Abstract
Biosynthesis using plant extract is known as one of the potential techniques to synthesize different zinc oxide nanoparticles (ZnO-NPs) in different size ranges. ZnO-NPs were synthesized using Plumeria leaf extract with laboratory chemical reagent Zn(CH3COO)2 and followed by the micro-encapsulation of biosynthesized ZnO-NPs using chitosan and cellulose with TEOF as a cross-linker employing freeze gelation method. Both neat and encapsulated ZnO-NPs have been characterized by FT-IR, UV spectroscopy, XRD, and SEM techniques. The UV-spectroscopic analysis confirmed the characteristic band of ZnO-NPs at 356.0 nm, and FIIR showed the peaks at 544 cm−1 and 545 cm−1 corresponding to the Zn–O bond. Powder XRD pattern showed the wurtzite structure of ZnO and gave the calculated average crystallite size as of 27.23 nm. In the case of encapsulated ZnO-NPs, the UV–visible spectrum showed two strong absorption peaks at 232.5 nm, 242.5 nm, and a weak peak at 357 nm. A broad peak at 3333 cm−1 in FT-IR spectra is either due to N–H stretching in the amide group of chitosan or hydroxyl group in encapsulated ZnO-NPs. It was observed that chitosan loaded ZnO-NPs had higher entrapment efficiency (81.98%) at 15 mL of plant extract. The kinetic profile in the release of ZnO particles out from encapsulated ZnO-NPs was observed to follow four kinetic paths in 120 min at pH 1.2. The particle release followed the zero-order kinetic in the first 50 min and then followed by Hixson–Crowell kinetic in the next 50 min with two different rate constants, 2.6 × 10−3 min−1 and 13 × 10−3 min−1, before it backs to the zero-order kinetics. This study shows that ZnO nanoparticles can easily be biosynthesized and encapsulated for use in the pharmaceutical industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanoparticles can be synthesized using many methods such as chemical, physical, biological, and hybrid techniques. Due to the severe toxicity and the harsh conditions in conventional methods, the research focus has been shifted towards eco-friendly protocols. Recently, many studies have shown that the plant extracts can act as a potential precursor for the synthesis of nanomaterials. There is a considerable number of works been reported on the successful use of plants in the synthesis of several greener nanoparticles such as cobalt [1], copper [2], silver [3], ZnO [4], and magnetite [5].
During the past decade, it is reported that in addition to the plants, many other biological systems such as algae [6], bacteria [7], yeast, fungi [8], and human cells [9] can transform inorganic metal ions into metal nanomaterials with the help of reductive capacities of the proteins and metabolites present in these organisms. The extended interest in the synthesis of metallic nanoparticles and also their derivatives such as metal oxides using biological materials are also due to their unusual optical [10] and chemical properties [11]. The synthesis and assembly of metallic and metal oxide nanoparticles would be beneficial towards the development of clean, non-toxic, and environmentally acceptable “green chemistry” procedures [12,13,14,15]. Nanobiotechnology is an alternative route for the synthesis of biocompatible stable nanoparticles [16, 17].
Plumeria obtusa is called by different names as frangipani, Singapore graveyard tree, and “araliya” in Sri Lanka. There is a wide range of medicinal use of the plant itself and its different parts reported in the many regions of the world [18]. Siddiqui group in their preliminary phytochemical screening of Plmeria obtusa revealed the presence of steroids, alkaloids, flavonoids, terpenoids, and glycosides [19].
Zn2+ is an essential nutrient in various aspects of metabolism in the human body. Usually, 11.0 and 9.0 mg of Zn2+ are recommended for a healthy adult man and a woman, respectively. The surface of ZnO is rich with –OH groups which can be functionalized by various surface decorating molecules. ZnO can be dissolved slowly in acidic and strong basic conditions. Further, ZnO nanostructures possess also semiconductor, optical, and piezoelectric properties [20].
Molecular encapsulation is used to incorporate food ingredients, pharmaceutical ingredients, enzymes, cells, or other materials on the micro or nanometric scale [21].
A large number of materials such as live cells, adhesives, flavours, agrochemicals, enzymes, and pharmaceuticals can be used as the polymer. The coating material should be cohesive and capable of forming a film with the core material, chemically compatible, and nonreactive with the core material providing the desired coating properties, such as strength, flexibility, impermeability, optical properties, and stability.
