Abstract
This study aimed to investigate the possibility of utilizing a locally available fly ash as a low-cost adsorbent material for the removal of copper from aqueous solution. Though copper is an essential element, it can be poisonous to human beings at higher concentrations. The removal efficiency of copper is studied under the different initial concentrations of copper (20–50 mg/L), pH (2–6), and fly ash dosage (20–80 g/L). A second-order polynomial model is proposed to study the interactive effects of the parameters. The result of the analysis of variance (ANOVA) confirms the quadratic model is very significant. The optimization of the process variables is carried out by using a full factorial rotatable Central Composite Design (CCD) in Response Surface Methodology (RSM) by Design-Expert Software (Version 6.0.8, State Ease, USA). The maximum removal of copper is achieved at an initial copper concentration of 43 mg/L, pH 6, and fly ash dosage 63 g/L. The value of the determination coefficient (R2) is found to be 0.9945. The value of Prob > F of the developed model is < 0.0001, indicating the applicability of the model. The fly ash has been analyzed by XRD, DTA, FTIR, and SEM/EDS techniques. The results of this study indicate that this fly ash can be used as a low-cost adsorbent to treat acidic wastewater generated from industries like electroplating, fertilizer, copper smelting, acid mine drainage, etc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Removal of heavy metals from wastewater is one of the most challenging environmental problems faced by several researchers worldwide. Unlike other organic pollutants, heavy metals’ degradation rate is very slow; thus, these metals become a part of the food chain and accumulated in a living organism’s body. Some of these heavy metals are very toxic, even in low concentrations. For example, exposer of copper above 1.3 mg/L for short periods of time causes intestinal and stomach problems [1]. Copper, the metal consider in this study, is widely used in various industrial processes such as electroplating, acid mine drainage, copper smelting, paint, paper factory, printed circuit board, copper smelting, fertilizer industry, etc. The concertation of copper present in these industrial wastewaters is shown in Table 1 [2,3,4,5,6,7,8]. Table 1 shows that these industrial wastewaters are the significant contributors of copper to the environment. That’s why it is essential to treat copper-containing wastewater before it was discharged into the atmosphere. Though copper is a vital trace nutrient for plants and animals, it can be poisonous to human beings and other living organisms when its concentration is high [9]. Copper is also found as contamination in food such as mushroom, nut, shellfish, etc. A recent study conducted by researchers showed that too much copper consumption throughout life might cause Alzheimer’s disease [10]. Because of this reason, the Indian standard recommends the permissible concentration of copper in drinking water in the absence of an alternative source is 1.5 mg/L [11].
Several methods are available in the literature to remove heavy metals from wastewater, such as chemical precipitation, solvent extraction, membrane filtration, ion exchange, reverse osmosis, adsorption, etc. As reported by various researchers, coagulation, ion exchange, chemical precipitation, and membrane filtration are the commonly used processes, but this method suffers from the disadvantages, such as expensive chemicals, high energy requirement, high capital and operating cost, and sludge disposal [12,13,14]. In particular, adsorption is the most well-known, influential, and economical treatment process for removing these metals from contaminated water. In recent years, several locally available low-cost adsorbent materials such as agricultural waste (olive cake [3], rice straw [4], bagasse ash [15], industrial by-products (red mud [16], fly ash [17,18,19,20], slag [21]), and natural materials have been utilized to remove copper from contaminated water. For example, Fernandez-Gonzalez et al. [3] have used a novel hydrolyzed olive cake to remove copper from fertilizer industry wastewater. Singha and Das [4] utilized rice straw to remove copper from electroplating wastewater. Gupta and Ali [15] investigated bagasse coal fly ash’s efficiency as a low-cost adsorbent to remove copper and zinc from industrial wastewater. Tsamo et al. [16] studied the adsorption capacity of raw and modified red mud as adsorbents to remove chromium, copper, and lead from contaminated water.
