Abstract
The solubility of NaCN and Na2CO3 in the water–ethanol mixture was determined through experiments. Also the polynomial fit was used to lead to the relationship between the solubility of NaCN/Na2CO3 and the solvent composition. In the meantime, the solvent molecular-electrolyte pair interaction parameters of electrolyte NRTL (eNRTL) model were estimated by using the data regression function of Aspen Plus software from solubility data of NaCN and Na2CO3 in water–ethanol mixed solvent. The process simulation for separation and purification of NaCN and Na2CO3 in water–ethanol mixed solvent was carried out by using Aspen Plus software. During the simulation, electrolyte equilibrium system in water–ethanol mixed solvent was observed by using eNRTL model property method, and the influence of several factors on the separation efficiency of NaCN was examined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
High purity NaCN is widely used in many fields including electroplating, precious metal smelting, catalyst industry and the demand for high purity NaCN is getting higher [1,2,3,4,5]. The crude NaCN usually obtained by the solid phase synthesis method contains sodium salts such as Na2CO3 and sodium formate (NaHCOO).
Many studies have been already carried out to produce pure NaCN from low-grade NaCN [6, 7]. One effective method to obtain pure electrolyte from a crude is by using the solubility difference between NaCN and other impurities in a suitable solvent [8, 9]. Solvents which dissolve NaCN but not for other impurities are already introduced. For example, it was known that methanol or ethanol could be used to dissolve NaCN and then the solution was evaporated to dryness to recover the purified form. Anhydrous ammonia is also well known solvent and a process for obtaining the pure cyanide by dissolving crude using anhydrous ammonia under pressure followed by evaporation of ammonia is also proposed. Due to not so high solubility of NaCN in anhydrous alcohol, anhydrous methanol or anhydrous ethanol consume a large amount of solvent in the dissolution step and a great deal of energy in the evaporation step.
Solubility and productivity of NaCN in solvent had been increased by using water–ethanol mixed solvent. The first thing in designing the purification process of NaCN in a water–ethanol mixed solvent is to determine the solubility of the materials in the mixed solvent. In this paper, solubility data for NaCN and Na2CO3 in water–ethanol mixed solvent were measured at different temperatures. These data have been used to simulate the process for separating NaCN and Na2CO3 in a water–ethanol mixed solvent.
The eNRTL model is widely used for phase equilibrium calculations in mixed solvent electrolyte systems [9,10,11]. This model was proposed by Chen [12] as an extension of the NRTL model of Renon and Prausnitz [13]. The eNRTL model was modified by Chen and Song [14] and recently supplemented by Bollas et al. [15]. The eNRTL model divides non-ideal contribution into a short range binary contribution and a long range electrostatic contribution. Activity coefficients are calculated based on asymmetry, where the reference state of the solvent is pure solution and the reference state of the solute is the infinitely diluted state in water. It is very important to precisely determine the interaction parameters of the solvent molecule–electrolyte pair when simulating electrolyte equilibria in mixed solvents using eNRTL models. Many parameters for eNRTL model have been stored in the databanks provided by Aspen Plus software. However, some parameters must be determined by experiment [16, 17]. In this paper, eNRTL model parameters were estimated from the solubility data of NaCN and Na2CO3 in water–ethanol mixed solvent. The estimated parameters were also used to simulate the process. Then, the process for separation of NaCN and Na2CO3 in water–ethanol mixed solvent was simulated by using Aspen Plus software.
Aspen Plus is a steady-state process simulator for predicting the behavior of a process or group of unit operations through existing relationships between them. Aspen Plus software process simulation is used in computer-aided design, process optimization (e.g., improving production and process efficiency, minimizing operational costs and emission of waste that may be contaminant, improving energy efficiency, etc.), solving operational problems, and so on [18,19,20]. In this paper, optimization of the process for the separation of NaCN and Na2CO3 mixtures in a water–ethanol mixed solvent was carried out.