Chitosan is a linear polysaccharide composed of β-1-4-D-glucosamine linked to N-acetyl-D-Glucosamine residues and has been widely used in the pharmaceutical industry in drug delivery systems in different forms, such as tablets, microspheres, micelles, vaccines, nucleic acids, hydrogels, nanoparticles, and conjugates. Chitosan and its derivatives can be used in drug delivery systems in both implantable and injectable forms orally, nasally. Besides, they facilitate trans-mucosal absorption, which is important in the nasal and oral administration of some polar drugs such as peptides along with protein vaccines for administration. It is commonly used as an excipient in the formulation of tablets for oral medications. High molecular weight chitosan is more viscous and delays the release of the active ingredient, prolongs the duration of drug activity, improves therapeutic efficacy, and reduces the side effects of oral tablets. The chitosan microspheres have been extensively investigated to determine the controlled release of drugs and vaccines through oral and nasal delivery [22].
In this study, we used the chitosan-cellulose which is proved to have advantageous in providing better mechanical, thermal, permeation, biocompatibility, rigid network structure, cell attachment, and cell viability properties. These underline properties are very inspiring as in biomaterial, drug delivery vehicles, and more importantly in skin tissue engineering. Shahzadi team reported a convenient and efficient new cross-linking method for chitosan-cellulose with ZnO-NPs using triethylorthoformate (TEOF) as a cross-linking agent first time [22]. Indeed, the development of new cross-linkers is highly demanding to achieve the biomaterials with of desired biodegradability and capability of handling properties [23].
To the best of our knowledge, there are no studies on the biosynthesis of ZnO nanoparticles using Plumeria extract as the plant material in which large amounts of alkaloids are reported to be present. Therefore in this study, we synthesized, encapsulated, characterized, and studied in vitro drug release of encapsulated ZnO-NPs. In subsequent sections, we report the biosynthesis of stable colloidal ZnO-NPs using leaf extract of Plumeria obtusa and followed by the details characterization, microencapsulation, and kinetic studies for potential medicinal uses.
2 Materials and methods
This section includes the complete procedures adopted in this study on the synthesis of ZnO-NPs, encapsulations, characterizations and kinetic studies on the release of ZnO particles out of the microcapsules.
2.1 Preparation of aqueous leaf extract
Leaves of Plumeria plant were collected from the University of Ruhuna, Matara, Sri Lanka. Fresh leaves of Plumeria plant were cleaned by washing several times with running tap-water and followed with distilled water. Leaves were allowed to dry at room temperature for 5 days so that 80% of the moisture was removed. 20.0 g of dried leaves were cut into small pieces and blended with 200 mL of distilled water for 10 min. The leaf decoction was stirred in a magnetic stirrer for 45 min at 80 °C temperature. Then the aqueous extract was cooled, filtered using Whatman No. 1 filter paper, and stored at 4 °C for further use [15].
2.2 Synthesis of ZnO nanoparticles
ZnO-NPs were synthesized by adopting the method described by Yedurkar with some modifications [15]. 50.00 mL of 0.5 M of Zn(CH3COO)2 solution was added separately into each of 6 beakers. Then 0, 1.00, 5.00, 10.00, 15.00, 20.00 mL of leaf extracts were added, respectively into each beaker, followed by 50.00 mL of 2 M NaOH to maintain the pH at 12, and stirred continuously for 2 h by magnetic stirrer maintaining the temperature at 60 °C separately. The precipitates were washed three times each with distilled water and ethanol. The precipitates were kept in an oven at 60 °C for 24 h. All the chemicals used were in analytical grade from Sigma Aldrich.
2.3 Characterization of ZnO nanoparticles
The final weights of ZnO samples at the different amounts of plant extract were measured using an analytical balance (OHAUS, Germany) and percentage yield was calculated. Several characterization methods, UV–visible spectroscopy, Fourier Transformed Infra-Red (FT-IR) spectroscopy, XRD, and SEM, were used to characterize the prepared ZnO-NPs.
2.3.1 UV–visible spectral analysis
Synthesized ZnO-NPs were suspended in distilled water with 10 times dilution. The absorption spectra of colloidal solutions of ZnO-NPs were obtained in the wavelength range 300–400 nm by UH5300 Hitachi spectrophotometer, USA (with the scanning range of 190–1100 nm).
