Abstract
Zn1−xFexO nanoparticles with different doping concentration i.e. (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) were successfully synthesized via hydrothermal method. The crystal structure, morphology, optical and magnetic properties were studied by X-ray diffraction, field emission scanning electron microscope, UV–visible, photoluminescence spectra (PL) and vibrating sample magnetometer. The experimental results show that all the samples possess a single phase hexagonal wurtzite structure, no impurity phase was observed. The crystallite size decreases with the increase in Fe concentration. Optical absorption analysis by UV–visible exhibits a slight blueshift in the bandgap with increasing doping concentration. PL spectra shows that the emission peak in the UV region is absent for all the samples. All the emission peaks are in the visible region. Shifting of PL emission peaks is not observed indicating that the intrinsic defects are consistent. The magnetic observation indicated that all the samples including pure zinc oxide exhibit room temperature ferromagnetism (RTFM). The observed RTFM is attributed to the bound magnetic polaron that arises due to the presence of singly ionized oxygen vacancy (Vo) as indicated by PL spectra.
Similar content being viewed by others
1 Introduction
Diluted magnetic semiconductors (DMSs) are the materials formed by replacing cations of nonmagnetic semiconductors by magnetic ions such as transition metals (TM) ions or rare earth ions [1]. Ferromagnetism observed in DMS has led to its intense research because of their potential applications in spintronic devices, which uses both the spin in magnetic materials and charge of electrons [2]. Moreover, doping of transition metal into wide band gap semiconductor also leads to the tuning of optical and ferromagnetic properties [3]. Intrinsic ferromagnetism with high Curie temperature (above room temperature) is the principal requirement of DMSs for the application in spintronic devices [4]. Thus, efforts are made to produce TM metal doped wide bandgap semiconductor exhibiting room temperature ferromagnetism (RTFM). Zinc oxide (ZnO) is II–VI wide band gap (Eg ~ 3.37 eV) and large exciton binding energy (60 meV) compound semiconductor with hexagonal wurtzite structure of P63mc space group [5]. Requirement of Curie temperature above room temperature (RT) and intrinsic ferromagnetism (FM), rather than the ferromagnetism because of magnetic clusters or secondary phases of dopants for spintronics application makes transition metal doped ZnO a favorable system owing to observation of RTFM in several ZnO based system including Mn-, Fe- and Co-doped ZnO [6]. Results quite contradictory to each other are reported for ferromagnetism in transition metal doped ZnO. Liu et al. [7] reported RTFM and explains it as the intrinsic property of Fe doped ZnO. Reports suggesting that transition metal does not play any role in the magnetism of Mn and Fe doped ZnO thin films are also available [8]. Bhargava et al. [9] observed ferromagnetism in Cr doped ZnO. Taher et al. [10] observed ferromagnetism in nanostructure Co-doped ZnO thin films reasonably caused by the oxygen vacancies introduced in the films during the hydrothermal growth. RTFM was also reported by Mishra et al. in Fe doped ZnO nanostructures synthesized using chemical root and attributed its cause to Zinc vacancy (Zni) while Wei et al. also observed ferromagnetism in Fe doped ZnO and attributed it to secondary phase for high doping concentration [11, 12]. Even though large numbers of reports are available in literature, there is a conflict for the origin of observed room temperature ferromagnetism in the transition metal doped ZnO DMS.
In the present work, our aim is to provide a better understanding of the origin of room temperature ferromagnetism. For this purpose, diluted magnetic semiconductor Zn1−xFexO with different doping concentration (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) is synthesized via hydrothermal method. The magnetic property is studied by using vibrating sample magnetometer (VSM). The structure and surface morphology is investigated by X-ray diffraction (XRD) and field emission scanning electron microscope (FESEM). The optical properties are studied with the help of UV–visible and photoluminescence (PL) spectra.
