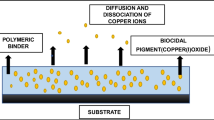
Abstract
Biofouling is one of the problems faced by cage aquaculture farmers, and its management is expensive. In this present study, polyethylene aquaculture cage nets were coated with the nano structured oxides of zinc and silicon incorporated with polyaniline. Fourier transform infrared spectroscopy and scanning electron microscope data evidenced that a thick coating of nano particle was formed on polyethylene. The oxides were attached to the quinoid part of polyaniline. The antifouling efficiency of treated cage net was tested by exposing in the Cochin estuary for three months. A webbing treated with 0.01% of each of the nano zinc oxide (ZnO) and silicon dioxide (SiO2) exhibited the highest biofouling resistance. Reinforcement of nano SiO2 with ZnO increased biofouling resistance as the former complemented the coating to make the system more efficient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In India, cage aquaculture is an emerging sector, and is growing synchronously with some problems such as a deficiency of good quality seed and feed, and biofouling. The fouling of cage nets by organisms including bacteria, algae, mollusc, bivalves, and crustaceans, increases the maintenance cost, pollutant accumulation (organic loads and nutrients) leading to eutrophication, and the lack of oxygen in culture systems. This problem eventually results in drastic ecological and economic implications [1]. Biofouling is a serious problem in aquacutlture because its management utilizes one fourth of total project expenses [2, 3].
Voluminous antifouling compounds and strategies are available globally, but many of these compounds cause environmental problems and influence non target organisms. Copper based biocides are highly effective against many foulers; however the effects of copper in aquatic systems are not widely known, and many algal foulers are copper resistant species and cannot be controlled [4]. The current antifouling strategies used under the marine environment are ineffectual and the development of competent technologies to prevent biofouling in the aquatic environment is required urgently [3].
High density polyethylene (PE) fibers are generally used to fabricate aquaculture cage nets. PE is a non polar molecule and very difficult to coat any antifouling biocides directly over it. Ashraf et al. [5] devised a coating method to coat polyaniline (PANI) over PE webbings. Moreover nano copper oxide has been coated on PANI, which provided an excellent biofouling resistance and it was explained due to the generation of hypochlorous acid [5, 6]. Nano technology and nano materials facilitated the exploration of biocides with high efficacy and low requirement of chemicals. Nano copper oxide coating on PANI [5] and nano copper oxide reinforced hydrogel coating on PE exhibited excellent biofouling resistance [6]. Although copper oxide is a micro nutrient, stakeholders do not use it because of regulatory restrictions. Mohan and Ashraf [7] synthesized nano silicon dioxide incorporated mixed charged zwitterionic hydrogel and demonstrated an improved biofouling resistance of aquaculture cagenet under the marine environment. Coating of nano silica functionalised zwitterionic sulfobetaine exhibited increased wettability, microorganism inhibition and decreased serum albumin protein fouling [8]. Nano SiO2 is considered an ecologically affable oxide and extensively used to incorporate as antibacterial agents [9]. SiO2-polystyrene-N-halamine nano particles used to destroy Escherichia coli (E. coli) cell surfaces. The SiO2 acted as a carrier and also improved the actvity of N-halamine [9]. Zwitterionic polymer-coated SixN4 surfaces exposed to a fibrinogen solution inhibited protein [10]. The coating of mesoporous silica on indium tin oxide electrode acted as molecular sieve and prevented the accumulation of haemoglobin during the detection of propranolol [11]. SiO2 incorporated matrices described above increased the antibacterial, fouling and protein inhibition efficiency.
Nano ZnO exhibits anti-microbial and antifouling properties. Al-Naamani et al. [12] synthesized chitosan- nano ZnO composite exhibiting diatom and bacterial resistance under the marine environment. Zhang et al. [13] fabricated super hydrophilic and hydrophobic surfaces by coating textured super hydrophobic nano ZnO on polydimethylsiloxane film, and the surface inhibited the oil accumulation. Nano ZnO rods were incorporated in the polyethersulfone membrane to improve the morphology, hydrophilicity and biofouling resistance [14]. A nano ZnO reinforced polysufone membrane exhibited excellent hydrophilicity, water permeability, increased porosity, and rejection of organic molecule adsorption implying its antifouling property [15].