2 Determination of solubility of NaCN and Na2CO3 in water–ethanol mixed solvents
2.1 Experimental
2.1.1 Chemicals and instrumentation
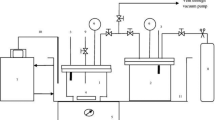
Ethanol was supplied by Merck and used without further purification (mass fraction purity >0.99). The purity of ethanol was checked by gas chromatography (GC). In this study, deionized and redistilled water was used. NaCN (>99 wt%)and anhydrous Na2CO3 (99 wt%) were purchased from Wako Pure Chemicals Industries, Ltd. (Osaka, Japan). In addition, 0.1000 mol/L Silver nitrate titration solution, 10% aqueous solution of potassium iodide and 25 wt% aqueous ammonia solution are used. All of these substances were of analytical grade. Aqueous solutions were prepared just before use by dissolving the reagent in ultrapure water.
As instrument, a magnetic stirrer (IKA RO10), electronic balance (Sartorius BT25S, ±0.000001 kg), constant temperature bath (FDLRTS-0A, ±0.01 °C), Vacuum drying oven (HD-E804-30B) were used in this study. Phase identification of solid phase was performed with X-ray diffraction analysis (XRD, Rigaku SmartLab, CuKα radiation from rotating anode X-ray tube, 1.5406 Å).
2.1.2 Experimental methods
First, a solid sample (NaCN and Na2CO3) was excessively added to pure water and various concentrations of a water–ethanol mixed solvent, and the mixture was thoroughly stirred by a magnetic stirrer. Next, the sample was placed in a constant temperature bath and allowed to stand at a constant temperature for 24 h or longer to reach an equilibrium state.
When the solid–liquid equilibrium is sufficiently reached, 1.00 mL of the supernatant is sampled from the sample, and the mass of the solution is weighed. The content of Na2CO3 in the solution was determined by evaporating the solution at 150 °C and weighing the remaining solid sample. The content of NaCN was determined by coordination titration using a 0.1000 mol/L silver nitrate solution as a titration solution and a 10 wt% potassium iodide aqueous solution as an indicator.
The solubility of NaCN and Na2CO3 at the respective temperatures and solvent compositions was determined from the mass of the solution and the amount of solid sample dissolved therein.
On the other hand, the remaining sample was separated by filtration to obtain a solid component. The crystal structure of the solid in solid–liquid equilibrium state was analyzed by powder X-ray diffraction analysis. The XRD patterns were taken in the angular range 2θ = 20°–60° with a scanning speed of 2°/min and step angle of 0.02°. Qualitative phase analysis has been performed using MDI JADE 9.0 software. All structural data for each of the phase are according to ICDD.
2.2 Results and discussions
The solubility of the NaCN electrolyte in the NaCN–H2O binary system at different temperatures are shown in Table 1. Where T is the temperature of the solution and S is the solubility of the salt in the liquid phase.
According to the X-ray analysis results, there are the two solid phases, NaCN·2H2O and NaCN, in the NaCN–H2O binary system: NaCN·2H2O in the low temperature, NaCN in the high temperature. All diffraction peaks of NaCN and NaCN·2H2O in the NaCN–H2O two-dimensional system are same with ICDD reference code 75-0872, 29-1206 respectively, and the space group of these two phases are belong to Immm of orthorhombic and P21/c of monoclinic respectively. Figure 1 shows the XRD patterns considering the change in the solid phase according to the temperature.
The relationship between the temperature and solubility of NaCN in pure water was regressed using Eq. (1) in Origin 8.1, and the following result was obtained.
A = 30.21327, B = −0.15778, C = 0.0231, D = −8.5487·10−5, δ = 0.97982.
Here, δ = ∑ [(Scal − Sexp)2/N]1/2, N is the number of test points.
On the other hand, the solubility measurement data for NaCN–C2H5OH–H2O ternary system at each temperature is shown in Table 2. Where C is the content of ethanol in the solvent and S is the solubility of NaCN in solution.