2 Experimental details
Zn1−xFexO (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) nanoparticles are prepared by hydrothermal method. An aqueous solution of 1 M zinc nitrate hexahydrate 5 M sodium hydroxide and 1 M ferric nitrate nonahydrate solutions are prepared in double distilled water. Zinc nitrate hexahydrate and ferric nitrate nonahydrate solutions are mixed according to the required amounts and stirred at room temperature. Sodium hydroxide solution is added dropwise to it. After being stirred for 2 h, the mixture is transferred into a 200 ml Teflon lined stainless steel autoclave and is kept in an oven at 100 °C for 6 h. It is then allowed to cool down naturally at room temperature. The obtained product is washed with distilled water and dried at 100 °C for 24 h. The samples are then heated at 500 °C for 2 h. Figure 1 shows the flowchart depicting hydrothermal method used for the synthesis of Zn1−xFexO (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05).
The structural and morphological characterizations of the samples are performed using X-ray diffractometer (Model: Smartlab XRD) and field emission scanning electron microscope (FESEM, Model: Carl Zeiss Ultra Plus). The optical properties are studied by using UV–visible spectrophotometer (Model: Cary 60 UV–Vis) and photoluminescence spectrophotometer (Model: Cary Eclipse Fluorescence Spectrophotometer). Vibrating sample magnetometer (VSM, Model: Quantum Design VERSALAB) was used for magnetic characterization.
3 Structural analysis
3.1 X-ray diffraction
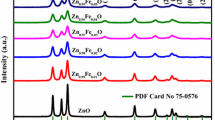
Figure 2a shows the XRD pattern of Zn1−xFexO nanoparticles. The diffraction peaks in the XRD pattern corresponds to the diffraction of (100), (002), (101), (102), (110) planes indicating the formation of hexagonal wurtzite structure of ZnO with a space group P63mc. Furthermore, no traces of impurity or secondary phases within the detection limit of XRD and no obvious diffraction patterns of Iron (Fe) species such as Fe, Fe2O3, and Fe3O4 are found in the samples of 1–5% Fe doped ZnO. The result indicates the successful incorporation of all Fe ions in the host ZnO crystal lattice. It is seen from the XRD pattern that with an increase Fe concentration the peak shifts towards the lower angle as compared to the pristine ZnO up to 3% doping and then again shift towards the higher angle being nearly equal to that for pristine ZnO at 4%. These results may be ascribed to the valence state of Fe ions. Fe ions are capable of existing as Fe2+, Fe3+ stably in compounds. In this paper, dopant ions are introduced into the host ZnO through ferric nitrate, so Fe would be in Fe3+ valence state. It is known that zinc act as a reducing agent in changing Fe3+–Fe2+ [24]. So, transition of Fe3+–Fe2+ is much expected as the host being ZnO in this case. The existence of Fe in Fe2+ is reported where the diffraction angle decreases with Fe concentration while the existence of iron as Fe3+ is reported where the diffraction angle increases [13,14,15]. Taking the peak position into consideration and from the existing reports, it can be inferred that Fe2+ and Fe3+ coexist in the Fe doped ZnO samples [16]. The shifting of peaks may also be due to the strain developed in the lattice due to doping. Broadening of peak, apart from its shift can be explained based on grain size reduction with Fe atom incorporation in ZnO. The ionic radii of Fe2+, Fe3+ and Zn2+ are 0.078, 0.068 and 0.074 nm respectively [17]. Substitution of Zn2+ with Fe2+, Fe3+ in the lattice site may cause lattice distortion resulting in strain in ZnO because of the difference in ionic radii of Fe2+ and Fe3+ with Zn2+ [18]. Fe ions need to have a balanced state of 2+ for maintaining the charge neutrality to properly substitute Zn2+ ionic sites. So, the balance state Fe3+ is likely to distort the lattice structure for maintaining the charge neutrality [19].