Fly ash, one of the most abundant waste materials from the combustion of coal, is a potential material for the removal of heavy metals present in wastewater. India produces about 130 million tons/yr. of fly ash from the burning of the enormous amount of coal, and by the year 2030, the fly ash production in India will be about 600 million tons/year [22]. The safe disposal of fly ash is a matter of great concern as it is not environment-friendly and requires a large amount of land to dispose of it in landfills. It has alkaline properties, and its surface is negatively charged at high pH. That is why it was used as a potential adsorbent for the treatment of wastewater containing heavy metals. Sočo and Kalembkiewicz [17] studied the copper and nickel adsorption properties of coal fly ash by varying the pH, contact time, and initial concentration. The adsorption process followed both Langmuir and Freundlich models. Raw and modified fly ash has been utilized as a potential adsorbent to remove copper from wastewater [18]. The adsorption ability of two different Turkish fly ashes to remove nickel, copper, and zinc from an aqueous solution was investigated by Bayat [19]. It was found the adsorption ability dependent on pH, fly ash origin, and initial metal concentration. Lin and Chang [20] used fly ash having different amounts of carbon and minerals to remove copper from contaminated water. Many researchers also utilized fly ash and modified fly ash to remove copper from wastewater [23,24,25].
After reviewing the published literature, it is found that copper’s adsorption by fly ash is predominantly affected by process parameters such as pH, fly ash dosage, and initial metal concentration. It is, therefore, essential to carefully optimize these process parameters. The majority of adsorption studies emphasized the traditional approach, i.e., one variable at a time (OVAT) approach to optimize these process parameters. But this type of system has several disadvantages, such as it is unable to address the effects of interactions between different factors, and it is also very much time-consuming. Therefore, to overcome these issues, an alternative approach involving statistical design should be considered. RSM technique combines mathematical and statistical methods to develop, improve, and optimize industrial/chemical processes. This technique is beneficial for modeling and analysis of processes where multiple independent variables potentially influence the response. In recent years, RSM techniques have been effectively used for the optimization of the various water treatment process [26, 27]. Kiran and Thanasekaran [28] employed RSM technique to optimize the copper adsorption capacity of an indigenously developed biosorbent. They have studied the influences of parameters like initial copper concentration, biosorbent dosage, and pH on copper adsorption. Özer et al. [29] applied the RSM technique combine with CCD to optimize the adsorption of copper by a novel biosorbent. However, no such work has been reported in the literature with the application of the RSM technique to remove copper from wastewater by fly ash.
Accordingly, this work emphasizes optimizing operating parameters to remove copper from wastewater by fly ash using RSM. To study the interactive effect of different operating parameters on copper removal efficiency, RSM combine with CCD of Design-Expert Software is used. The results are analyzed to predict the copper uptake, and comparison is made between model prediction data and experimental data. X-ray fluorescence (XRF), FE-Scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR) are used to characterize the fly ash.
2 Materials and methods
2.1 Chemicals
The following chemicals are used in the experimental work: NaOH (RFCL, New Delhi), HCl (Rankem, New Delhi), Cu(NO3)2.3H2O (Merck, Mumbai), H2SO4 (RFCL, New Delhi), HF (Merck, Mumbai)
2.2 Fly ash
Fly ash used in the present experiment is collected from a coal-based power plant operated by the Hindalco industry located at Renukoot (Uttar Pradesh, India) after receiving relevant permissions for scientific use from the responsible authorities. Before the investigation, the fly ash is washed several times with distilled water and dried at 105 °C [30]. This dried sample is used throughout the study. The chemical composition of this fly ash is determined by the XRF (Model: Bruker, S8 Tiger Eco) technique. The heavy metals present in the fly ash is analyzed by ICP-optical emission spectrometry (ICP-OES, Model: Prodigy XP, Teledyne Leeman Labs) after hydrofluoric acid digestion of fly ash in a microwave accelerated reaction system (Model: CEM MARS Xpress). The soluble concentration of various heavy metals present in fly ash is also determined to have insight into this fly ash’s environmental impact. One gram of fly