NaCN–C2H5OH–H2O ternary system, as in the NaCN–H2O binary system, there are two solid phases of NaCN·2H2O and NaCN present, the higher the temperature and ethanol content, the higher the NaCN content.
The results of Eq. (2) regression of the solubility relationship of NaCN according to the solvent composition are shown in Table 3.
In the equation, wROH is the mass fraction of ethanol in the mixed solvent.
Next, the solubility measurement data for Na2CO3–C2H5OH–H2O ternary system at each temperature is shown in Table 4. The liquid-phase equilibrium data for the Na2CO3–H2O binary system have not been reported here because they have already been reported in the prior literatures. Where C is the content of ethanol in the solvent and S is the solubility of Na2CO3 in solution.
Figure 2 shows X-ray patterns in accordance with temperature in the NaCN–C2H5OH–H2O ternary system of the content of 64.9% ethanol. In the Na2CO3–C2H5OH–H2O ternary system, there may be exist at least one of the three solid phases, Na2CO3·10H2O, Na2CO3·7H2O, and Na2CO3·H2O, depending on the conditions of temperature and the changes in ethanol content. According to the X-ray diffraction analysis, all diffraction peaks of these phases are same with ICDD reference code 75-7991, 70-2148 and 09-3809 respectively, and the space group of these three phases are belong to C1c1 of monoclinic, Pbca and P21ab of orthorhombic respectively.
The results of Eq. (2) regression of the solubility relationship of Na2CO3 according to the solvent composition are shown in Table 5.
3 Estimation of interaction parameters between solvent molecule–electrolyte pair interaction of eNRTL model
eNRTL model is a multidimensional model for calculating electrolyte activity coefficient and not only can be expressed in the water-soluble electrolyte system but also can be expressed in the mixed electrolyte system over the entire electrolyte concentration range by using the various interaction parameters.
In this model, the asymmetric Pitzer–Debye–Huckel model and the Born equation are used to denote contributions due to remote ion–ion interactions, and the NRTL model is used to indicate contribution due to local interaction (Figs. 3 and 4).
In general, the parameters for the eNRTL model include:
-
(a)
The dielectric constant of a pure component in a non-aqueous solvent.
-
(b)
Born radius of ion species.
-
(c)
NRTL parameters for molecule–molecule, molecule–electrolyte, electrolyte–electrolyte pair.
In the non-aqueous solvent, the dielectric constant of the pure component and the born radius of the ionic species are required only in the mixed solvent electrolyte system. All forms of eNRTL parameters are consist of two non-random factors α and an energy parameter τ.
Many parameters are included in the eNRTL model parameter databank provided by Aspen. First, the databank contains most of the binary parameters (NRTLs) for molecular–molecule interactions. There is also a certain amount of data on the non-coincidence factor (GMELCN) and the energy parameter (GMELCC, GMELCD, GMELCE) for the molecule–electrolyte pair and the electrolyte–electrolyte pair.
However, the values of some of these parameters are not given and in most cases they should be estimated through experiments. In particular, the Aspen Plus databank does not include molecular–electrolyte interaction parameters we are trying to estimate, except H2O–(Na+, CO32−).
Therefore, we have estimated the interaction parameters between solvent molecules (water or ethanol)–electrolytes (NaCN or Na2CO3) pairs based on solubility data of NaCN and Na2CO3 in a water–ethanol mixed solvent experimentally determined previously.
In this study, the data regression function provided by Aspen Plus (v8.4) was used to regress the solvent molecule–electrolyte pair interaction parameters of the eNRTL model from solubility experimental data.