Figure 2b shows a large difference in intensity of XRD peaks for 1% and 3% Fe doped ZnO. The difference in intensity is due to the difference in their crystalline quality [13, 20]. The full width half maximum (FWHM) increases for 1% Fe doped ZnO indicating the decrease in the crystalline quality compares to pristine ZnO. The decrease in crystalline quality may be because of the lattice disorder and strain experience due to the incorporation of Fe ions in ZnO matrix [21]. But as the doping of Fe increases to 2%, FWHM value decreases suggesting an increase in crystalline quality. The crystalline quality then again keeps on declining with the increase in doping up to 5% as implied by the increase in FWHM value. The increase in the crystallinity of the 2% Fe doped ZnO in our experiment may be ascribed to the formation of favorable nucleation centers from the dopant atoms for ZnO crystal growth. The decline in crystalline quality in 5% doping may be because of the saturation of newer nucleation centers or because of the ionic radii difference of Zn2+ and Fe ions [18]. Substitution of a large number of Fe ions in Zn2+ lattice sites leads to an increase in lattice distortion causing a lager strain which affects the crystal growth of ZnO.
The (101) preferred orientation with maximum intensity has been observed in the XRD pattern. The average crystallite size (D) of the samples is estimated from XRD line width of (101) peak by using Scherrer’s equation [22]. The crystalline size value of pristine and Fe doped ZnO is shown in Fig. 3. The crystallite size is in accordance with the FWHM values. The calculated values of a, c, c/a, D, d, volume, bond length, internal parameter (u) and distortion degree (R) are given in Table 1 [22].
The c/a ratio of pristine ZnO as well as Fe doped samples is less than the c/a ratio of ideal stoichiometry wurtzite structure ratio i.e. 1.633 indicating the presence of oxygen vacancies and zinc vacancies [23]. The calculated values of bond length vary from 1.9692 to 1.9735. Variation of bond length is not monotonous with doping concentration. The non-monotonous variation of bond length may be because of the strain present in the samples and a slight change in R is also observed [24].
3.2 SEM analysis
The morphology of the synthesized Zn1−xFexO (x = 0, 0.03, 0.05) materials are studied by field emission scanning electron microscope (FESEM). The FESEM images imply the change of shape and morphology of ZnO nanoparticles with doping. Figure 4a shows formation of a mixture of rod like and nanoflakes like morphology in pristine ZnO. In contrast, the Zn0.97Fe0.03O and Zn0.95Fe0.05O doped ZnO in Fig. 4b, c respectively showed cone-like particles that are formed may be due to the asymmetrical and multidimensional growth of ZnO particles [25]. It is difficult to say something about the average grain size of the pristine and doped samples as the particles seems to be agglomerated for these images.
Energy dispersive X-ray analysis (EDAX) study is carried out for observing the elemental composition of synthesized samples and to verify the successful incorporation of dopants and the formation of ZnO nanoparticles. EDAX signals of pristine ZnO and Zn0.95Fe0.05O are shown in Fig. 5. For pristine ZnO nanoparticles, sharp signals of Zn and oxygen were found confirming the formation of ZnO nanoparticles. For Fe doped sample, Fe signals in addition to Zn and oxygen were also found, confirming the successfully incorporated of Fe in the ZnO.
4 Optical properties
4.1 UV–visible analysis
We explore and analyze pristine and Fe doped ZnO nanoparticles energy bandgap using UV–visible spectroscopy. The optical bandgap of the pristine and Fe doped sample is calculated by using the Tauc relation. Figure 6a shows the Tauc plot and the variation of bandgap with doping concentration is shown in Fig. 6b. Values of the bandgap are also tabulated in Table 2.