2.3.2 Fourier transformed infra-red (FT-IR) spectroscopy
FTIR spectra of biosynthesized ZnO-NPs were obtained using the FT-IR instrument (Thermo Scientific-Nicolet iS10 with 7800–350 cm−1 optimized, mid-infrared KBr beam-splitter) after spreading a small amount of sample powder evenly on the sample platform and scanning in the range of 400–4000 cm−1.
2.3.3 XRD analysis
The particle size and the nature of the ZnO-NPs were determined using the normal focus diffractometer, RigakuUltima IV XRD (with 2–90° 2θ measuring range and 2° min−1 scan speed). It was operated with Cu target in the range 3θ–80θ at 40 kV and 30 mA at the scan rate of 4°/min. The data were analyzed using Origin Pro 8.0 software. The size of the ZnO-NPs was determined by Debye Sherrer’s equation.
where D—Average crystalline size, β—line broadening in radians, θ—Bragg angle in radians and λ—X-ray wavelength.
2.3.4 Scanning electron microscope (SEM)
The samples were imaged using the field emission scanning electron microscope (FE-SEM); Model: Carl-Zeiss Evoma 15 (with a maximum resolution of 200 nm) from the Material Science Department, University of Moratuwa, Sri Lanka. The synthesized ZnO-NPs were placed in the sample holders with carbon sticky tapes and then placed in a coat sputter to inject a thin layer of gold coat. Then sample holder was placed in the FE-SEM machine and exposed to a highly focused beam of electrons from an electron gun. This beam of electrons is focused on a small spot on the sample surface using the SEM objective lens. The NPs were checked between the size range (100–200) nm.
2.3.5 Preparation of chitosan encapsulated ZnO nanoparticles
Chitosan encapsulated ZnO-NPs were prepared following the method reported by Shahzadi et al. [22, 23]. Initially, the chitosan solution was prepared by dissolving approximately 1.2 g of chitosan in 48.00 mL of 1% acetic acid solution at room temperature for 2 h. Similarly, approximately 1.2 g of cellulose was prepared by dissolving in 12.0 mL of 1% acetic acid maintaining the temperature at 80 °C. Both solutions were mixed thoroughly and stirred further overnight at room temperature. This homogeneous mixture was further treated with 1.2 mL of glacial acid and 3.0 mL of triethylorthoformate (TEOF) at 80 °C for 4 h. Then approximately 100 mg ZnO was added to the resulting solution and stirred for 2 h. The homogeneous solution was transferred to a petri-dish and allowed to cool to room temperature. Then petri-dish was placed in a freezer at − 20 °C for 8 h. The frozen membrane was immersed in a pre-cooled 3 M NaOH ethanolic solution and stored again in the freezer at − 20 °C overnight. Finally, the frozen membrane was washed thrice with 50% ethanol and a few times with distilled water until neutral pH was reached. The characterization of encapsulated ZnO-NPs was performed using UV–Visible spectroscopy, Fourier Transformed Infra-Red (FT-IR) spectroscopy, and as described earlier.
2.3.6 Tablets preparation
About 100 mg of encapsulated ZnO-NPs were prepared and sintered into tablets by using a pressing machine. The dimensions of all the tablets were measured by using Vernier Calliper and Micrometre screw gauge. Then the weight of each tablet of encapsulated ZnO-NPs was measured by analytical balance (OHAUS, Switzerland) [23].
2.3.7 Entrapment efficiency of encapsulated ZnO-NPs
The amount of unincorporated ZnO-NPs in encapsulation was measured by taking the absorbance of the supernatant solution at 356.0 nm. The ZnO percent entrapment efficiency (EE%) was calculated by subtracting the mass of free ZnO-NPs in the supernatant from the initial mass of the ZnO-NPs used as given below equation [23].
where w initial ZnO—Initial mass of ZnO-NPs used for the encapsulation, W free ZnO—Mass of ZnO-NPs in the supernatant [25].
2.3.8 In-vitro release study of ZnO-NPs
The dissolution of the ZnO-NPs in tablet form was investigated using Levy’s beaker and stirrer method [24]. The ZnO-NPs tablet was added to a 400 mL of dissolution medium containing 0.1 M HCl acid solution (pH 1.2). The mixture was continuously stirred by a magnetic stirrer at 37 ± 0.5 °C. A 5 mL sample aliquot from the dissolution mixture was withdrawn at different time intervals, filtered, and assayed at 356.0 nm on the UV–visible spectrophotometer. The ZnO-NPs concentration in each sample after each aliquot was taken out was corrected considering the concentrations in the previous sample. The dissolution test was repeated 3 times to obtain mean values. The cumulative percentage of the released ZnO-NPs versus time was plotted. The shape of each plot was fitted to one of five kinetic models given in Table 1 to understand the kinetic model for the particle release [23].
