Abstract
Chromium (VI) is one of the toxic heavy metals causing various human ailments like asthma to severe forms of cancer; hence, its removal from industrial effluents is essential. The present study demonstrates ameliorating techniques for the removal of Cr(VI). A chromium-reducing bacterium (CRB) identified as Ochrobactrum pseudintermedium ADV31 through 16S rRNA gene sequencing, was found to remove concentrations of Cr(VI) up to 600 mg/L in nutrient medium. Calcium alginate (CA) and polyurethane foam (PUF) were the two different materials used for the immobilization of Ochrobactrum pseudintermedium ADV31. The efficiency of the immobilized cells and free form of bacterial cells with inoculum concentrations of 1% and 5% were compared for the removal of Cr(VI). Calcium alginate with 5% inoculum concentrations showed removal of 82% of 600 mg/L in 5 days, while PUF with 5% inoculum size showed removal up to 86% of 600 mg/L in 5 days. Free form of bacterial cell was able to remove 36.7%. The bacterium was able to tolerate a wide range of pH ranging from 6 to 9 and had an optimum temperature of 45 °C. The results confirmed that both forms of immobilization methods are equally effective for the removal of hexavalent chromium and can be used for various biotechnological processes for the metal bioremediation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chromium occurs naturally in animals, plants, rocks, soils, volcanic eruptions and gases. The different oxidation states of chromium range from divalent to hexavalent with Cr(III) and Cr(VI) being the most stable states. Trivalent chromium (Cr III), when taken in moderate amounts, is an essential trace element [1], whereas Cr(VI) is highly toxic, carcinogenic and mutagenic [2,3,4]. Anthropogenic sources of chromium contaminating the environment are industries that involve electroplating, steel production, leather tanning, wood treatment and textile dyeing activities which release large amounts of chromium in its effluent [5, 6]. Cr(VI) being highly soluble tends to contaminate groundwater sources, and its concentration goes beyond its permissible limit (0.05 mg/L) in drinking water [7]. On the other hand, occupational exposure to it can cause asthma, dermatitis, skin and nasal ulceration and other allergic reactions [8]. At the cellular level, Cr(VI) finds its way into the cells via the sulfate uptake pathway due to its structural similarity with sulfate [9]. Once Cr(VI) crosses the plasma membrane, it gets reduced mediated by soluble cytosolic reductases. During the process, various reactive oxygen species such as singlet oxygen, superoxide [10], hydrogen peroxide radicals and hydroxyl ion [11] are produced which easily combine with DNA, thus disrupting the normal physiological processes [12].
Owing to various adverse effects of chromium, removal of chromium (VI) from contaminated environments and preventing its release into the ecosystem from anthropogenic sources is required (US EPA) [13]. One such treatment approach is bioremediation which is environment-friendly and cost-effective as compared to conventional physico-chemical methods which have drawbacks such as the generation of toxic sludge, higher reagent and incomplete metal removal. Bioremediation involves the removal of pollutants from the environment using microorganisms and plants [14,15,16]. Bacteria capable of reducing Cr(VI) are named chromium-reducing bacteria (CRB) [17]. Pseudomonas dechromaticans, isolated from sewage sludge, was the first to be found to reduce Cr VI [18]. Earlier more focus was given on facultative anaerobic bacteria such as Aeromonas, Aerococcus and Micrococcus [19]. Later, bacteria capable of reducing Cr(VI) aerobically like Thermus scotoductus were identified [20]. Polti et al. 2007 [21] identified ten genera of Streptomyces and one Amycolatopsis which were Cr(VI) resistant. Various mechanisms have been developed by microbes to resist the toxic chromium, regulation of sulfate uptake shuttle systems that are involved in cellular accumulation [22], and bacteria cell surfaces possess functional groups which reduce Cr(VI) to Cr(III) extracellularly [23]. It also includes adsorption of Cr(VI) to functional group followed by reduction in the cell membrane [20, 24], activation of scavenging enzymes such as catalase, superoxide dismutase to counter chromate-induced oxidative stress [25, 26] and DNA damage repair system by SOS response [26, 27]. Therefore, the microbial response to the toxicity of heavy metal plays an important role in re-establishing the polluted areas. For better operational stability, reusability and higher efficiency, the application of immobilized enzymes or whole cell is proved to be advantageous.