The exciton Bohr radius of ZnO is much larger than the obtained average crystallite size. Therefore, the quantum confinement effect is not the major reason for the change in the bandgap of prepared samples [26]. The increase in bandgap may be due to the strain experienced by the doped sample. The result obtained is in agreement with the result reported by Parra-Palomino et al. [27] where they observed a large blueshift in bandgap as the concentration of the dopant increase. The obtained bandgap is also in accordance with the result obtained by Hammad et al. [15] who explain the blue shift behavior based on Moss–Burstein theory. According to this theory, ZnO nanoparticle is n-type semiconductor and due to doping the Fermi level shifts inside the conduction band. The absorption edge shifts to higher energy as the states below such shifting in the conduction band are filled leading to the widening of bandgap. The shifting of Fermi level towards the conduction band may be attributed to the additional carriers or d–d transition from Fe. Rekha et al. and Arun et al. also explain the blue shift behavior of their sample based on the Moss–Burstein theory [28, 29]. The increase in bandgap or the blue shift behavior for the Fe doped sample with respect to that of pristine ZnO may also be because of the sp-d exchange interaction of localized d electron and band electrons as explained by Pal Singh et al. [30].
4.2 Photoluminescence (PL) characteristic
The PL properties of pristine and Fe-doped ZnO nanoparticles are also studied. The photoluminescence is recorded at room temperature with an excitation wavelength of 330 nm. Figure 7 shows the PL spectra at RT for all the samples. The inset of Fig. 7 represents the PL spectra of pristine ZnO deconvoluted into six peaks. Even though PL spectra of ZnO have been studied extensively, the source of visible range emission bands remains controversial. The typical photoluminescence spectra of ZnO have emission bands in the UV and visible regions. Usually, the UV luminescence peak is considered as the characteristics emission of ZnO and it is ascribed to the band edge emission or transition of exciton [31]. It is exciting to note that there is no UV emission peak in the PL spectra of prepared samples. This signifies that the recombination of exciton pairs of the prepared samples occurs at the non-radiative centers [32]. The disappearance of UV emission peak may also be attributed to a much higher intensity of visible emission due to an increase in defect density or it may also be because its excitation energy is significantly lower than its bandgap energy [33].
PL spectra confirmed that all the samples exhibited six peaks in the visible region. The peak near 416 nm (2.98 eV) in the PL spectra arises due to violet emission. This may arise because of the transition from conduction band (CB) to deep holes trapped levels above the valence band (VB) [34]. A single distinct peak centered about 458 nm (2.70 eV) in the blue band (2.75–2.63 eV) may be attributed to the direct recombination of a conduction electron in the CB and a hole in the VB [35]. The blue green emission peak centered about 486 nm (2.55 eV) may occur because of the electronic transition of donor level Zni to acceptor level Zinc vacancy (VZn) [36]. Green emission peak centered about 528 nm (2.35 eV) is credited to defects related to oxygen vacancies because of the recombination of photogenerated holes and electron trapped by singly ionized oxygen vacancies (Vo) [37]. Vo are known as color centers (or F centers) and are also an important set of point defects in oxides. In the ZnO lattice, it shows three dissimilar charged states as F++, F+, F0. The F+ oxygen vacancy which is singly occupied can contribute in activating bound magnetic polarons (BMP) and magnetic moment in DMSs. However, F++ and F0 vacancies do not induce FM in ZnO since they have spin zero ground states [14]. The broad peak centered about 584 nm (2.12 eV) in the range 550–600 nm (2.25–2.06 eV) is dedicated to the defect related transition originating from excess oxygen. Non-shifting of luminescence peaks in the visible range indicates that the intrinsic defects are consistent in all the samples [24]. Quenching of the luminescence peaks may be because of non-radiative recombination or may be due to the preferential occupation of Fe3+ ions by photogenerated electrons inducing trap centers [38].