Nano mixtures having different properties coated over steel exhibited improved corrosion resistance [16] and few studies have been conducted using the nano materials of different properties in a mixture to prevent biofouling. Nano SiO2 and ZnO are considered safe, due to their toxicity characteristics and environmental friendliness. Nano ZnO deterred microorganisms through photocatalytic activity by forming OH radical (OH) by reacting with water [17]. The study on penetration of UV visible solar radiation in the oceanic waters revealed that 30–40% deeper penetration than predicted euphotic zone. The UVA (315–380 nm) radiation penetrate about 5–7 m deep in an oligotrophic water of depth having 50–70 m [18]. The electron rich materials like reduced graphene, nano composites of semiconductors, and carbon nano tubes were used to reduce the band gap of TiO2 and ZnO. This improved the photocatalytic effect of the above materials [19,20,21,22,23]. In the present study ZnO is coated over electron rich PANI and this may improve photocatalytic effect of ZnO. Coating of nano SiO2 and ZnO on an electron rich PANI may increase the antibacterial and biofouling resistance. Similar works are considerably limited especially in the development of strategies for preventing biofouling. A two step process was employed in this study instead of PANI–ZnO—SiO2 composite coating over PE since the latter exhibited poor biofouling inhibition due to the utilisation of all electronic states of ZnO and SiO2 for forming the composite. The study aimed to coat nano SiO2 and ZnO mixture of varied concentrations on PANI treated PE aquaculture cage net and to test its biofouling resistance.
2 Material and methods
Polyethylene (PE) aquaculture cage net, a material frequently used in cage aquaculture, was purchased with 1 × 3 ply and a mesh size of 25 mm procured from Matsyafed Net Factory, Cochin, India. Colored PE netting was used for the experiment since farmers prefer colored nettings. Aniline, ammonium persulphate, and HCl were purchased from CDH, India. Nano ZnO and hydrophilic nano SiO2 were purchased from Reinste Nano ventures, India. The size, specific surface area and purity of nano ZnO was 14 nm, 30 ± 5 m2/g and 99% respectively. Size, specifc area, bulk density and purity of hydrophilic nano SiO2 was 7–14 nm, > 200 m2 g−1, 0.048 g cm−2 and 99.8% respectively.
Polyaniline (PANI) was synthesised in situ on PE aquaculture cage nets by following the procedure provided by Ashraf et al.[5]. The surface activation of the PE cage net was carried out by immersing the net overnight in acidified aniline (0.3 M in 0.1 M HCl) solution and the following day ammonium persulfate (0.3 M in 0.1 M HCl) was added dropwise along the sides of container without disturbing the container. This will enable the formation of nano sized and rod shaped PANI over the net. Kept the reaction mixture overnight, and the following day the net samples were taken out, allowed to dry in air. Excess PANI was removed by washing with water. The PANI coated polyethylene (PE-PANI) aquaculture cage nets were immersed overnight in varied concentrations of aqueous nano ZnO and SiO2 mixtures (Table 1). On subsequent day, samples were taken out and air dried. This step will enable the nano materials to adsorb over the PANI. Pre-weighed, and tagged treated nets were tied in a PVC frame and immersed in the test site at Cochin estuary, 1 m below the low tide height for 3 months. The samples were retrieved once in every month for evaluating their fouling resistance through biomass accumulation and microscopic studies. The retrieved samples were cleaned by running water, air dried and microscopic analysis were carried out. The biomass accumulated over the pre weighed cage net was recorded after air drying using an electronic balance with 0.001 g sensitivity. The difference in weight was used to calculate the accumulation in per kg of cage net. Due to the limited space in the test site, fearing the loss of uniformity in exposure (depth within 0.75–1.25 m), and need to expose the rack in length wise, prevented to incorporate more replicates in the experiment.
The surface characteristics of treated webbings were analyzed using Scanning electron microscope (SEM). The SEM micrographs of PE netting were recorded after sputtering with gold by using JEOL Model JSM–6390LV SEM. Fourier transform infrared (FTIR) spectra were obtained by using Thermo Nicolet Si10 FTIR. The materials were scanned from 650 to 4000 cm−1 at 4 cm−1 resolution was done by using zinc selenium Nicolet iTR accessory and to study the absorption in lower frequencies for identifying the inorganic part, another scan from 400 to 4000 cm−1 using FTIR fitted with microarray sampling accessory. The material was scanned 50 times and the average was showed as final plot. The data was processed using Omnic software available with the FTIR. The The images of nettings were examined through Leica MZ16A stereo microscope at 25X magnification.