The present study envisages isolation and identification of a CRB from sewage which can efficiently remove higher concentrations of Cr(VI) aerobically. Further increase in removal efficiency is attempted through immobilization of the bacterium via entrapment and adsorption on inert materials.
2 Materials and methods
2.1 Isolation of chromium-resistant bacteria
The bacterial cell culture was isolated from sewage in a previous study that was carried out by CSIR NEERI, Mumbai, India
2.2 Maximum tolerance concentration
This test was performed by agar well diffusion method in which bacterial culture was bulk seeded in nutrient agar followed by the addition of varying Cr(VI) concentrations in mg/L (200, 400, 600, 800, 1000) in the bored wells. The maximum tolerance level is the maximum concentration of chromium in the nutrient medium in which the growth of the organism is supported.
2.3 Growth studies of isolated bacterial culture
The isolated bacterial strain was grown in tryptic soy broth, and the cells were separated by centrifugation (5000 g for 10 min at 10 °C). After washing the cells twice with saline water, they were resuspended in the phosphate buffer (0.1 M NaH2PO4-Na2HPO4; pH, 7.2). The bacterial cells were inoculated in conical flasks containing 100 mL sterile nutrient broth having three different concentrations (200, 400, 600 mg/L) of chromium as chromate salt. It was then incubated in shaking conditions at 120 rpm at 35 ± 2 °C. Optical density was measured at 600 nm at intervals of 30 min. Absorbance was checked till constant optical density was reached. Optical density versus time graph was plotted.
2.4 Identification of isolated bacterial culture
The identification of bacterial culture was done using 16S rRNA sequencing. Spin column kit (HiMedia, India) was used for the extraction of chromosomal DNA. Using a polymerase chain reaction, the bacterial 16S rRNA gene (1500 bp) was amplified in a thermal cycler. The purification of extracted DNA was done using Exonuclease I Shrimp Alkaline Phosphatase (Exo-SAP). The sequencing of purified amplicons was done by the Sanger method in ABI 3500xL genetic analyzer (Life Technologies, USA). Sequencing files (ab1) were edited using CHROMASLITE (version 1.5) and further analyzed by BLAST (Basic Local Alignment Search Tool) with the closest culture sequence obtained from the NCBI (National Centre for Biotechnology Information database) that finds regions of local similarity between sequences.
2.5 Optimum pH and temperature in presence and absence of varying amount of chromium (VI)
The influence of pH and temperature on growth was assessed. pH ranging from 4 to 9 was used to check the optimum pH, whereas exposure to different temperatures such as 4 °C, 15 °C, 30 °C, 45 °C and 70 °C was used to determine the optimum temperature for the growth of the bacterium. The test system consisted of sterile nutrient broth supplemented with varying concentrations of Cr(VI). Eighteen-hour-old actively grown culture was inoculated in the test system, and the tubes were incubated at respective temperatures for 24 h. Media control was prepared in the same way as the test system but without the addition of culture. Positive and negative control was also prepared. Growth in the form of turbidity was measured at 660 nm.
2.6 Immobilization of the isolated bacteria
Two different materials, calcium alginate (CA) and polyurethane foam (PUF), were used for the immobilization of bacterial culture. In total, 1% sodium alginate was prepared by dissolving 1 g of alginate in 100 mL hot distilled water with constant stirring. At room temperature, bacterial culture was added to the slurry of sodium alginate at two different concentrations (1% and 5%) under constant stirring conditions for even dispersal. Sterile syringe of 10 mL was used, and the slurry solution was dispersed dropwise into 0.2 M calcium chloride solution. Curing of gel beads was done for 2 h at 4 °C and then was washed thoroughly with distilled water. Control beads were prepared similarly but without the addition of bacterial culture. In total, 50 mL of sterile nutrient broth was inoculated with two inoculum sizes of 1% and 5% for PUF with varying surface areas (30 cm2, 60 cm2 and 90 cm2). Flasks were incubated at 37 °C under shaking conditions for 48 h for the formation of biofilm on PUF. After 48 h, PUF was washed with sterile distilled water to remove the unattached cells.