To do this, we selected the electrolyte process standard simulation model in the Aspen Plus (v8.4) software and set the data regression method as the run mode. We then defined the required components in the [Components] folder of the [Properties] guide list and used the [Elec wizard] technique to define the various chemical reactions that can occur in the electrolyte system and the resulting species. In addition, the ELECNRTL model is set up by the physical property method, and the model parameters provided in the Aspen Plus databank are searched and defined. Next, in the [Data] folder of the [Properties], the experimental data for the solid–liquid phase equilibrium in each electrolyte system (H2O–NaCN, H2O–C2H5OH–NaCN and H2O–C2H5OH–Na2CO3) were entered. Then, regression was performed after setting the initial value, the upper limit value, and the lower limit value of the molecular-electrolyte pair interaction parameters in the [Regression] folder.
The interaction parameters of the molecule–electrolyte pairs that are regressed using the Aspen Plus software are shown in Tables 6, 7, and 8, respectively.
The solubility of NaCN and Na2CO3 in water and water–ethanol mixture that simulated by using the molecular–electrolyte interaction parameters obtained by regression of the solubility data was compared with experimental data. The results are shown in Figs. 5 and 6, respectively.
As can be seen from Figs. 5 and 6, the simulation results are comparable to the experimental results. Therefore, the previously estimated solvent molecule–electrolyte pair interaction parameters can be used to simulate the NaCN and Na2CO3 separation processes in a water–ethanol mixed solvent.
4 Process simulation for separation of NaCN and Na2CO3 mixture by using water–ethanol mixed solvent
4.1 Establishment of process model
4.1.1 Create process diagram
The process of separating materials using solubility differences in mixed solvents generally consists of dissolving, filtering, evaporating and drying the raw material mixture.
The Aspen Plus process diagram for separating NaCN and Na2CO3 mixture in water–ethanol mixed solvent is shown in Fig. 7. As shown in Fig. 7, the major unit operating models used in the process simulation are Flash2 (gas–liquid separator), CFuge (crystallizer), and Dryer (dryer). In the process diagram, the SOLUTION model (Flash2) simulates the electrolyte equilibrium process when the raw materials (NaCN and Na2CO3 mixture) are dissolved in a water–ethanol mixed solvent. The SEPARATE model (CFuge) simulates the centrifugation process of Na2CO3 precipitates that are not dissolved in mixed solvents. The EVAPOR model (Crystallizer) simulates the process of obtaining NaCN crystals by evaporating and concentrating the NaCN solution. The DRYER model simulates the process of completely removing the moisture contained in NaCN crystals from the crystallizer.
Definition of component
First, NaCN and Na2CO3, H2O, C2H5OH are defined as general components. Next, since NaCN, Na2CO3, and H2O exist in the electrolyte form, they use the Electrolyte Wizard function provided by Aspen Plus to define electrolyte dissociation equilibrium and all other components obtained during the salt formation process. The results are shown in Table 9.
4.1.2 Selection of physical properties and determination of parameters
The correct choice of physical properties is a key part of the process simulation.
The eNRTL model is best known as a thermodynamic model for simulating the electrolyte equilibrium process in a mixed solvent system. The eNRTL model is a multivariate model for calculating the activity coefficient, which can be used not only in a water-soluble electrolyte system, but also in a mixed solvent–electrolyte system over a full range of electrolyte concentrations. This model can calculate the activity coefficient for ion species and molecular species in water soluble electrolyte systems and mixed solvent electrolyte systems.
Therefore, in this paper, the eNRTL model was used to simulate the separation process of NaCN and Na2CO3 mixtures in a water–ethanol mixed solvent.
In Aspen PLUS, The eNRTL model parameters of a number of substances required for electrolytic activity coefficient calculation are already documented. However, there are many unknown parameters. So, Based on the measurement of the solubility of NaCN and Na2CO3 in the water–ethanol mixture, we have estimated the solvent molecules (water or ethanol)–electrolytes (NaCN and Na2CO3) pair interaction parameters by using data regression function of Aspen Plus.
4.2 Process simulation and determination of optimum conditions
4.2.1 Set initial input value
Table 10 shows the composition of the initial raw materials (mixture of NaCN and Na2CO3) and mixed solvents used in the process simulations. As shown in the table, the content of NaCN in the initial raw material is 50 wt%, the content of ethanol in the mixed solvent is 70 wt%, and the ratio of the solid to liquid is 1:6.67.