3 Results and discussion
A detailed explanation of the characterization of synthesized and encapsulated ZnO-NPs and kinetic models in ZnO-NPs release based on the results obtained from the performance of the procedures explained above will be presented in the following subsequent sections.
3.1 Percentage yield of ZnO nanoparticles
The percentage yields for a series of different plant extracts were measured keeping the other chemical reagents invariable [50.00 mL of 2 M NaOH and 50 mL of 0.5 M Zn(CH3COO)2] and are given in Online Resource 1. The highest percentage yield of 97.59% was obtained for the 1.00 mL of plant extract, while the lowest percentage yield of 97.30% was obtained for the 10.00 mL of plant extract. This shows that there are no significant variations in the percentage yields with the concentrations of the plant extracts. The 1.00 mL plant extract with the highest percentage yield was then used in reporting the characterization.
3.2 Characterization of ZnO nanoparticles
Figure 1 presents the UV–visible absorption spectra of ZnO-NPs synthesized using different amounts of plant extracts (plot b, c, d, and f) along with the ZnO-NPs prepared without the presence of plant extract (plot a). All the spectra showed the maximum absorption peak at 356.0 nm corresponding to the characteristic band of ZnO-NPs. The shape of the UV–visible spectrum is quite similar to the spectra reported in previous studies [12,13,14]. There is no change in the position of the λmax of the absorption spectra at the different amounts of plant extracts indicating that the particle size of the nanoparticles is independent of the concentration of the plant extract. In contrast, λmax, for the ZnO-NPs prepared without the presence of plant extract appears at 364.0 nm (plot a). This deviation of wavelength may be due to the reducing and/or the capping ability of the different alkaloids present in the plant [25].
The FT-IR spectra in Fig. 2 give the information on the vibrational and rotational modes in the ZnO-NPs. The characteristic absorption by Zn–O stretching vibrations for the biosynthesized ZnO-NPs with 1 mL and 20 mL of plant extracts are at 544 cm−1, and 545 cm−1, respectively. The broad absorption peaks attributed to the characteristic absorption of the hydroxyl group in the particles synthesized at two different concentrations, 1 mL and 20 mL are at 3377 cm−1 and 3213 cm−1, respectively. Even though the shapes of the peaks of two biosynthesized nanoparticles are quite similar, the peak’s transmittance intensity of the spectrum corresponding to the 20 mL of plant extract, which consists of more biological molecules than 1 mL extract, is higher than that of the spectrum corresponding to the 1 mL of plant extract. This intensity increase appears in most of the peaks in the spectrum. This observation proposes that the biological molecules could perform dual functions: the formation and stabilization of ZnO-NPs in the aqueous medium. These may be due to the adsorption of phytochemicals onto the surface of metal nanoparticles as reducing and capping agents. These peak shapes and its positions cannot be exactly compared with the previous studies since the functional groups which are present in plant vary on the types of the plants, but broad O–H stretching band could be observed in many previous studies [12,13,14, 26].
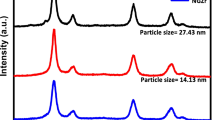
The X-ray diffraction (XRD) patterns of the biosynthesized ZnO-NPs at 1 mL and 20 mL of plant extracts were obtained and that for 1 mL, which gives the highest percentage yield, is shown in Fig. 3. X-ray diffraction pattern shows 2θ values at 31.71°, 34.39°, 36.24°, 47.52°, 56.56°, 62.86°, 67.91°, and 69.02°. The respective 2θ could be referred to (100), (002), (101), (102), (110), (103), (112) and (201) miller planes of the crystal structure. All the evident peaks are indexed as the ZnO-wurtzite structure. The wurtzite structure is most stable at ambient conditions and thus it is the most common form of ZnO. This confirms also that the synthesized nanopowder is free of impurities as it does not contain any characteristic XRD peak other than peaks corresponding to zinc oxide. This peak pattern is in agreement with the previous studies [12,13,14]. The average size of the nanoparticles was estimated by applying Debye Sherrer’s equation (1) to the most intense band. The most intense band [(101) plane] of ZnO-NPs synthesized by 1 mL and 20 mL plant extracts are at 36.24° and 36.28°, and then the average sizes calculated corresponding to those two extracts are 27.23 nm and 25.92 nm, respectively.