ash sample is stirred with 200 ml of distilled water for 2 h and filtered [19]. Then, the heavy metal concentration of filtrate is determined by ICP-OES. The loss on ignition (LOI) of fly ash sample is determined by heating the pre-weighted air-dried, finely ground sample at 1000 ± 25 °C for 20–30 min in a platinum crucible as per IS 1727-1967 [31]. The fly ash sample’s specific gravity is determined by Le chatelier flask as per IS 1727-1967 [31]. The surface area of fly ash is determined by Brunauer–Emmett–Teller (BET) method (Model: ASAP 2020 Micromeritics) by nitrogen adsorption isotherm technique. Mineralogical constituent and phase proportions of this fly ash are determined by powder XRD (Model: Bruker, D-8). XRD analysis is carried at room temperature out in the scan rate of 2°/min and range of 5–80° with nickel filter CuKα radiation (λ = 1.54060 Å) and scanning speed 4°/min. The XRD pattern is matched with JCPDS and ICSD data files for phase identification. An FE-SEM attached with an energy dispersive spectroscopy is used to study the fly ash sample’s surface morphology (Model: Carl Zeiss Ultra Plus). FTIR spectrometer (Nicolet Model Avtar 370Csl, Thermo Electron Corporation) has been utilized to identify the fly ash’s functional groups range from 4000 to 500 cm−1. Thermogravimetric analysis (Model: Seiko Exstar 6300) of this fly ash has been carried out under an inert atmosphere (Nitrogen). The particle size of fly ash is determined by the laser scattering technique (Model: Horbia, LA-950).
2.3 Point of zero charge
The point of zero charges (pHPZC) of this fly ash is measured by adding 0.5 g of sample in deionized water (100 ml) in a series of 250-ml conical flask. The slurry’s pH values are adjusted from 2 to 10 by HCl (0.1 M) or NaOH (0.1 M). The slurry is agitated continuously for 2 h, and the zeta potential of the slurry has been recorded by a zeta sizer (Model: Malvern Nano ZS90) at different pH values.
2.4 Batch study
The batch study is undertaken for the removal of copper from contaminated water. Stock contaminated water of 1000 mg/L is prepared synthetically in the laboratory by dissolving cupric nitrate trihydrate salt in deionized water purified by Millipore milli-Q system and standardized [32]. Solutions of different concentrations are prepared by diluting the stock solution. Then, 50 ml of these solutions are mixed with fly ash in a conical flask and placed in a water shaker bath for 3 h for proper mixing to attain the equilibrium. Batch experiments are conducted in a series of the conical flask containing a variable amount of copper ion and fly ash at different pHs. Then, the samples are taken out of the stirrer and filtered. The filtrate thus obtained is tested for the remaining copper ion concentration using ICP-OES. The removal efficiency of the process is determined by using Eq. 1.
The amount of copper adsorbed (q mg/g) by fly is calculated by using Eq. 2.
where E is metal removal efficiency (%), Co is the initial copper concentration in contaminated water (mg/L), Ct is the final copper concentration in treated water (mg/L), m is the mass of fly ash (g), and V is the volume of solution (L). All the experiments are carried out thrice, and the average values of the results are reported.
2.5 Experimental design
After detailed literature survey it is found that copper removal is predominantly influenced by three independent process variables, namely initial metal concentration, pH, and fly ash dosage. In order to optimize the experimental conditions and to collect data for modeling the ranges of variables are chosen as initial metal concentration (20–50 mg/L), pH (2–6), and fly ash dosage (20–80 g/L). A three factorial five-level CCD is developed for this study. For statistical calculations, these parameters (Xi) are coded as xi according to Eq. 3 and shown in Table 2.
where xi is the coded value, Xi is the actual value of the ith process variable, \(\partial x\) is the step change, an X0 is the value of the process variable at the center point.
For three independent parameters, a total number of experimental combinations are 20 according to 2n + 2n + 6. Here, n is the number of independent parameters. This design includes 6 replication at the center point, 6 axial points, and 8 full factorial points.
Data Analysis: The following second-order quadratic polynomial Eq. is employed to attain the interaction between the independent and dependent variables.
Here, Y is the dependent variable (response i.e., removal efficiency).Xi and Xj are the independent process variables (i = 1, 2 and 3; j = 1, 2 and 3; i ≠ j). b0, bi, bii, bij are the constant regression coefficient of the model. Experimental data are evaluated by ANOVA. The quality of the polynomial model’s fit is assessed by the value of the determination coefficient (R2) and adjusted R2.