3 Results and discussion
PE aquaculture cage net surface was modified by in situ synthesised PANI, and then nano ZnO–SiO2 mixture were coated as described in materials and methods. Figure 1 and Table 2 presents the FTIR characteristics of PE-PANI, SiO2 treated PE-PANI (PE-PANI SiO2), SiO2 and ZnO treated PE-PANI (PE-PANI SiO2 + ZnO), ZnO treated PE-PANI (PE-PANI ZnO). The characteristic peaks of PE, shifted slightly after PANI and nano material treatments. Visible differences were obtained for the CH2 wagging and stretching band at 1176 and 2919 cm−1 respectively. PE-PANI SiO2 and PE-PANI ZnO exhibited a shift in peaks at 2908 and 2911 cm−1 respectively. Similarly PE-PANI SiO2 and PE-PANI ZnO exhibited a shift in absorption from 1176 to 1143 cm−1. The shifting was due to the influence of PANI, SiO2 and ZnO in the polyethylene matrix.
The FTIR absorption characteristics of PANI changed after ZnO and SiO2 treatments. Quinoid and benzenoid ring vibrations at 1585 cm−1 shifted to the lower frequency at ~ 1566 cm−1, indicating that ZnO and SiO2 were attached to the quinoid ring. Observed absorptions for ZnO stretching and Zn–O deformation were 548 [24] and 620 cm−1 [25] respectively, and the similar results were obtained at 562 and 610 cm−1 respectively, which indicated the interaction of ZnO with PANI. All the three characteristic peaks of SiO2 were obtained in the spectra of the SiO2 treated matrix. A shift appeared in the infrared absorption of SiO2 mainly due to the influence of PANI and nano particles. The results revealed that both the metal oxides interacted strongly with the quinoid ring of PANI in the matrix.
SEM micrographs of PE, PE-PANI, and PE-PANI with metal oxides are presented in Fig. 2. The micrographs indicated that strong interaction of PANI nano rods formed on the PE matirx with PE and the former uniformly coated on the surface. SiO2 particles attached onto PANI nano rods were visible. SiO2 particles were distributed uniformly in the matrix. The matrix treated with the mixture of ZnO and SiO2 exhibited a uniformly distributed coating. The netting with equal concentration of ZnO–SiO2 coating treatment exhibited a different pattern. The ZnO coating on PANI exhibited an uniform distribution of ZnO flakes which was visible.
3.1 Field studies
The treated aquaculture cage nets PE-PANI, PE-PANI SiO2, PE-PANI SiO2 + ZnO and PE-PANI ZnO were subjected to the estuarine environment for three months and retrieved after first, second and third month for their performance evaluation (Fig. 3). Fouling accumulated on aquaculture cage nets of different treatments after various periods were illustrated in Fig. 3. On most samples retrieved after 1 month, showed numerous foulers were attached. The samples retrieved after second and third month exhibited uneven fouling pattern. The 0.01% (w/v) each of nano ZnO and SiO2 treated net (S4) sample retrieved after second and third month exhibited the lowest biofouling. The accumulation pattern of fouling organism was different in ZnO + SiO2 treated samples. Studies have reported that the fouling diversity in the Cochin estuary varied with seasons [28]. The biomass accumulation observed after different treatments were shown in Fig. 4. The results did not provide any definite pattern for biofouling accumulation. Variations in the first, second and third months probably occurred due to climate change experienced at exposure sites during that perod. Field experimental environments undergo unusual variations which researchers could not have controlled. Microscopic images and biomass evaluation revealed that the nets treated with 0.01% (w/v) of nano ZnO and SiO2 exhibited the highest biofouling resistance and nano ZnO and SiO2 acted synergistically to inhibit biofoulers accumulation on the net. On examining biomass accumulation on the nets treated with 0.01 and 0.02% (w/v) SiO2, exhibited increased fouling biomass accumulation than control upto 2 months (Fig. 4). This finding indicated that in the PANI, PE-PANI + SiO2 (S2 and S3) can encourage foulers attachment, where as 0.01 and 0.02% (w/v) PE-PANI + ZnO (S8 and S9) treated net exhibited comparatively low biomass accumulation because of the antifouling property of nano ZnO. The PE-PANI + ZnO + SiO2 treatment (S4 to S7) reduced the accumulation of fouling biomass. Figure 4 revealed that the three month sample exhibited lower accumulation of biomass than the first and second month sample did, which may have been caused by the sloughing off foulers because of rain in the region, which changed the salinity of the test site. The results highlighted ZnO is an effective inhibitor of fouling organism, addition of nano SiO2 further increased the biofouling resistance. ZnO and SiO2 synergistically acted to decrease the accumulation of foulers. Incorporation of nano SiO2 with N-halamine, zwitterionic polymer and indium tin oxide electrode respectively inhibited E. Coli, protein and haemoglobin [9,10,11]. The results in the present study correlated with the above findings that reinforcement of SiO2 along with nano ZnO increased the biofouling resistance.