3.3 Characterization of encapsulated ZnO nanoparticles
The absorption spectrum was recorded for the encapsulated ZnO-NPs in the range of 200–400 nm. The spectrum shows two strong absorption peaks at 232.5 nm, 242.5 nm, and a weak peak at 357 nm. Figure 4 compares the absorption band of the chitosan encapsulated ZnO-NPs with that of the neat chitosan [27].
The characteristic peak of ZnO-NPs at 364 nm in the chitosan encapsulated ZnO-NPs (spectrum b), the absence of it in the spectrum of the neat chitosan, and the blue shift of the absorption band corresponding to the chitosan encapsulated ZnO-NPs evidence the formation of chitosan/ZnO nanoparticle composite. Li et al. reported a similar blue-shifted absorption band in the composite [25].
FT-IR spectra of neat ZnO-NPs and chitosan encapsulated ZnO-NPs at for 1 mL of plant extract are shown in Fig. 5. In the FT-IR spectrum, the intensities of peaks corresponding to the ZnO-NPs become intense with the encapsulation as similar to the intensity increase observed above when the plant extract increased from 1 mL to 20 mL for the neat ZnO-NPs. Therefore, this further evidences the role of the biological molecules/chitosan on the ZnO surface. The broad peak that was due to the hydroxyl group stretching observed before the encapsulation has red shifted and overlapped with the broad peak at 3333 cm−1 due to the N–H stretching in the amide group of chitosan polymer or completely diminished. The peak at 1420 cm−1 in the spectrum before the encapsulation also has red shifted without a notable intensity change upon the encapsulation. Most importantly, the confirmation of the formation of encapsulation is evidenced by the newly formed highly intense unique peak at 1030 cm−1 which is due to the C–N stretching of the bond between chitosan and cellulose with TEOF as a cross-linker. The peaks’ shapes and positions are in agreement with the work reported by Shahzad et al. [26].
The XRD patterns of the neat ZnO-NPs and the chitosan encapsulated ZnO-NPs at the plant extract of 1 mL are compared in Fig. 6. The X-ray diffraction pattern shows 2θ values at 15.69°, 22.55°, 26.71°, and 34.51°. All the evident peaks, except the last peak at 34.51°, could be due to the presence of chitosan. The peak at 2θ equals 34.51° is from the (002) plane of the crystal structure of zinc oxide. The peak at 22.55° could be attributed to the chitosan molecule and the intensity of this peak is high compared to the intensity of the peaks due to ZnO as a result of the use of a comparatively high amount of the chitosan over ZnO during the encapsulation, and thus as a result, ZnO peaks were buried under the high intense chitosan peaks [28]. The unchanged peak position of ZnO corresponding to (002) plane under the presence of chitosan confirms the unchanged structure of ZnO upon the encapsulation which is in agreement with the work reported by Raman Dhivya et al. [29].
The surface characteristics of ZnO-NPs and the chitosan encapsulated ZnO-NPs were examined using the scan electron microscope (SEM), and the image obtained is shown in Fig. 7 (See also the original images in Online Resource 2). The EDX spectra and the data are given in Online Resource 3. The biosynthesized ZnO-NPs show a smooth dispersed spectrum of nanoparticles in the sizes between 62 and 73 nm of the particle diameter (7a). In the encapsulation, the ZnO-NPs have aggregated into the microscale in the size range of 5–14 μm (7b). The encapsulated ZnO-NPs are irregular in shape and they are well separated apart promising an effective release.
3.4 Entrapment efficiency of encapsulated ZnO nanoparticles
Particle entrapment efficiency (PEE) was expressed as the percentage of ZnO-NPs entrapped in the chitosan shell compared to the initial amount of ZnO-NPs used in the encapsulation. The entrapment efficiency was calculated for the series of plant extracts used in the biosynthesis of ZnO-NPs. The efficiencies corresponding to the ZnO-NPs originated from each of 0, 1, 5, 10, 15 and 20 mL of Plumeria extract were 65%, 73%, 77%, 80%, 82%, and 82%, respectively. The highest entrapment efficiency percentage with the value 82% is found in the encapsulated ZnO-NPs originated at 15 mL of plant extract. The lowest entrapment efficiency percentage with the value 65% is found in the encapsulated ZnO-NPs without plant extract in synthesis. The entrapment efficiency gradually increases with the amount of plant extract and eventually reaches the constant value of ~ 82% at 15 mL of plant extract. This increase in DEE is also following the wavelength decrease observed above in the UV–visible spectra from 364 nm to 356 nm due to the reducing and/or the capping ability of biomolecules present in the plant. The biological molecules such as plant alkaloids capped on the surface of the ZnO-NPs may have enhanced the interaction between ZnO nanosurface and the chitosan molecules, and hence the DEE increases with the concentration of the plant extract.