The initial process conditions set in the unit operation models are shown in Table 11.
4.2.2 Process simulation results and discussions
Table 12 shows the results of simulations using the initial raw materials and mixed solvent composition and process conditions. In Table 12, the material flow LEACHATE contains the content of each material component in equilibrium when the raw material is dissolved in the mixed solvent. The material flow Na2CO3 contains the content of the substance components contained in the precipitate taken from Centrifuge. The material flow FILTRATE contains the content of each substance component contained in the supernatant taken from the centrifuge. The content of each of the material components contained in the concentrate obtained by evaporation concentration in the crystallizer is included in the material flow TODRYER. Substance flow PRODUCT contains the content of each substance component in the final product obtained through the drying process.
As shown in Table 12, when the mixture of NaCN and Na2CO3 was dissolved in a mixed solvent of water and ethanol, NaCN was completely dissolved and Na2CO3 was almost completely precipitated as a Na2CO3∙H2O (SALT2) crystal hydrate. Also, the purity of the separated Na2CO3 precipitate and the final NaCN product was very high, more than 99 wt%. From this, it can be seen that a mixed solvent can be used to effectively separate NaCN and Na2CO3.
4.2.3 Optimization of process conditions
In the process simulation, the purity and yield of NaCN products were investigated in various raw material conditions (NaCN contents are 20 wt%, 30 wt%, 40 wt%, 50 wt%, respectively) with varying solid–liquid ratio, solvent composition and dissolution temperature.
-
Influence of solid–liquid ratio
The ethanol content in the mixed solvent was 70 wt%, and the dissolution temperature was fixed at 35 °C, and the separation efficiency of the raw material mixture was examined while varying the solid–liquid ratio. The results are shown in Table 13. Where CNaCN is content of NaCN, R is the solid–liquid ratio, P is the purity, and Y is the yield.
In the simulation, the total amount of raw material was fixed to 6 kg and the amount of solvent was changed. Other conditions are same as Tables 9 and 10.
As shown in Table 13, as the solid–liquid ratio decreases, the purity of the NaCN product does not change, but the yield decreases. This is because, as the solid–liquid ratio decreases, NaCN does not completely dissolve in the mixed solvent and remains a precipitate with Na2CO3. On the other hand, as shown in Table 13, the optimum solid–liquid ratio decreases as the NaCN content in the raw material decreases. That is, when the content of cyanide in the raw material is 50 wt%, the optimum solid–liquid ratio is 1:6.5 and when the content is 40 wt%, it is 1:5.5. When the content of cyanide is 30 wt%, the optimum solid–liquid ratio is 1:4.5 and when the content is 20 wt%, it is 1:3.5.
-
Influence of Solvent Composition
According to the composition of the raw material, the solid–liquid ratio is changed to the optimum solid–liquid ratio and the dissolution temperature is kept constant at 35 °C.
Then, the results of examine the purity and yield of NaCN according to the content of ethanol in the mixed solvent is shown as Table 14. Where CNaCN is the NaCN content in solution, Cethanol is content of Ethanol in mixed solvents, R is the solid–liquid ratio, P is the purity, and y is the yield. In the simulation, the amount of solid raw material was kept constant and only the mixed solvent content was changed. Other conditions are the same as above. As shown in Table 14, it can be seen that as the ethanol content in the solvent decreases, the purity of the NaCN product decreases, and when the ethanol content increases, the NaCN yield decreases sharply. This is because the low ethanol content does not cause a significant difference in solubility between the two electrolytes, so the product contains a large amount of Na2CO3. And, when the ethanol content is too high, the solubility of NaCN is drastically lowered so that a large amount of NaCN is not dissolved and remains as a precipitate. As shown in Table 14, reasonable solvent composition is when the ethanol content of 60–70 wt%.
