEDTA. Asia-Pac J Chem Eng. https://doi.org/10.1002/apj.2397
Fang P, Cen C, Tang Z, Zhong P, Chen D, Chen Z (2011) Simultaneous removal of SO2 and NOX by wet scrubbing using urea solution. Chem Eng J 168:52–59
Zhong Y, Gao X, Huo W, Luo Z, Ni M, Cen K (2008) A model for performance optimization of wet flue gas desulfurization systems of power plants. Fuel Process Technol 89:1025–1032
Jia Y, Du D, Zhang X, Ding X, Zhong O (2013) Simultaneous removal of SO2 and NOX with ammonia absorbent in a packed column. Korean J Chem Eng 30:1735–1740
Yan J, Bao J, Yang L, Fan F, Shen X (2011) The formation and removal characteristics of aerosols in ammonia-based wet flue gas desulfurization. J Aerosol Sci 42:604–614
Gao X, Ding HL, Du Z, Wu ZL, Fang MX, Luo ZY, Ce KF (2010) Gas-liquid absorption reaction between (NH4)2SO3 solution and SO2 for ammonia-based wet flue gas desulfurization. Appl Energy 87:2647–2651
Fan L, Jiang X, Jiang W, Guo J, Chen J (2014) Physicochemical properties and desulfurization activities of metal oxide/biomass-based activated carbons prepared by blending method. Adsorption 20:747–756
Johnstone HF (1935) Recovery of sulfur dioxide from waste gases-equilibrium partial vapor pressures over solutions of the ammonia-sulfur dioxide-water system. Ind Eng Chem 27:587–591
Wu ZB, Liu Y, Sheng ZY, Wang HQ, Wang YJ, Tang N (2010) Experimental study on main affecting factors of flue gas desulphurzation process by ammonia method. Environ Sci Technol 33:135–137
Jia Y, Zhong Q, Fan XY, Chen QQ, Sun HB (2011) Modeling of ammonia-based wet flue gas desulfurization in the spray scrubber. Korean J Chem Eng 28:1058–1064
Liu GR, Wang ZW, Wang YL, Ji QL (2010) experimental study and modeling for ammonia desulphurization in spray tower. CIESC J 61:2463–2467
Chen MQ, He BS, Chen GH, Fan LJ, Liu SM (2005) Chemical kinetics based analyses on SO2 removal reactions by ammonia scrubbing1. Acta Scien Circum 25:886–890
Sada E, Kumazawa H, Butt MA (1980) Absorption of sulfur dioxide in aqueous slurries of sparingly soluble fine particles. Chem Eng Sci 19:131–138
Deshwal BR, Lee HK (2009) Mass transfer in the absorption of SO2 and NOx using aqueous euchlorine scrubbing solution. J Environ Sci 21:155–161
Ebrahimi CS, Picioreanu R, Kleerebezem J (2003) Rate-based modelling of SO2 absorption into aqueous NaHCO3/Na2CO3 solutions accompanied by the desorption of CO2. Chem Eng Sci 58:3589–3600
Wang J, Mai B, Li X (1989) Performance of perforated packings(VI)-effective interfacial area. Chem Eng (China) 25:21–25
Li X, Tan Q, Li A (1980) Liquid side mass transfer coefficients of three tower packings perforated ring. J Chem Ind Eng (China) 4:375–386
Funding
This work was funded by the Talent Introduction Project of Kunming University of Science and Technology (kksy201305110).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All author declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, J., Yao, W. & Yang, Z. Prediction of SO2 removal efficiency for ammonia-based wet flue gas desulfurization in a packed tower. SN Appl. Sci. 2, 360 (2020). https://doi.org/10.1007/s42452-020-2147-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2147-z