5 Magnetic properties
To study the effect of Fe doping on the magnetic behavior of ZnO, the magnetization versus applied magnetic field curve obtain from VSM data performed at RT is studied. Figure 8 shows the M–H curves of Zn1−xFexO (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) nanoparticles. It is observed that the M–H curves of the pristine and doped samples do not saturate even at high applied fields. The M–H curve for all the samples comprises a linear part possibly because of the small paramagnetic (PM) and superparamagnetic (SPM) contribution in addition to a distinct ferromagnetic ordering confirmed by hysteresis behavior of the curve at lower field region [39]. Pristine ZnO ferromagnetism termed as d0 ferromagnetism may arise because of the presence of Vo defects and is supported by the PL spectra [40]. There are also reports indicating the role of Zni and Vo in maintaining pure ZnO magnetic behavior. RTFM may be due to extrinsic phenomena such as the formation of cluster or secondary phase of dopant ions and intrinsic phenomena such as exchange interaction [30]. The XRD pattern indicates that there is no formation of secondary phases such as Fe2O3, Fe3O4, etc. Therefore, the observed ferromagnetism in our sample cannot be attributed to the formation of the secondary phase or cluster due to the doping of Fe. So, it may be because of the intrinsic phenomena and not due to the extrinsic phenomena. Number of models and concepts has been proposed to explain the room temperature ferromagnetism such as (a) hole mediated Zener mean-field model (b) carrier-mediated RKKY (Ruderman–Kittel–Kasuya–Yosida) mechanism (c) donor impurity band exchange model, in this, the FM in DMSs is due to an indirect exchange donor electrons and this form a BMPs and (d) direct interactions (such as double or superexchange mechanism). RKKY is not valid as it is based on free electrons and ZnO is a semiconductor and cannot be converted into metal, double exchange mechanism is also not possible because of the very dilute doping of the sample [41]. Hole mediated Zener field model of ferromagnetism may also be excluded since ZnO is an n-type semiconductor [42]. So, the observed ferromagnetism for our sample can be centered on the substitutional behavior of Fe and can be understood based on bound magnetic polarons (BMPs) due to defects. F+ is known to be paramagnetic that can activate bound magnetic polaron and these BMPs interact leading to the formation of magnetic domains [43]. As a result, the singly occupied Vo may assume to be the cause of ferromagnetism. The interaction between Vo and Fe ions may lead to the formation of BMP in doped samples. The saturation magnetization (MS) is obtained by drawing a tangential line from the high field loop to the zero-field and is extrapolated. The intercept of the tangent on magnetization axis is taken as the value of Ms. Ms value increases up to 4% Fe doped samples and then decreases for 5% Fe doped ZnO. The enhancement in magnetization may be because of the overlapping of more Fe ions into the ferromagnetic domain with the increase in volume occupied by BMPs in accordance with the explanation given by Hsu et al. for Co-doped ZnO [44]. The decrease in magnetization may be because of the random distribution of some Fe ions leading to the decrease in net magnetization as mention by Iqbal et al. [45]. The result obtained is in accordance with the report given by Agarwal [46] where they observed a decrease in magnetization at higher Cu concentration. Table 3 shows the value of saturation magnetization (Ms), coercivity (Hc), remanence (Mr).
Models describing the FM and SPM/PM behavior of magnetization curves are not found. The non-saturating hysteresis loop is a phenomenon commonly observed in thin films magnetic oxides. The slope of non-saturation term is known as high field susceptibility. Non-saturation of magnetization is also observed by Margulies [47] in Fe3O4 films grown by sputter deposition on crystalline substrate and is term as anomalous. The approach to saturation magnetization problem has been discussed in detail by Chakazumi and Charap [48].
Assuming that the presence of restoring force increases with the advance of magnetization towards saturation, the law of approach to saturation magnetization can be expressed as
where \(M\) is the actual magnetization value observe at H and the value of constant Q corresponds to its saturation value. a, b, c and e are constants. The term \(\frac{a}{\sqrt H }\) is credited to the point defects or magnetic anisotropy fluctuation on the atomic scale. The term \(\frac{b}{H}\) originated from the local internal stresses caused by dislocation and to voids or nonmagnetic inclusions. \(\frac{c}{{H^{2} }}\) is credited to the weak and large scale fluctuations in the bulk anisotropy. The last term eH is negligible and hence ignored. It is caused by spontaneous magnetization by the external field. The high field susceptibility fitting curve is shown in Fig. 9. Table 4 shows the value of Q, a, b, and c.