-
Influence of dissolution temperature
According to the composition of the raw materials, the optimum mixing ratio was set as the solid–liquid ratio, and the ethanol content was fixed to 70 wt% in the mixed solvent. Then, the purity and deposition rate of the NaCN product were examined while varying the dissolution temperature. The results are shown in Table 15. As can be seen from Table 15, the lowering of the dissolution temperature leads to a lower yield of NaCN. This is because when the temperature is lowered, the solubility of NaCN is lowered and a part of NaCN precipitates without being dissolved in the mixed solvent. From this, the dissolution temperature should be raised to 35 °C or higher in order to increase the yield of NaCN. However, if the temperature is excessively high, the hydrolysis of NaCN will be accelerated, which will also affect the yield. Therefore, the most reasonable dissolution temperature is 35 °C.
Thus, the most reasonable process conditions for separating and purifying the mixture of NaCN and Na2CO3 by using a water–ethanol mixed solvent from the Aspen Plus simulation are described.
5 Conclusion
The solubility of NaCN and Sodium Na2CO3 in the water–ethanol mixture was determined through experiments and the relationship between the solubility of NaCN/Na2CO3 and the solvent composition are led. And the solvent molecular-electrolyte pair interaction parameters of eNRTL model were estimated by using the data regression function of Aspen Plus software from solubility data of NaCN and Na2CO3 in water–ethanol mixed solvent.
The process simulation for separation and purification of NaCN and Na2CO3 in water–ethanol mixed solvent was carried out by using Aspen Plus software. During the simulation, electrolyte equilibrium system in water–ethanol mixed solvent was observed by using eNRTL model property method, and the influence of several factors on the separation efficiency of NaCN was examined.
Based on the experimental results, when the content of cyanide in the raw material is 20–50 wt%, the optimum solid–liquid ratio is 1:3.5–1:6.5. Also, the concentration of ethanol in mixed solvents most suitable for NaCN separation is 60–70 wt%, most reasonable dissolution temperature is 35 °C.
References
Singhal S, Jain SL, Sain B (2010) Heterogeneously catalyzed oxidative cyanation of tertiary amines with sodium cyanide/hydrogen peroxide using polymer-supported iron(II) phthalocyanines as catalyst. Adv Synth Catal 352:1338–1344. https://doi.org/10.1002/adsc.201000007
Murahashi SI, Komiya N, Terai H, Nakae T (2003) Aerobic ruthenium-catalyzed oxidative cyanation of tertiary amines with sodium cyanide. J Am Chem Soc 125:15312–15313. https://doi.org/10.1021/ja0390303
Murahashi S-I, Komiya N, Terai H (2005) Ruthenium-catalyzed oxidative cyanation of tertiary amines with hydrogen peroxide and sodium cyanide. Angew Chem 117:7091–7093. https://doi.org/10.1002/ange.200501496
Murahashi SI, Nakae T, Terai H, Komiya N (2008) Ruthenium-catalyzed oxidative cyanation of tertiary amines with molecular oxygen or hydrogen peroxide and sodium cyanide: Sp3 C-H bond activation and carbon-carbon bond formation. J Am Chem Soc 130:11005–11012. https://doi.org/10.1021/ja8017362
Leisner P, Zanella C, Belov I et al (2017) Control of silver throwing power by pulse reverse electroplating. Trans Inst Met Finish 95:25–30. https://doi.org/10.1080/00202967.2017.1260895
Pan R, Tong M, Li J, et al. Preparation and purification of hydrogen cyanide, involves preheating ammonia gas, mixing with inert gas and compressed air, entering mixture into tube furnace from methanol feed nozzle, vaporizing methanol, and mixing with methanol. CN106745066-A