Statistical significance and the adequacy of the employed model are evaluated by the model Fisher variation ratio (F-value), a probability value (Prob > F), and adequate precision value.
3 Results and discussion
3.1 Fly ash characterization
The physical–chemical properties of the fly ash sample are presented in Table 3. The results specify that this is class F-type fly ash as the combined weight percentage of SiO2 and Al2O3 is more than 70% and lime content is less than 10%, according to ASTM C 618 [33]. Fly ash is slightly alkaline as the pH value of a 2% aqueous solution is 8.4. It is evident from Table 3 that the heavy metals present in the fly ash are Cu, Cr, Pb, Ni, and Mn, which constitute a small fraction as compared to other oxides. The soluble concentration of elements present in fly ash is presented in Table 4. The results indicate that the probability of leaching of heavy metals is significantly less, and this fly ash can be classified as non-hazardous waste. Similar observations were reported previously by other researchers. [19, 34, 35]. The particle size distribution curve of fly ash is presented in Fig. 1. It is found that particle size ranges from 0.389 to 200 µm. It is also observed that 90% of the particles are smaller than 81.64 µm and 10% of the particle is smaller than 10.75 µm. A similar type of particle size distribution curve has also been found in the previous study [36].
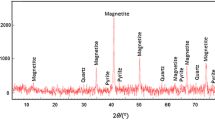
The XRD patterns revealed that the fly ash mainly contains Quartz (SiO2), a moderate amount of Mullite (Al4.75Si1.25O9.63), and a meager amount of Rutile (TiO2) and Hematite (Fe2O3) (Fig. 2). The individual phases have been matched and analyzed by the PDF-2 database from X’pert high score plus software. TGA curve shows that mass loss occurs in two stages (Fig. 3). In the first stage, about 0.14% of mass loss occurs as the sample is heated up to 200 °C. This is maybe due to absorbed moisture and interstitial water in the fly ash. In the second region, 200–925 °C, about 2.8% of weight loss occurs. This is maybe due to the release of volatile matter from the sample.
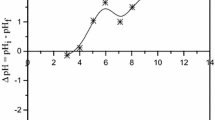
pHPZC plays a critical role in the adsorption of heavy metal ions. pHPZC is the pH value at which the net surface charge is zero, and the surface of the adsorbent neither exhibits a positive charge nor a negative charge. The zeta potential measurement of this fly ash at different pH’s is shown in Fig. 4. From the graph, it has been observed that pHPZC of this fly ash is 2.9. Researchers have found that the cation’s adsorption is favored at a pH greater than pHPZC, while an anion is preferred at a pH lower than pHPZC [37, 38]. The BET surface area of fly ash used in the study is found to be 4.89 m2/g. [38].
3.2 SEM/EDS analysis of fly ash before and after adsorption
Figure 5a and b shows the FE-SEM and EDS analysis result of fly ash before and after adsorption, respectively. From the SEM image, it is clear that the majority of the particles ranged in size from approximately 1 to 100 µm and consists of solid spheres. This fly ash contains a considerable number of small flakes (pleurospheres), and the surface is microporous. Regular and spherical shapes of cenospheres are also found. Furthermore, the surface morphology of this fly ash reveals loose aggregates with severely nonuniform layered structures. This microporous structure is responsible for the adsorption of heavy metal ions. When this raw fly ash is used for copper adsorption, the copper ions adsorbed over the fly ash surface and formed a smooth film covering the surface [39]. This film is seen in Fig. 5b. From the EDS analysis, it is clear that before adsorption, copper ion is absent in fly ash surface, but when this fly ash is used for the treatment of wastewater, approximately 1.39% copper is present on the fly ash surface.