Nano ZnO is a known antimicrobial oxide and attacks the microroganisms by synthesising reactive oxygen species [29]. An incident UV light causes interfacial movement of electrons in oxygen and zinc vacancies, which leads to the formation electrons and holes in the conduction and valence bands, respectively [30, 31]. Holes react with the water molecules attached to ZnO and form hydroxyl radical (OH•). Montazer and Amiri [17] described the reaction mechanism of this process in detail. It has already demonstrated that the biocidal activity of OH•, and it is adequate to kill the microbes present in the biofilms, fungi and phytoplankton [32]. A high photocatalytic activity of ZnO depends on the number of active sites. Studies by Lee et al. [18] predicted that UV radiation penetrates upto 10% of the euphotic depth of oligotrophic waters. The concentration of UV light may be low compared to the visible radiations in an aquatic system. Thus band gap reduction is necessary to activate ZnO under visible light. Electron rich particles like chemically reduced graphene, carbon nanotubes and the nano composites of semiconductors, were employed to reduce the band gap [19,20,21,22,23]. In the present study the electron rich conductng polymer, PANI, was used as the substrate to coat the oxides. The nano ZnO was strongly adsorbed over PANI and this might have reduced the band gap of ZnO and hence increased the photocatalytic effect [23, 33]. Mao et al. [34] reported the synergistic effect of nano ZnO and SiO2 to block the UV radiation in cotton fibres. In the present study, the SiO2 might have influenced synergistically with ZnO to inhibit biofouling. In presence of PANI and SiO2 the e−/h+ recombination rate of ZnO increased and this might have resulted increased photocatalytic effect and biofouling resistance [35,36,37]. This finding was correlated with the results of bioaccumulation pattern of nano SiO2 and ZnO coated cage net. Increased bioaccumulation was showed in SiO2 coated net than ZnO coated net, where as PANI–ZnO–SiO2 system exhibited more efficient biofouling resistance.
4 Conclusion
Biofouling is a serious problem in aquacutlture because resolving it utilizes one fourth of total project expenses. PE is a non polar molecule commonly used for aquaculture cage nets and is highly demanded for coating antifouling biocides directly over it. This study reported the applications of nano silicon and zinc oxides with PANI coated on PE to prevent biofouling in the aquatic environment. The suface morphology analysis confirmed the formation of nano zinc and silicon oxides on PE –PANI. The results obtained from field exposure experiments revealed that treating PE-PANI net with 0.01% (w/v) each of the nano ZnO and SiO2 provided highest biofouling resistance and the nano ZnO and SiO2 acted synergistically to inhibit biofoulers accumulation on PE net. The results must be assessed further through extensive field exposures in aquaculture farms.
Data availablity
The data pertaining to this research paper is available with the corresponding author and will be shared the same after a reasonable request.