3.5 In-vitro dissolution kinetic studies
The dissolution of ZnO-NPs in a tablet form was investigated using Levy’s beaker and stirrer methods. The in vitro release profiles of the encapsulated ZnO-NPs originated from 0 to 20 mL of plant extracts were carried out at pH 1.2 for 2 h and the cumulative particle release (CDR %) profiles are shown in Fig. 8. According to the United States Pharmacopeia (USP) 24, upon exposing to 0.1 M HCl (pH 1.2) medium, all the enteric-coated dosage form should not release more than 10% of its drug load in the initial period of 2 h [25]. Figure 8 reveals that there is a slow release of ZnO-NPs from the tablet in pH 1.2 medium and the tablets release only about 9.84% at the end of 2 h, hence the ZnO-NPs kinetic profile is following the specifications of the USP24 [29]. The low percentage of the particle release at pH 1.2 ensures the high bioavailability of ZnO-NPs to the small intestines.
The encapsulated ZnO-Nps synthesized without plant extract gives the lowest cumulative particle release (9.10%) while those synthesized at different amounts of plant extracts show no significant difference after 2 h. This slightly increased cumulative release of the encapsulated nanoparticles derived with plant extracts may be due to the interactions between organic molecules capped on the surface of the ZnO-NPs and the solvent species. The shapes of all the plots, especially relevant to the ZnO-NPs synthesized with the plant extracts, follow an identical pattern with four regions in 2 h revealing that the particle release occurs through four different kinetics. The inserted-figures in Fig. 8 show the best of five kinetic models (Table 1) that fitted to the kinetic data in each region of 0–50, 50–75, 75–100, and 100–120 min while data correlation coefficients (R2) and the kinetic constants corresponding to four regions are given in Table 2.
In the first region (0–50 min), the release profile follows the zero-order kinetics (k0 = 1.179 × 10−1 min−1) which is in accordance with the slow release of drug from the coated capsule and the rate is independent of the size of the ZnO-NPs dose. The next 50 min (from 50 min to 100 min) follow Hixon Crowell kinetic model with two different Hixon Crowell constants (kHC) before it backs to the zero-order from 100 min to 120 min. The rate constant (kHC) in the time interval from 50 min to 75 min is 2.6 × 10−3 min−1 and it from 75 min to 100 min is 13 × 10−3 min−1 which is five times greater than the rate constant for the first half from 50 min to 75 min.
Similarly, the study on in vitro particle release profiles was carried out at higher pH (6.8) for the encapsulated ZnO-NPs originated from 20 mL of plant extract for 2 h mimicking pH below the stomach of the digest system. The cumulative particle release profiles are shown in Fig. 9 and the kinetic models and parameters obtained by fitting the experimental data are given in Table 3. The ZnO-NPs release passed the recommended maximum limit (USP-24) for the stomach within the first 50 min. At the end of the 2 h, the chitosan encapsulated ZnO-NP tablets reach the cumulative drug particle release (CDR%) to 17.84% which ensures the bioavailability of ZnO-NPs in the small intestine rather than the stomach.
Similar to Fig. 8 corresponding to the drug release at 1.2 pH, Fig. 9 shows for different kinetics in 2 h. The inserted-figures in Fig. 9 show the best kinetic model corresponding to each region of 0–35, 35–50, 50–85, and 95–120 min. The shape of the graph itself and the larger rate constants evidenced that the change in particle release kinetics and the rate of particle release are faster compared to it is at 1.2 pH.
4 Conclusion
The biological synthesis using leaf extract of Plumeria provides an environmentally friendly, simple, and efficient route for the synthesis of ZnO-NPs. The prepared ZnO-NPs with a high yield of 97% on average were characterized using XRD, FT-IR, and UV–visible absorption spectroscopic techniques. The blue-shifted UV–visible absorption peak at 356.0 nm and also the characteristic absorption peak of Zn–O bond stretching in the FT-IR spectrum at 544 cm−1 confirm the formation of ZnO-NPs. X-ray diffraction confirmed the formation of the pure hexagonal wurtzite phase which is the most stable form of the zinc oxide at ambient conditions. The average particle size of the ZnO-NPs was found as 27.23 nm which indicates that there is a uniform size distribution which is also evidenced by the dispersed SEM spectrum of the particles.