3.3 FTIR analysis of fly ash before and after adsorption
Figure 6 shows the FTIR spectra of fly ash before adsorption and after adsorption. In the raw fly ash, a broad band at 3432 cm−1 confirms the hydroxyl group’s presence. The bond stretching is due to O–H stretching vibration of the silanol group of fly ash (Si–OH) and HO–H vibration of adsorbed water molecules on the fly ash surface. The medium intensity bands at 1646 and 1488 cm−1 are due to C = O, and –CH bending vibration. The sharp peak at 1010 cm−1 corresponds to the asymmetric stretching of T-O-T (T = Al, Si) tetrahedral sheets. Furthermore, the bands at 798, 684, and 634 cm−1 indicate quartz’s presence in the fly ash. It was evident from Fig. 6 that the intensity of spectra differs from raw fly ash, and also the positions of peaks are shifted while fly ash adsorbed copper. The percentage transmittance of the stretching vibration spectra of O-H at 3432 cm−1 is diminished after the adsorption of copper. This is due to the formation of a silanol copper complex as per Eq. 7 [34]. Furthermore, the transmittance of the peaks at 1010, 798, 684, and 634 cm−1 are increased after the adsorption of copper. This phenomenon indicates copper is adsorbed on fly ash’s active sites (silica and alumina), which results in a decrease in silica and alumina present on fly ash. These results suggested copper is adsorbed on fly ash surface by chemisorption, probably indicating fly ash/copper complexation [34, 40].
3.4 Process modeling and statistical analysis
The individual and interactive effect of the three process variables (initial metal concentration, pH, and fly ash dosage) on copper removal efficiency are investigated using the CCD model by conducting 20 experiments and presented in Table 5. Based upon the calculation of regression coefficient copper removal efficiency in terms of coded factor is modeled as:
Statistical significance and model adequacy of the predicted model has been analyzed by employing the ANOVA and presented in Table 6. As evident from this table, the model F-value is 201.25, confirming the model is very much significant. Only a 0.01% chance that a model F-value this large could occur due to noise. This model also has a very low probability value (p > F value less than 0.0001), which implies that model terms are significant. The adequate precision value of this model’s is 54.97, indicating an adequate signal of the model. For a significant model, this should be greater than 4. In this study, X1, X2, X3, X 21 , X 22 , X 23 , X1X2, and X1X3 are significant model terms as their “prob > F” values are less than 0.0500 while other terms are not significant, (In this case, only X2X3 is not significant.) The R2 value of the model is 0.995, indicates that this model could not explain only 0.5% of the total variation. In this model, the predicted R2 value (0.9544) shows a reasonable agreement with the adjusted R2 value (0.9896), which indicates all the terms depicted in this model are very significant. These statistical values indicate that this statistical model can perfectly simulate this fly ash’s copper adsorption behavior. It is found that the plot of Normal % probability versus studentized residual is following a straight line, which indicates data are normally distributed (Fig. 7a). The experimental values and model-predicted values of removal efficiency as per Eq. 5 showed a high correlation with each other and shown in Fig. 7b.
To study the interactive relation between the process variables and removal efficiency, the 3D response surface is plotted based on the predicted removal efficiency. Two process variables are varying, and the other is kept constant.
3.5 Effect of initial copper ion concentration and fly ash dosage on removal efficiency
Figure 8a illustrates the effect of initial copper ion concentration and fly ash dosage on removal efficiency at a contact time of 3 h and a pH of 4. It is observed that at a particular fly ash dosage, the removal efficiency shares an inverse relationship with copper concentration as it increases with a decrease in copper concentration and vice versa. This is because, at a particular fly ash dosage, total active sites available for the metal ion sorption are constant. It is also found that removal efficiency increases with an increase in fly ash dosage. The removal efficiency increased from 48% to 62% when the fly ash dosage increased from 35 to 65 g/L at an initial copper concentration of 27.5 g/L and pH 4. The increase is attributed to the availability of metal adsorption sites [18, 39].