References
Armstrong E, Boyd KG, Pisacane A, Peppiatt CJ, Burgess JG (2000) Marine microbial natural products in antifouling coatings. Biofouling 16(2–4):215–224. https://doi.org/10.1080/08927010009378446
Fitridge I, Dempster T, Guenther J, De Nys R (2012) The impact and control of biofouling in marine aquaculture: a review. Biofouling 28(7):649–669
Braithwaite RA, McEvoy LA (2005) Marine biofouling on fish farms and its remediation. Adv Mar Biol 47:215–252
Lewis JA (1998) Marine biofouling and its prevention. Mater Forum 22:41–46
Ashraf PM, Sasikala KG, Thomas SN, Edwin L (2020) Biofouling resistant polyethylene cage aquaculture nettings: a new approach using polyaniline and nano copper oxide. Arab J Chem 13:875–882. https://doi.org/10.1016/j.arabjc.2017.08.006
Chen S, Zhu J, Zhou T, He B, Huang W, Wang B (2012) Preparation and properties study of polyaniline conductive anti-fouling coatings. Int J Electrochem Sci 7(9):8170
Mohan A, Ashraf PM (2019) Biofouling Control using nano silicon dioxide reinforced mixed-charged zwitterionic hydrogel in aquaculture cage nets. Langmuir 35(12):4328–4335
Knowles BR, Wagner P, Maclaughlin S, Higgins MJ, Molino PJ (2017) Silica nanoparticles functionalized with zwitterionic sulfobetaine siloxane for application as a versatile antifouling coating system. ACS Appl Mater Interfaces 9(22):18584–18594
Dong A, Huang J, Lan S, Wang T, Xiao L, Wang W, Zhao T, Zheng X, Liu F, Gao G, Chen Y (2011) Synthesis of N-halamine-functionalized silica–polymer core–shell nanoparticles and their enhanced antibacterial activity. Nanotechnology 22(29):295602
Nguyen Ai T, Baggerman J, Paulusse JMJ, van Rijn CJM, Zuilhof H (2011) Stable protein-repellent zwitterionic polymer brushes grafted from silicon nitride. Langmuir 27(6):2587–2594
Serrano MB, Despas C, Herzog G, Walcarius A (2015) Mesoporous silica thin films for molecular sieving and electrode surface protection against biofouling. Electrochem Commun 52:34–36
Al-Naamani L, Dobretsov S, Dutta J, Burgess JG (2017) Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 168:408–417
Zhang J, Pu G, Severtson SJ (2010) Fabrication of Zinc oxide/polydimethylsiloxane composite surfaces demonstrating oil-fouling-resistant superhydrophobicity. ACS Appl Mater Interfaces 2(10):2880–2883
Rajabi H, Ghaemi N, Madaeni SS, Daraei P, Astinchap B, Zinadini S, Razavizadeh SH (2015) Nano-ZnO embedded mixed matrix polyethersulfone (PES) membrane: influence of nanofiller shape on characterization and fouling resistance. Appl Surf Sci 349:66–77
Chung YT, Ba-Abbad MM, Mohammad AW, Benamor A (2016) Functionalization of zinc oxide (ZnO) nanoparticles and its effects on polysulfone-ZnO membranes. Desalin Water Treat 57(17):7801–7811
Ashraf PM, Anuradha R (2018) Corrosion resistance of BIS 2062-grade steel coated with nano-metal-oxide mixtures of iron, cerium, and titanium in the marine environment. Appl Nanosci 8(1–2):41–51
Montazer M, Amiri MM (2014) ZnO nano reactor on textiles and polymers: ex situ and in situ synthesis, application, and characterization. J Phys Chem B 118:1453–1470
Lee Z, Hu C, Shang S, Du K, Lewis M, Arnone R, Brewin R (2013) Penetration of UV-visible solar radiation in the global oceans: insights from ocean color remote sensing. J Geophys Res Oceans 118:4241–4255. https://doi.org/10.1002/jgrc.20308
Zhang Y, Tang ZR, Fu X, Xu YJ (2011) Engineering the unique 2D mat of graphene to achieve graphene-TiO2 nanocomposite for photocatalytic selective transformation: what advantage does graphene have over its forebear carbon nanotube? ACS Nano 5(9):7426–7435
Zhang Y, Tang ZR, Fu X, Xu YJ (2010) TiO2_graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: is TiO2_graphene truly different from other TiO2_carbon composite materials? ACS Nano 4:7303–7314
Zhang Y, Zhang N, Tang ZR, Xu YJ (2012) Graphene transforms wide band gap ZnS to a visible light photocatalyst. the new role of graphene as a macromolecular photosensitizer. ACS Nano 6:9777–9789