The microencapsulated ZnO-NPs were characterized by XRD, FT-IR, SEM, and UV–visible absorption techniques. The chitosan loaded ZnO-NPs have higher entrapment efficiency (about 82% at 15 mL of plant extract). The kinetic studies on cumulative particle release at 1.2 pH show that the coated ZnO-NPs do not release more than 10% of its original load in the tablet ensuring that the bioavailability of the coated ZnO-NPs remains until it reaches the small intestine passing the stomach, in contrast at higher pH (6.8), the tablet achieves 17.84% of cumulative particle release.
This study shows that ZnO nanoparticles can easily be biosynthesized and encapsulated for use in the pharmaceutical industry. ZnO-NPs prepared can supplement Zn2+ ions, which is an essential nutrient in various aspects of metabolism in the human body.
References
Vijayanandan A, Balakrishnan R (2018) Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J Environ Manag 218:442–450. https://doi.org/10.1016/j.jenvman.2018.04.032
Thiruvengadam M, Chung I, Gomathi T et al (2019) Synthesis, characterization and pharmacological potential of green synthesized copper nanoparticles. Bioprocess Biosyst Eng 42:1769–1777. https://doi.org/10.1007/s00449-019-02173-y
Shankar SS, Ahmad A, Sastry M (2003) Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog 19(6):1627–1631. https://doi.org/10.1021/bp034070w
Azizi S, Ahmad M, Namvar F, Mohamad R (2014) Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassummuticum aqueous extract. Mater Lett 116:275–277. https://doi.org/10.1016/j.matlet.2013.11.038
Haratifar E, Shahverdi H, Shakibaie M, Mollazadeh MK, Amini M, Montazeri H, Shahverdi AJ (2009) Semi-biosynthesis of magnetite-gold composite nanoparticles using an ethanol extract of Eucalyptus camaldulensis. J Nanomater 2009:1–5. https://doi.org/10.1155/2009/962021
Govindaraju K, Basha S, Kumar V, Singaravelu G (2008) Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J Mater Sci 43(15):5115–5122. https://doi.org/10.1007/s10853-008-2745-4
Kowshik M, Deshmukh N, Vogel W, Urban J, Kulkarni S, Paknikar K (2002) Microbial synthesis of semiconductor CdS nanoparticles their characterization and their use in the fabrication of an ideal diode. Biotechnol Bioeng 78(5):583–588. https://doi.org/10.1002/bit.10233
Rautaray D, Ahmad A, Sastry M (2003) Biosynthesis of CaCO3 crystals of complex morphology using a fungus and an actinomycete. J Am Chem Soc 125(48):14656–14657. https://doi.org/10.1021/ja0374877
Söderstjerna E, Johansson F, Klefbohm B, Englund JU (2013) Gold- and silver nanoparticles affect the growth characteristics of human embryonic neural precursor cells. PLoS ONE 8(3):58211. https://doi.org/10.1371/journal.pone.0058211
Królikowska A, Kudelski A, Michota A, Bukowska J (2003) SERS studies on the structure of thioglycolic acid monolayers on silver and gold. Surf Sci 532–535:227–232. https://doi.org/10.1016/s0039-6028(03)00094-3
Kumar A, Mandal S, Selvakannan P, Pasricha R, Mandale A, Sastry M (2003) Investigation into the interaction between surface-bound alkylamines and goldnanoparticles. Langmuir 19(15):6277–6282. https://doi.org/10.1021/la034209c
Yedurkar S, Maurya C, Mahanwar P (2016) Biosynthesis of zinc oxide nanoparticles using ixoracoccinea leaf extract—a green approach. OJSTA 5:1–14. https://doi.org/10.4236/ojsta.2016.51001
Verma PR, Khan F, Banerjee S (2020) Salvadorapersica root extract-mediated fabrication of ZnO nanoparticles and characterization. Inorg Nano-Met Chem. https://doi.org/10.1080/24701556.2020.1793355
Agarwal H, Kumar SV, Rajeshkumar S (2017) A review on green synthesis of zinc oxide nanoparticles—an eco-friendly approach. Res-Eff Tech 3(4):406–413. https://doi.org/10.1016/j.reffit.2017.03.002