2.7 SEM analysis of polyurethane foam
PUF from the control and test system was removed and washed with phosphate buffer (0.1 M NaH2PO4-Na2HPO4; pH, 7.2). The internal structure of PUF was observed under a scanning electron microscope for the formation of the biofilm.
2.8 Batch studies for removal of chromium (VI)
Batch studies were carried out in triplicates. In total, 7.062 g of K2Cr2O7 was weighed and dissolved in 50 mL of distilled water giving a concentration of 50,000 μg/mL of Cr (VI). The stock solution was further diluted as per the desired concentration. Batch studies were carried out in 250-mL Erlenmeyer flask. Experiments were conducted for varying concentrations of chromium (VI) (200, 400, 600 mg/L). The flasks were incubated at room temperature (30 °C) with continuous shaking at 150 rpm. At time interval of 1, 3 and 5 days, aliquots of the samples were removed and filtered through bacteria proof filter (0.45 microns) and the concentration of chromium in the filtrate was checked using Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES, Thermo Scientific, Model: iCAP 6300 Duo) which measures the total chromium. The efficiency of chromium (VI) removal by immobilized cells was further compared with the removal by free bacterial cells.
2.9 Analysis of chromium (VI)
Absorbance of reference solutions and samples was measured using ICP-OES, and the background correction was performed with a blank solution. The percentage removal of chromium (VI) was calculated [28].
3 Results and discussion
3.1 Maximum tolerance concentration
The tolerance to Cr(VI) by the isolate was tested with different concentrations of Cr(VI) ranging from 200 mg/L to 1000 mg/L. The isolate showed a maximum tolerance concentration (MTC) value of 600 mg/L of Cr(VI) (Fig. 1). At a concentration of 800 and 1000 mg/L, a zone of inhibition was observed, indicating the inability of the isolated bacterial strain to grow at these concentrations. Other researchers have also isolated bacterial species for chromium removal, but they tolerate chromium of lower concentration than what has been reported in this study. Megharaj et al. 2003 [29] observed that the bacterial strain Arthrobacter spp. could resist 100 mg/L Cr(VI), while Bacillus sp. resisted only 10 mg/L. It was also seen that chromium-resistant bacteria isolated from tanneries could resist up to 250 mg/L [30]. Upadhyay et al. [31] found that Bacillus subtilis MNU 16 tolerated and reduced up to 36.77 mg/L of chromium concentration within 72 h.
3.2 Growth study of culture in the presence of chromium (VI)
The growth curve was made by inoculating the culture in nutrient broth with different concentrations of Cr(VI) and taking readings at intervals of 30 min. The growth of bacteria in the nutrient media without Cr(VI) was rapid and achieved its logarithmic phase within 2 h. On the contrary, the lag phase in the presence of Cr(VI) was found to be 8 h (Fig. 2). Similarly, Faisal et al. [32] in his study found that the attainment of the log phase by Brevibacterium CrT-13 took 4 h when nutrient media were supplemented with K2CrO4. Guo et al., 2010 [33] studied endophytic bacteria for removal of various heavy metals. It was found that bacteria showed a high amount of ATPase activity when the media were supplemented with toxic metals. It is well known that there is a great demand for energy to cope with the toxicity of the compounds [34]. Similarly, the bacterial species in the present study took a longer lag phase which is probably to generate high energy to overcome the toxic effects of Cr(VI).
3.3 Identification of isolated bacterial culture
The bacterial culture was identified as Ochrobactrum psuedintermedium strain ADV31 by 16S rRNA sequencing (through neighbor-joining method) with a total score of 2353 and 99.39% similarity index (Fig. 3). Ochrobactrum psuedintermedium is gram-negative, motile and nonspore-forming bacteria. It belongs to α-proteobacteria subclass. Ochrobactrum spp. like Ochrobactrum sp. strain CSCr-3 [35], Ochrobactrum anthropi [36], Ochrobactrum intermedium SDCr-5 [37] are widely used for bioremediation of heavy metals and have been previously isolated from chromium-contaminated soils. However, Ochrobactrum psuedintermedium ADV31 for removal of Cr(VI) has not been previously mentioned in the literature.