Zhang N, Zhang Y, Xu YJ (2012) Recent progress on graphene- based photocatalysts: current status and future perspectives. Nanoscale 4:5792–5813
Chen Z, Zhang N, Xu YJ (2013) Synthesis of graphene-ZnO nanorod nanocomposites with improved photocatalytic and anti- photocorrosion. Cryst Eng Comm 15:3022–3030
Zhu Y, Apostoluk A, Gautier P, Valette A, Omar L, Cornier T, Bluet JM, Masenelli-Varlot K, Daniele S, Masenelli B (2016) Intense visible emission from ZnO/PAAX (X= H or Na) nanocomposite synthesized via a simple and scalable sol-gel method. Sci Rep 6:23557
Kumar H, Rani R (2013) Structural and optical characterization of ZnO nanoparticles synthesized by microemulsion route. Int Lett Chem Phys Astron 14:26–36. https://doi.org/10.18052/www.scipress.com/ILCPA.19.26
Beganskienė A, Sirutkaitis V, Kurtinaitienė M, Juškėnas R, Kareiva A (2004) FTIR, TEM and NMR investigations of Stöber silica nanoparticles. Mater Sci (Medžiagotyra) 10:287–290
Tang X, Alavi S, Herald TJ (2008) Effects of plasticizers on the structure and properties of starch–clay nanocomposite films. Carbohydr Polym 74(3):552–558
Geetha PN, Nandan SB (2014) Ecology, diversity, and abundance of macrobenthic crustaceans in Cochin Estuary, India. Res J Recent Sci 3:137–148
Sharma D, Rajput J, Kaith BS, Kaur M, Sharma S (2010) Synthesis of ZnO Nanoparticles and study of their antibacterial and antifungal properties. Thin Sol Films 519:1224–1229
Yatmaz HC, Akyol A, Bayramoglu M (2004) Kinetics of the photocatalytic decolorization of an azo reactive dye in aqueous ZnO suspensions. Ind Eng Chem Res 43:6035–6039
Nair G, Nirmala M, Rekha K, Anukaliani A (2011) Structural, optical, photo catalytic and antibacterial activity of ZnO and Co doped ZnO nanoparticles. J Mater Lett 65:1797–1800
Van Dijken A, Meulenkamp EA, Vanmaekelbergh D, Meijerink A (2000) The kinetics of the radiative and nonradiative processes in nanocrystalline ZnO particles upon photoexcitation. J Phys Chem B 104:1715–1723
Zhang Y, Chen Z, Liu S, Xu YJ (2013) Size effect induced activity enhancement and anti-photocorrosion of reduced graphene Oxide/ZnO composites for degradation of organic dyes and reduction of Cr (VI) in water. Appl Catal B 140–141:598–607
Mao ZP, Shi QP, Zhang LP, Cao HT (2009) The formation and UV-blocking property of needle-shaped ZnO nanorod on cotton fabric. Thin Sol Films 57:2681–2686
Zhou M, Yu J, Cheng B (2006) Effects of Fe-doping on the photocatalytic activity of mesoporous TiO2 powders prepared by an ultrasonic method. J Hazard Mater 137(3):1838–1847
Klingshirn CF (2007) ZnO: material, physics and applications. ChemPhysChem 8:782–803
Estrellan CR, Salim C, Hinode H (2009) Photocatalytic activity of sol-gel derived TiO2 co-doped with iron and niobium. React Kinet Catal Lett 98:187–192
Acknowledgements
The authors thank the Director of ICAR Central Institute of Fisheries Technology for providing facilities, the technical staff of the Fishing Technology Division of ICAR-CIFT, STIC, CUSAT for extending SEM facilities and Head Microbiology Division of ICAR CIFT for providing FTIR facilities. Thanks to Editing India for English correction of the manuscript.
Author information
Authors and Affiliations
Contributions
Experiments were designed by P.M.A, S.N.T, L.E and N.M.L. Experiments performed by N.M.L, PMA and A.K.K. Manuscript written by N.M.L and P.M.A
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no Conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lekshmi, N.M., Ashraf, P.M., Keerthana, A.K. et al. Biofouling inhibition for aquaculture cage nets through a coating nano zinc and silicon oxides incorporated with polyaniline. SN Appl. Sci. 2, 2034 (2020). https://doi.org/10.1007/s42452-020-03788-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03788-7