Narayanan KB, Sakthivel N (2011) Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv Colloid Interface Sci 169(2):59–79. https://doi.org/10.1016/j.cis.2011.08.004
Singh M, Manikandan S, Kumaraguru AR (2011) Nanoparticles: a new technology with wide applications. Re J Nanosci 1:1–11. https://doi.org/10.3923/rjnn.2011.1.11
Narwariya P (2017) Comprehensive overview of Plumeriaobtusa. World J Pharm Res 8:664–676. https://doi.org/10.20959/wjpr20174-8212
Sibi G, Venkategowda A, Gowda L (2014) Isolation and characterization of antimicrobial alkaloids from plumeria alba flowers against food borne pathogens. Am J Life Sci 2(6-1):1–6. https://doi.org/10.11648/j.ajls.s.2014020601.11
Siddiqui B, Ilyas F, Rasheed M, Begum S (2004) Chemical constituents of leaves and stem bark of Plumeria obtuse. Phytochemistry 65(14):2077–2084. https://doi.org/10.1016/j.phytochem.2004.04.024
Ahmad J, Bazaka K, Anderson LJ, White RD, Jacob MV (2013) Materials and methods for encapsulation of OPV: a review. Renew Sust Energ Rev 27:104–117. https://doi.org/10.1016/j.rser.2013.06.027
Suganya V, Anuradha V (2017) Microencapsulation and nanoencapsulation: a review. IJPCR 9(3):233–239. https://doi.org/10.25258/ijpcr.v9i3.8324
Shahzadi L, Chaudhry A, Aleem A, Malik M, Ijaz K, Akhtar H, Alvi F, Khan A, Rehman I, Yar M (2018) Development of K-doped ZnO nanoparticles encapsulated cross linked chitosan based new membranes to stimulate angiogenesis in tissue engineered skin grafts. Int J Biol Macromol 120:721–728. https://doi.org/10.1016/j.ijbiomac.2018.08.103
Hii YS, Jeevanandam J, Chan YS (2018) Plant mediated green synthesis and nanoencapsulation of MgO nanoparticle from Calotropisgigantea: characterisation and kinetic release studies. Inorg Nano-Met Chem 48(12):620–631. https://doi.org/10.1080/24701556.2019.1569053
Levy G (1961) Comparison of dissolution and absorption rates of different commercial aspirin tablets. J Pharm Sci 50:388–392. https://doi.org/10.1002/jps.2600500503
Li L, Deng J, Deng H, Liu Z, Xin L (2010) Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr Res 345:994–998. https://doi.org/10.1016/j.carres.2010.03.019
Shahzad S, Siddiqi S, Mahmood N, Rauf A, Anwar M, Chaudhry A, Rehman I (2015) Triethylorthoformate mediated a novel crosslinking method for the preparation of hydrogels for tissue engineering a lications: characterization and in vitro cytocompatibility analysis. Mater Sci Eng C 56:154–164. https://doi.org/10.1016/j.msec.2015.06.021
Vaseeharan B, Sivakamavalli J, Thaya R (2013) Synthesis and characterization of chitosan-ZnO composite and its antibiofilm activity against aquatic bacteria. J Compos Mater 49(2):177–184. https://doi.org/10.1177/0021998313515289
Kendall R, Alhnan M, Nilkumhang S, Murdan S, Basit A (2009) Fabrication and in vivo evaluation of highly pH-responsive acrylic microparticles for targeted gastrointestinal delivery. Eur J Pharm Sci 37:284–290. https://doi.org/10.1016/j.ejps.2009.02.015
Dhivya R, Ranjani J, Rajendhran J, Mayandi J, Annaraj J (2018) Enhancing the anti-gastric cancer activity of curcumin with biocompatible and pH sensitive PMMA-AA/ZnO nanoparticles. Mater Sci Eng C 82:182–189. https://doi.org/10.1016/j.msec.2017.08.058
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Halanayake, K.D., Kalutharage, N.K. & Hewage, J.W. Microencapsulation of biosynthesized zinc oxide nanoparticles (ZnO-NPs) using Plumeria leaf extract and kinetic studies in the release of ZnO-NPs from microcapsules. SN Appl. Sci. 3, 17 (2021). https://doi.org/10.1007/s42452-020-04100-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-04100-3