Phylogenetic tree of isolated bacteria and closely related species based on 16S rRNA gene sequences. The tree was constructed using the neighbor-joining method. The optimal tree with the sum of branch length = 0.32362990 is shown. The numbers at nodes indicate the percentages of occurrence of the branching order in 1000 bootstrapped trees. The evolutionary distances were computed using Jukes–Cantor method
3.4 Optimum pH and temperature in the presence of varying amounts of Cr(VI)
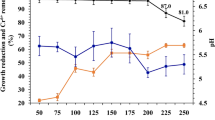
Heavy metal tolerance capacity can be affected by certain environmental parameters, such as temperature and pH. Under optimum conditions the growth is faster as well as enzymatic activity is higher. Optimum pH for Ochrobactrum pseudintermedium ADV31 was found to be 6 (Figure 4a). However, this bacterium could resist and grow in the pH range from 6 to 9. The reason for investigating the effect of pH on heavy metal tolerance by bacteria is because at very low pH (< 4) the heavy metal tolerance ability is reduced, and this is due to competition of hydronium ions (H3O+) with heavy metals for binding sites on the microbial surface [38]. Another environmental parameter which affects heavy metal tolerance is temperature since it is directly linked to microbial growth and metabolism. The results obtained showed that 45 °C was found to be the optimum temperature (Fig. 4b).
3.5 SEM analysis of polyurethane foam
The internal structure of control PUF and test PUF was analyzed using a scanning electron microscope (Fig. 5). PUF has a porous structure. It is chemically inert and mechanically stable concerning abrasion. The microscopic figure clearly shows that the PUF, used as a support for biofilm formation, showed dense bacterial growth of Ochrobactrum pseudintermedium ADV31. The size of the bacteria was between 1.36 and 2.085 µm. From the above results, it can be concluded that PUF proves to be an excellent support material for biofilm formation.
3.6 Batch studies for removal of chromium (VI)
Calcium alginate and PUF were used for the immobilization of Ochrobactrum pseudintermedium ADV31 cells, and their performance with respect to Cr(VI) removal and bead integrity for calcium alginate was observed. It was found that calcium alginate and PUF were much more efficient as compared to the free form of the bacterial cells. At a lower concentration of 200 mg/L of Cr(VI), free cells were found to be as effective as immobilized cells. As the concentration of Cr(VI) was increased, the toxicity to free bacterial cells was much more than to the immobilized cells (Figs. 6, 7, 8). This is attributed to the entrapment of the cells inside the matrix which serves as a protective barrier for the bacterial cells. Although the duration for biofilm formation given was only 48 h, efficient chromium removal was observed. However, an increase in the incubation period for biofilm formation may give much more effective results. Kavita et al. [39] in her study found that Ochrobactrum intermedium BCR400 could reduce 31.2% of 400 mg/L Cr(VI) in 52 h, whereas our study reveals that Ochrobacterum pseudintermedium ADV31 was able to remove 45% and 59% of 400 mg/L by free bacterial cells and PUF, respectively, in 24 h. Also, Rehman et al. [40] reported a 91% reduction of 100 mg/L of Cr(VI) in 96 h, while O. pseudintermedium ADV31could remove 91.5% of 200 mg/L Cr(VI) in 72 h when immobilized on PUF. It was also seen that 1% calcium alginate beads were found to be stable in the present study for a period of 5 days. In a study carried out by Pal et al. [41], the reduction of chromate by B. sphaericus cells was performed using different inert materials. It was observed that 87.5% of 20 µM Cr(VI) was achieved by PVA-alginate immobilized cells after incubation for 24 h. Agar–agar, agarose and PVA borate beads lost its integrity and were unstable within 18–24 h.
4 Conclusion
It is evident from the present findings that Ochrobactrum pseudintermedium ADV31 is not only resistant to chromium (VI) toxicity, but can grow and is capable of removing toxic Cr(VI) up to 600 mg/L. It was also observed that the immobilized cells are more efficient than the free form of a bacterial cell. The effectiveness of the immobilized cells increased as the inoculum size increased. Also, the bacterium was able to grow in a wide range of pH. This indicates that the bacteria can resist pH shock and can effectively remove Cr(VI) from industrial effluents with varying pH. A temperature of 45 °C was found to be optimum, indicating that Ochrobactrum pseudintermedium ADV31 is mesophilic. Since industrial effluents are released in large quantities, the use of biosorbents in immobilized form can prove to be an excellent low-cost means for bioremediation of toxic metals present in the environment.