Lv S, Han Y, Wen X, et al. Purification of gold-extracting cyanide fluid involves adding cyanide fluid with sodium magnesium salt of xanthogenate, conducting ion-exchange reaction to form xanthate deposit, and separating cyanide fluid from precipitate. CN101186374-A
Cui RF, Hu MC, Jin LH et al (2007) Activity coefficients of rubidium chloride and cesium chloride in methanol-water mixtures and a comparative study of Pitzer and Pitzer-Simonson-Clegg models (298.15 K). Fluid Phase Equilib 251:137–144. https://doi.org/10.1016/j.fluid.2006.11.016
Wang P, Anderko A, Young RD (2002) A speciation-based model for mixed-solvent electrolyte systems. Fluid Phase Equilib 203:141–176. https://doi.org/10.1016/S0378-3812(02)00178-4
Liu C, Tang G, Ding H et al (2015) Determination of the solubility and thermodynamic properties of wedelolactone in a binary solvent of ethanol and water. Fluid Phase Equilib 385:139–146. https://doi.org/10.1016/J.FLUID.2014.10.031
Long B, Zhao D, Liu W (2012) Thermodynamics studies on the solubility of inorganic salt in organic solvents: application to KI in organic solvents and water-ethanol mixtures. Ind Eng Chem Res 51:9456–9467. https://doi.org/10.1021/ie301000b
Chen CC (1980) Computer simulation of chemical processes with electrolytes. PhD thesis, Massachusetts Institute of Technology. http://dspace.mit.edu/handle/1721.1/15864
Renon H, Prausnitz JM (1968) Local compositions in thermodynamic excess functions for liquid mixtures. AICHE J 14:135–144. https://doi.org/10.1002/aic.690140124
Chen CC, Song Y (2004) Solubility modeling with a nonrandom two-liquid segment activity coefficient model. Ind Eng Chem Res 43:8354–8362. https://doi.org/10.1021/ie049463u
Bollas GM, Chen CC, Barton PI (2008) Refined electrolyte-NRTL model: activity coefficient expressions for application to multi-electrolyte systems. AICHE J 54:1608–1624. https://doi.org/10.1002/aic.11485
Wang W, Zeng D, Yin X, Chen Q (2012) Prediction and measurement of gypsum solubility in the systems CaSO4 + HMSO4 + H2SO4 + H2O (HM = Cu, Zn, Ni, Mn) at 298.15 K. Ind Eng Chem Res 51:5124–5134. https://doi.org/10.1021/ie201721m
Wang W, Zeng D, Wang J et al (2014) Experimental determination and modeling of the solubility of CaSO4 2H2O and CaSO4 in the quaternary system CaSO4 + MgSO4 + H2SO4 + H2O. Ind Eng Chem Res 53:12839–12847. https://doi.org/10.1021/ie5021365
Cavalaglio G, Coccia V, Cotana F et al (2018) Energy from poultry waste: an Aspen Plus-based approach to the thermo-chemical processes. Waste Manag 73:496–503. https://doi.org/10.1016/J.WASMAN.2017.05.037
Puig-Gamero M, Argudo-Santamaria J, Valverde JL et al (2018) Three integrated process simulation using Aspen Plus®: pine gasification, syngas cleaning and methanol synthesis. Energy Convers Manag 177:416–427. https://doi.org/10.1016/J.ENCONMAN.2018.09.088
Larbi F, García A, del Valle LJ et al (2018) Simulation basis for a techno-economic evaluation of chitin nanomaterials production process using Aspen Plus® software. Data Brief 20:1556–1560. https://doi.org/10.1016/J.DIB.2018.08.130
Acknowledgements
We are thankful to researchers of Analytical Research Institute at Kim Il Sung University for their assistance with this research completeness.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Experimental design, data collection and wrote the manuscript were performed by K-IK and J-HR. Instrumental analysis including XRD analysis were performed by S-UK and data analysis were performed by I-HK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, KI., Ri, JH., Kim, SU. et al. Estimation of interaction parameters of electrolyte NRTL model based on NaCN and Na2CO3 solubility in water–ethanol mixed solvent and process simulation for separation of NaCN/Na2CO3. SN Appl. Sci. 2, 2112 (2020). https://doi.org/10.1007/s42452-020-03914-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03914-5