6 Conclusion
In this work structural, optical and magnetic properties of Zn1−xFexO (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) nanoparticles synthesized by hydrothermal method has been studied. XRD results show that the particles exhibited a hexagonal wurtzite structure without any impurity phase and suggest that Fe2+ and Fe3+ ion coexist. Blueshift of optical band gap has been observed for the increase in Fe concentration. PL spectra show the absence of near band edge and all the observed emission peaks are in the visible region. Weak room temperature ferromagnetism even for the pure ZnO has been observed. The observed hysteresis loop has not been saturated even at high field, may be due to PM and SPM contribution. The observed ferromagnetism is intrinsic and is attributed to BMP.
References
Chen AJ, Wu XM, Sha ZD, Zhuge LJ, Meng YD (2006) Structure and photoluminescence properties of Fe-doped ZnO thin films. J Phys D Appl Phys 39(22):4762
Liu C, Yun F, Morkoc H (2005) Ferromagnetism of ZnO and GaN: a review. J Mater Sci Mater Electron 16(9):555
Kumar S, Tiwari N, Jha SN, Chatterjee S, Bhattacharyya D, Sahoo NK, Ghosh AK (2015) Insight into the origin of ferromagnetism in Fe-doped ZnO diluted magnetic semiconductor nanocrystals: an EXFAS study of local structure. RSC Adv 5(115):94658–94669
Sinha S, Singh M, Singh RP (2015) ZnO based diluted magnetic semiconductors for spintronic device applications: a review. Int J Emerg Res Manag Technol 4(7):16–20
Benramache S, Arif A, Belahssen O, Guettaf A (2013) Study on the correlation between crystallite size and optical gap energy of doped ZnO thin film. J Nanostruct Chem 3(1):80
Zhang ZH, Wang X, Xu JB, Muller S, Ronning C, Li Q (2009) Evidence of intrinsic ferromagnetism in individuals dilute magnetic semiconducting nanostructures. Nat Nanotechnol 4(8):523
Liu H, Yang J, Zhang Y, Wang Y, Wei M (2008) Ferromagnetism and exchange bias in Fe-doped ZnO nanocrystals. Mater Chem Phys 112(3):1021–1023
Hong NH, Sakai J, Brizé V (2007) Observation of ferromagnetism at room temperature in ZnO thin films. J Phys Condens Matter 19(3):036219
Bhargava R, Sharma PK, Chawla AK, Kumar S, Chandra R, Pandey AC, Kumar N (2011) Variation in structural, optical and magnetic properties of Zn1−xCrxO (x = 0.0, 0.10, 0.15, and 0.20) nanoparticles: role of dopant concentration on non-saturation of magnetization. Mater Chem Physi 125(3):664–671
Taher FA, Abdeltwab E (2018) Shape-controlled synthesis of nanostructured Co-doped ZnO thin films and their magnetic properties. CrystEngComm 20(38):5844–5856
Mishra AK, Das D (2010) Investigation on Fe-doped ZnO nanostructures prepared by a chemical route. Mater Sci Eng B 171(1–3):5–10
Wei XX, Song C, Geng KW, Zeng F, He B, Pan F (2006) Local Fe structure and ferromagnetism in Fe-doped ZnO films. J Phys Condens Matter 18(31):7471
Xu L, Li X (2010) Influence of Fe-doping on the structural and optical properties of ZnO thin films prepared by sol–gel method. J Cryst Growth 312(6):851–855
Chen ZC, Zhuge LJ, Wu XM, Meng YD (2007) Initial study on the structure and optical properties of Zn1−xFexO films. Thin Solid Films 515(13):5462–5465