References
Bahijri SMA, Mufti AMB (2002) Beneficial effects of chromium in people with type 2 diabetes, and urinary chromium response to glucose load as a possible indicator of status. Biol Trace Element Res 85:97–110
Costa M (1997) Toxicology and carcinogenicity of Cr(VI) in animal models and humans. Cr Rev Toxicol 27:431–442
Wang P, Mori T, Toda K, Ohtake H (1990) Membrane-associated chromate reductase activity from Enterobacter cloacae. J Bacteriol 172:1670–1672
Nishioka H (1975) Mutagenic activity of metal compounds in bacteria. Mutat Res 31:185–189
Lauwerys R, Haufroid V, Hoet P, Lison D (2007) Toxicologie industrielle et intoxications professionnelles, 5th edn. Elsevier, Paris
Agarwal A, Kumar V, Pandey BD (2008) Remediation options for the treatment of electroplating and leather tanning effluent containing chromium—a review. Miner Process Extr Metall Rev 272:99–130
World Health Organization (2004) Guidelines for drinking water quality. WHO, Geneva
Halasova E, Matakova T, Kavcova E, Musak L, Letkova L, Adamkov M, Ondrusova M, Bukovska E, Singliar A (2009) Human lung cancer and hexavalent chromium exposure. Neuro Endocrinol Lett 1:182–185
Ksheminska H, Fedorovych D, Babyak L, Yanovych D, Kaszycki P, Koloczek H (2005) Chromium (III) and (VI) tolerance and bioaccumulation in yeast: a survey of cellular chromium content in selected strains of representative genera. Process Biochem 40:1565–1572
Cheng Y, Xie Y, Zheng J, Wu Z, Chen Z, Ma X, Li B, Lin Z (2009) Identification and characterization of the chromium(VI) responding protein from a newly isolated Ochrobactrum anthropi CTS-325. J Environ Sci 21:1673–1678
McNeill L, McLean J (2012) State of the science of hexavalent chromium in drinking water. Pollut Eng 44:5
Cervantes C, Campos-Garcia J, Devars S, Gutierrez-Corona F, Loza-Tavera H, Torres Guzman JC, Moreno-Sanchez R (2001) Interaction of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347
USEPA (2009) National primary drinking water regulations. Environmental Protection Agency, Washington, D.C
Dixit R, Malaviya D, Pandiyan K, Singh B, Sahu A, Shukla R, Singh BP, Rai JP, Sharma PK, Lade H (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7:2189–2212
Mani D, Kumar C (2014) Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: an overview with special reference to phytoremediation. Int J Environ Sci Technol 11:843–872
Akcil A, Erust C, Ozdemiroglu S, Fonti V, Beolchini F (2015) A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod 86:24–36
Somasundaram V, Philip L, Bhallamudi SM (2009) Experimental and mathematical modeling studies on Cr(VI) reduction by CRB, SRB, and IRB, individually and in combination. J Hazard Mater 172:606–617
Romanenko VI, Korenkov VN (1977) A pure culture of bacteria utilizing chromates and bichromates as hydrogen acceptors in growth under anaerobic conditions. Mikrobiologiya 43:414–417
Srinath T, Khare S, Ramteke PW (2001) Isolation of hexavalent chromium reducing Cr-tolerant facultative anaerobes from tannery effluent. J Gen Appl Microbiol 47:307–312
Opperman DJ, Heerden E (2008) A membrane-associated protein with Cr(VI)-reducing activity from Thermus scotoductus SA-01. FEMS Microbiol Lett 280(2):210–218
Polti MA, Amoroso MJ, Abate CM (2007) Chromium(VI) resistance and removal by actinomycete strains isolated from sediments. Chemosphere 67:660–667
Brown SD, Thompson MR, Verberkmoes NC, Chourey K, Shah M, Zhou JZ, Hettich RL, Thompson DK (2006) Molecular dynamics of the Shewanella oneidensis response to chromate stress. Mol Cell Proteomics 5:1054–1071
Ngwenya N, Chirwa EMN (2011) Biological removal of cationic fission products from nuclear wastewater. Water Sci Technol 63:124–128