3.6 Effect of initial copper concentration and pH on removal efficiency
The interactive effect of different levels of initial copper concentration and pH of solution on removal efficiency is shown in Fig. 8b. It is found that the pH of the solution has a significant impact on removal efficiency as it plays a vital role in determining the degree of ionization of metal ion and surface charge of fly ash. It is observed that removal efficiency increases significantly with the increase in pH and initial copper concentration. The adsorption of metal ion on the fly ash surface occurs mainly due to functional oxide groups such as silica and alumina. This fly ash’s major constituents are silica and alumina. At pH values lower than the pHZPC, the adsorbent surface is positively charged, and for pH values greater than the pHZPC, the surface is negatively charged, which favors the metal ion’s adsorption. It is found that the removal efficiency of this fly ash at pH 2 is 21%. In comparison, the removal efficiency is 89% at pH 6 at a fly ash dosage and initial concentration of 50 g/L and 35 mg/L, respectively. As the pH values increases beyond 2.9, fly ash’s surface becomes more negatively charged as per Eq. 6, allowing copper ion to be chemisorbed.
As observed by various researchers [34, 41] removal of copper at pH higher than pHZPC takes place as per the following Eq.
pH values lower than pHZPC protonation of the superficial hydroxyl groups take place as per Eq. 9.
Due to repulsion caused by this positively charges hydroxyl group and Cu2+, the very low removal efficiency is observed at a pH value of 2.
As suggested by various research pH values beyond 6, copper ion precipitation became predominant, indicating that removal of copper ion below pH 6 is mainly accomplished by adsorption [34, 39].
3.7 Effect of fly ash dosage and pH on removal efficiency
Figure 8c shows the interactive effect of pH and fly ash dosage on removal efficiency at a copper concentration of 35 mg/L. It has been observed that the percentage removal increases on increasing pH from 3 to 5. The percentage removal rises from 28% to 55% when the pH increased from 3 to 5 at a 35 g/L fly dosage. Removal efficiency also increases with an increase in fly ash dosage.
3.8 Optimization
The main objective of this study is to maximize copper removal efficiency. Optimization of the process variables has been carried out by using the desirability function approach. This approach is one of the most widely used methods for optimizing multiple response processes. In this method, for each response process Yi(x), a desirability function di (Yi) is assigned, and its value lies in between 0 to 1 [42]. And the overall desirability is obtained as a geometric mean of individual desirability as per the following Eq. 10:
where n denotes the number of responses. It should be noted that if any response Yi is completely undesirable (di(Yi) = 0), then the overall desirability is zero.
Each process variable’s desired goal is kept within range, whereas it is kept at a maximum for the response. A set of solutions with different desirability is provided by design to find out the optimum condition. Based on the solutions, an optimum condition having desirability 1.0 is chosen, and its parametric constraints with model prediction and experimental data are presented in Table 7. To find out the accuracy of the model, further experiments in triplicate are carried out at laboratory scale at optimum conditions, and the average value of the same is reported in Table 7. It is found that the experimental value shows a small deviation of 1.9%. This confirms the validity of the proposed CCD model for maximizing the copper removal efficiency by fly ash.
3.9 Comparison of adsorption capacity
Table 8 compares this fly ash’s maximum adsorption capacity with those of others. It is observed that the adsorption capacity of this fly ash is similar to other fly ashes used by different researches [15, 19, 20, 23,24,25, 43, 44]. Although the fly ash used in this study exhibits low adsorption capacity compared to activated carbon and other bio-adsorbent, but it is abundantly available at a very low cost. That’s why it can be used as a low-cost adsorbent to treat acidic wastewater as an acid-neutralizing agent.
4 Conclusion
The current study is undertaken to use solid waste, namely fly ash, as a cost-effective adsorbent to remove copper from contaminated water. Fly ash is found to be a potential adsorbent for copper removal. Fly ash used in the present study is class F-type, and pHZPC of this fly ash is 2.9. This study’s main objective is to find and optimize the copper removal potential of fly ash using the RSM approach. The initial copper concentration, pH, and fly ash dosage significantly influence the copper removal efficiency. It is found that the model obtained by CCD shows a high correlation between the experimental value (Actual value) and model-predicted value. The optimum level of 93.8% removal efficiency of copper is achieved at an initial copper concentration of 43 mg/L, pH 6, and fly ash dosage 63 g/L. The results of this study indicate that this fly ash can be used to treat acidic wastewater generated from industries like electroplating, fertilizer, copper smelting, acid mine drainage, etc. The copper loaded fly ash can be securely disposed of by cement fixation and may be used as a secondary construction material.