Hammad TM, Griesing S, Wotocek M, Kuhn S, Hempelmann R, Hartmann U, Salem JK (2013) Optical and magnetic properties of Fe-doped ZnO nanoparticles prepared by the sol–gel method. Int J Nanoparticles 6(4):324
Kim KJ, Park YR (2004) Optical investigation of Zn1–xFexO films grown on Al2O3 (0001) by radio-frequency sputtering. J Appl Phys 96(8):4150–4153
Abdel-Baset TA, Fang YW, Anis B, Duan CG, Abdel-Hafiez M (2016) Structural and magnetic properties of transition-metal-doped Zn1–xFexO. Nanoscale Res Lett 11(1):115
Salaken SM, Farzana E, Podder J (2013) Effect of Fe-doping on the structural and optical properties of ZnO thin films prepared by spray pyrolysis. J Semiconduct 34(7):073003
Cheng W, Ma X (2009) Structural, optical and magnetic properties of Fe-doped ZnO. J Phys Conf Ser 152(1):012039
Kafle BP, Acharya S, Thapa S, Poudel S (2016) Structural and optical properties of Fe-doped ZnO transparent thin films. Ceram Int 42(1):1133–1139
Wang C, Chen Z, He Y, Li L, Zhang D (2009) Structure, morphology and properties of Fe-doped ZnO films prepared by facing-target magnetron sputtering system. Appl Surf Sci 255(15):6881–6887
Cullity BD (1956) Elements of X-ray diffraction. Addison-Wesley Publishing, Boston, MA
Beltrán JJ, Barrero CA, Punnoose A (2015) Understanding the role of iron in the magnetism of Fe doped ZnO nanoparticles. Phys Chem Chem Phys 17(23):15284–15296
Srivastava A, Kumar N, Khare S (2014) Enhancement in UV emission and bandgap by Fe doping in ZnO thin films. Opto-Electron Rev 22(1):68–76
Pung SY, Lee WP, Aziz A (2012) Kinetic study of organic dye degradation using ZnO particles with different morphologies as a photocatalyst. Int J Inorg Chem. https://doi.org/10.1155/2012/608183
Sharma D, Jha R (2017) Transition metal (Co,Mn) co-doped ZnO nanoparticles: effect on structural and optical properties. J Alloys Compd 698:532–538
Parra-Palomino A, Perales-Perez O, Singhal R, Tomar M, Hwang J, Voyles PM (2008) Structural, optical, and magnetic characterization of monodisperse Fe-doped ZnO nanocrystals. J Appl Phys 103(7):07D121
Rekha K, Nirmala M, Nair MG, Anukaliani A (2010) Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Phys B 405(15):3180–3185
Menon AS, Kalarikkal N, Thomas S (2013) Studies on structural and optical properties of ZnO and Mn-doped ZnO nanopowders. Indian J Nanosci 1(1):16–24
Singh RPP, Hudiara IS, Rana SB (2016) Effect of calcination temperature on the structural, optical and magnetic properties of pure and Fe-doped ZnO nanoparticles. Mater Sci Poland 34(2):451–459
Lu YM, Li XP, Cao PJ, Su SC, Jia F, Han S, Liu WJ, Zhu DL, Ma XC (2012) Study of ultraviolet emission spectra in ZnO thin films. J Spectrosc. https://doi.org/10.1155/2013/797232
Ischenko V, Polarz S, Grote D, Stavarache V, Fink K, Driess M (2005) Zinc oxide nanoparticles with defects. Adv Funct Mater 15(12):1945–1954
Dorranian D, Solati E, Dejam L (2012) Photoluminescence of ZnO nanoparticles generated by laser ablation in deionized water. Appl Phys A 109(2):307–314