Tahri Joutey N, Bahafid W, Sayel H, El Ghachtouli N (2013) Biodegradation: involved microorganisms and genetically engineered microorganisms. In: Chamy R (ed) Biodegradation—life of science. InTech, Cambridge, p 289
Ackerley DF, Barak Y, Lynch SV, Curtin J, Matin A (2006) Effect of chromate stress on Escherichia coli K-12. J Bacteriol 188:3371–3381
Cervantes C, Campos-Garcıa J (2007) Reduction and efflux of chromate by bacteria. Molecular microbiology of heavy metals. Microbiol Monogr 6:407–419
Hu P, Brodie EL, Suzuki Y, McAdams HH, Andersen GL (2005) Whole-genome transcriptional analysis of heavy metal stresses in Caulobacter crescentus. J Bacteriol 187:8437–8449
APHA (2005) Standard methods for the examination of water and waste water, 21st edn. American Public Health Association, Washington DC
Megharaj M, Avudainayagam S, Neidu R (2003) Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol 47:51–54
Basu M, Bhattacharya S, Paul AK (1997) Isolation and characterization of chromium resistant bacteria from tannery effluent. Bull Environ Contam Toxicol 58:535–542
Upadhyay N, Vishwakarma K, Singh J, Mishra M, Kumar V, Rani R, Mishra R, Chauhan D, Tripathi D, Sharma S (2017) Tolerance and reduction of chromium(VI) by Bacillus sp. MNU16 isolated from contaminated coal mining soil. Front Plant Sci 8:1–13
Faisal H, Hasnain S (2004) Comparative study of Cr(VI) uptake and reduction in industrial effluent by Ochrobactrum intermedium and Brevibacterium sp. Biotechnol Lett 26:1623–1628
Guo H, Luo S, Chen L, Xiao X, Xi Q, Wei W, Zeng G, Liu C, Wan Y, Chen J, He Y (2010) Bioremediation of heavy metals by growing hyperaccumulator endophytic bacterium Bacillus sp. L14. Bioresour Technol 101(22):8599–8605
Leedjarv A, Ivask A, Virta M (2008) The interplay of different transporters in the mediation of divalent heavy metal resistance in Pseudomonas putida KT2440. J Bacteriol 190:2680–2689
He Z, Gao F, Sha T, Hu Y, He C (2009) Isolation and characterization of a Cr(VI)-reducing Ochrobactrum sp. strain CSCr-3 from chromium landfill. J Hazard Mater 163:869–873
Ozdemir G, Ozturk T, Ceyhan N, Isler R, Cosar T (2003) Heavy metal biosorption by biomass of Ochrobactrum anthropi producing exopolysaccharide in activated sludge. Bioresour Technol 90:71–74
Sultan S, Hasnain S (2007) Reduction of toxic hexavalent chromium by Ochrobactrum intermedium strain SDCr-5 stimulated by heavy metals. Bioresour Technol 98:340–344
Karthikeyan S, Palaniappan P, Sabhanayakam S (2007) Influence of pH and water hardness upon nickel accumulation in edible fish Cirrhinus mrigala. J Environ Biol Acad Environ Biol India 28:489–492
Kavita B, Keharia H (2012) Reduction of hexavalent chromium by Ochrobactrum intermedium BCR400 isolated from a chromium-contaminated soil. 3 Biotech 2:79–87
Rehman A, Zahoor A, Muneer B, Hasnain S (2008) Chromium tolerance and reduction potential of a Bacillus sp. ev3 isolated from metal-contaminated wastewater. Bull Environ Contam Toxicol 81:25–29
Pal A, Datta S, Paul Amal K (2013) Hexavalent chromium reduction by immobilized cells of Bacillus sphaericus AND 303. Braz Arch Biol Technol 56(3):505–512
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, I, Dr. Shalini A Tandon states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tandon, S., Jha, M. & Dudhwadkar, S. Study on Ochrobactrum pseudintermedium ADV31 for the removal of hexavalent chromium through different immobilization techniques. SN Appl. Sci. 2, 296 (2020). https://doi.org/10.1007/s42452-020-2103-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2103-y