References
Steenkamp GC, Keizer K, Neomagus H et al (2002) Copper (II) removal from polluted water with alumina/chitosan composite membranes. J Membr Sci 197(1–2):147–156
Naghizadeh A, Mousavi SJ, Derakhshani E et al (2018) Fabrication of polypyrrole composite on perlite zeolite surface and its application for removal of copper from wood and paper factories wastewater. Korean J Chem Eng 35(3):662–670
Fernández-González R, Martín-Lara MA, Blázquez G et al (2020) Hydrolyzed olive cake as novel adsorbent for copper removal from fertilizer industry wastewater. J Clean Prod 268:121935
Singha B, Das SK (2013) Adsorptive removal of Cu (II) from aqueous solution and industrial effluent using natural/agricultural wastes. Colloids Surf B Biointerfaces 107:97–106
Akbal F, Camcı S (2011) Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 269(1–3):214–222
Dib A, Makhloufi L (2004) Cementation treatment of copper in wastewater: mass transfer in a fixed bed of iron spheres. Chem Eng Process 43(10):1265–1273
Patterson JW, Jancuk W (1977) Cementation treatment of copper in wastewater. In: Proceedings of the 32nd Industrial Waste Conference, pp 853–865
Basha CA, Bhadrinarayana NS, Anantharaman N et al (2008) Heavy metal removal from copper smelting effluent using electrochemical cylindrical flow reactor. J Hazard Mater 152(1):71–78
Lopez FA, Martın MI, Perez C et al (2003) Removal of copper ions from aqueous solutions by a steel-making by-product. Water Res 37(16):3883–3890
Singha I, Sagarea AP, Comaa M et al (2013) Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance. PANS Early Edition 110(36):14771–14777
IS 10500 (2012), Drinking Water—Specification
Basha CA, Saravanathamizhan R, Nandakumar V et al (2013) Copper recovery and simultaneous COD removal from copper phthalocyanine dye effluent using bipolar disc reactor. Chem Eng Res Des 91(3):552–559
Barakat MA, Schmidt E (2010) Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater. Desalination 256(1–3):90–93
Tang SY, Qiu YR (2018) Removal of copper (II) ions from aqueous solutions by complexation–ultrafiltration using rotating disk membrane and the shear stability of PAA–Cu complex. Chem Eng Res Des 13:712–720
Gupta VK, Ali I (2000) Utilisation of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater. Sep Purif Technol 18(2):131–140
Tsamo C, Djonga PD, Dikdim JD et al (2018) Kinetic and equilibrium studies of Cr(VI), Cu (II) and Pb(II) removal from aqueous solution using red mud, a low-cost adsorbent. Arab J Sci Eng 43(5):2353–2368
Sočo E, Kalembkiewicz J (2013) Adsorption of nickel (II) and copper (II) ions from aqueous solution by coal fly ash. J Environ Chem Eng 1(3):581–588
Hsu TC, Yu CC, Ye CM (2008) Adsorption of Cu2+ from water using raw and modified coal fly ashes. Fuel 87(7):1355–1359
Bayat B (2002) Comparative study of adsorption properties of Turkish fly ashes: I. The case of nickel (II), copper (II) and zinc (II). J Hazard Mater 95(3):251–273
Lin CJ, Chang JE (2001) Effect of fly ash characteristics on the removal of Cu (II) from aqueous solution. Chemosphere 44(5):1185–1192
Feng D, Van Deventer JSJ, Aldrich C (2004) Removal of pollutants from acid mine wastewater using metallurgical by-product slags. Sep Purif Technol 40(1):61–67
Chattopadhyay GC (2015) Issues in utilization of ash by thermal power plants in the country. J. Government Audit and Accounts 3:1–4
Crini G (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci 30(1):38–70
Ayala J, Blanco F, García P et al (1998) Asturian fly ash as a heavy metals removal material. Fuel 77(11):1147–1154
Panday KK, Prasad G, Singh VN (1985) Copper (II) removal from aqueous solutions by fly ash. Water Res 19(7):869–873
Renu Agarwal M, Singh K (2018) Removal of copper, cadmium, and chromium from wastewater by modified wheat bran using Box-Behnken design: kinetics and isotherm. Sep Sci Technol 53(10):1476–1489