Kundu S, Sain S, Satpati B, Bhattacharyya SR, Pradhan SK (2015) Structural interpretation, growth mechanism and optical properties of ZnO nanorods synthesized by a simple wet chemical route. RSC Adv 5(29):23101–23113
Wu X, Wei Z, Zhang L, Wang X, Yang H, Jiang J (2014) Optical and magnetic properties of Fe doped ZnO nanoparticles obtained by hydrothermal synthesis. J Nanomater 2014:1–6
Ghosh A, Choudhary RNP (2009) Structural evolution and visible photoluminescence of ZnO nanophosphor. Phys Status Solidi (a) 206(3):535–539
Das J, Mishra DK, Srinivasu VV, Sahu DR, Roul BK (2015) Photoluminescence and Raman studies for the confirmation of oxygen vacancies to induce ferromagnetism in Fe doped Mn:ZnO compound. J Magn Magn Mater 382:111–116
Ovhal MM, Kumar AS, Khullar P, Kumar M, Abhyankar AC (2017) Photoluminescence quenching and enhanced spin relaxation in Fe doped ZnO nanoparticles. Mater Chem Phys 195:58–66
Chanda A, Gupta S, Vasundhara M, Joshi SR, Mutta GR, Singh J (2017) Study of structural, optical and magnetic properties of cobalt doped ZnO nanorods. RSC Adv 7(80):50527–50536
Sundaresan A, Bhargavi R, Rangarajan N, Siddesh U, Rao CNR (2006) Ferromagnetism as a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys Rev B 74(16):161306
Rana AK, Kumar Y, Rajput P, Jha SN, Bhattacharyya D, Shirage PM (2017) Search for the origin of room temperature ferromagnetism properties in Ni-doped ZnO nanostructure. ACS Appl Mater Interfaces 9(8):7691–7700
Ramay SM, Saleem M, Atiq S, Siddiqi SA, Imran M, Al-Zeghayer YS, Al-Awadi AS, Haider S, Mahmood A (2017) RKKY magnetic interactions in chemically synthesized Zn0.95−xFe0.05AlxO (x = 0, 0.03, 0.05, 0.07) nanocrystallites. Arab J Chem 10:S1204–S1208
Zhan P, Wang W, Liu C, Hu Y, Li Z, Zhang Z, Zhang P, Wang B, Cao X (2012) Oxygen vacancy-induced ferromagnetism in un-doped ZnO thin films. J Appl Phys 111(3):033501
Hsu HS, Huang JCA, Huang YH, Liao YF, Lin MZ, Lee CH, Lee JF, Chen SF, Lai LY, Liu CP (2006) Evidence of oxygen vacancy enhanced room-temperature ferromagnetism in Co-doped ZnO. Appl Phys Lett 88(24):242507
Iqbal J, Wang B, Liu X, Yu D, He B, Yu R (2009) Oxygen-vacancy-induced green emission and room-temperature ferromagnetism in Ni-doped ZnO nanorods. New J Phys 11(6):063009
Agarwal DC, Singh UB, Gupta S, Singhal R, Kulriya PK, Singh F, Tripathi A, Singh J, Joshi US, Avasthi DK (2019) Enhanced room temperature ferromagnetism and green photoluminescence in Cu doped ZnO thin film synthesised by neutral beam sputtering. Sci Rep 9(1):1–12
Margulies DT, Parker FT, Spada FE, Goldman RS, Li J, Sinclair R, Berkowitz AE (1996) Anomalous moment and anisotropy behavior in Fe3O4 films. Phys Rev B 53(14):9175
Chikazumi S (1955) Physics of magnetism (Wiley, New York, 1964), p. 554. W. Känzig, Phys Rev 9: 98–549
Acknowledgements
Authors would like to acknowledge Dr. Naresh Kumar, MNNIT Allahabad, Prayagraj, India for providing the characterization facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maibam, B., Baruah, S. & Kumar, S. Photoluminescence and intrinsic ferromagnetism of Fe doped zinc oxide. SN Appl. Sci. 2, 1712 (2020). https://doi.org/10.1007/s42452-020-03519-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03519-y