Tetteh EK, Rathilal S, Naidoo DB (2020) Photocatalytic degradation of oily waste and phenol from a local South Africa oil refinery wastewater using response methodology. Sci Rep 10(1):1–12
Kiran B, Thanasekaran K (2011) Copper biosorption on Lyngbya putealis: application of response surface methodology (RSM). Int Biodeterior Biodegradation 65(6):840–845
Özer A, Gürbüz G, Çalimli A et al (2009) Biosorption of copper (II) ions on Enteromorpha prolifera: application of response surface methodology (RSM). Chem Eng J 146(3):377–387
Alinnor IJ (2007) Adsorption of heavy metal ions from aqueous solution by fly ash. Fuel 86(5–6):853–857
IS: 1727 (1967) Methods of Tests for Pozzolanic Materials
Jeffery GH, Bassett J, Mendham J et al (1988) Vogel’s quantitative inorganic analysis, 5th edn. ELBS, Berlin
ASTM C 618 (2003) Standard Specification for Fly Ash and Raw or Claimed Natural Pozzolan for Use as Mineral Admixture in Portland cement Concrete
Mohan S, Gandhimathi R (2009) Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. J Hazard Mater 169:351–359
Mollamahmutoğlu M, Yilmaz Y (2001) Potential use of fly ash and bentonite mixture as liner or cover at waste disposal areas. Environ Geol 40(11–12):1316–1324
Li HL, Liu GL, Cao Y (2014) Content and distribution of trace elements and polycyclic aromatic hydrocarbons in fly ash from a coal-fired CHP plant. Aerosol Air Qual Res 14(4):1179–1188
Srivastava VC, Mall ID, Mishra IM (2006) Characterization of mesoporous rice husk ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. J Hazard Mater 134(1):257–267
Weng CH, Huang C (2004) Adsorption characteristics of Zn (II) from dilute aqueous solution by fly ash. Colloids Surf A Physicochem Eng Asp 247(1):137–143
Ghosh A, Saha PD (2012) Optimization of copper adsorption by chemically modified fly ash using response surface methodology modelling. Desalination Water Treat 49(1–3):218–226
Visa M, Isac L, Duta A (2012) Fly ash adsorbents for multi-cation wastewater treatment. Appl Surf Sci 258(17):6345–6352
Tofan L, Paduraru C, Bilbia D, Rotoriu M (2008) Thermal power plants ash as sorbent for the removal of Cu(II) and Zn(II) ions from wastewaters. J Hazard Mater 156:1–8
Myers RH, Montgomery CM (1995) Response Surfaces Methodology: Process and Product Optimization using Designed Experiments. Wiley, New York
Yu B, Zhang Y, Shukla A, Shukla SS et al (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper. J Hazard Mater 80(1–3):33–42
Saeed A, Akhter MW, Iqbal M (2005) Removal and recovery of heavy metals from aqueous solution using papaya wood as a new biosorbent. Sep Purif Technol 45(1):25–31
Acknowledgments
The authors express their sincere thanks to the Director, Central Building Research Institute, Roorkee for his support, encouragement to publish this paper. The authors thank M/S Hindalco Industries Limited, Renukoot, for providing the fly ash free of cost for the experiment. The authors are also very grateful to the Department of Chemical Engineering, Indian Institute of Technology Roorkee, for providing technical facilities.
Funding
This research work was funded by the Council of Scientific and Industrial Research
Author information
Authors and Affiliations
Contributions
SM (PhD student) carried out all the experiments. SM, AKM, and BP performed the analysis. SM wrote the paper. BP and AKM reviewed and edited the paper. BP provided a nurturing environment and technical inputs.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there is no conflict of interest in the preparation of this article
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maiti, S., Prasad, B. & Minocha, A.K. Optimization of copper removal from wastewater by fly ash using central composite design of Response surface methodology. SN Appl. Sci. 2, 2151 (2020). https://doi.org/10.1007/s42452-020-03892-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03892-8