Abstract
Coral reefs are the foundation to our social, cultural, and economic life; however, reefs around the world are currently being threatened by many local and global impacts. Ongoing increases in seawater temperature pose significant threats to the integrity of these valuable ecosystems through extensive coral bleaching events. Therefore, we developed a coral health reference card, the Hawaiian Koʻa (coral) Card, to assess and quantify coral bleaching and to educate the community about its impacts in Hawai‘i. To accurately quantify the change in coral color during bleaching, controlled laboratory studies followed by field validations and surveys were conducted. Colors presented on the Hawaiian Koʻa Card have been linked to physiological state and health (e.g., symbiont density, chlorophyll levels, photosynthetic performance) of common coral species in Hawaiʻi due to bleaching. The Hawaiian Koʻa Card provides a technical solution to inform and improve management of our nearshore resources through collaborative monitoring efforts by community members, educators, researchers, and managers on a state-wide scale, which will assist in determining management efficacy, identifying regions and species of resilience, establishing baselines and focus areas, and developing and executing rapid response plans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rising global seawater temperatures are threatening the health of coral reef ecosystems worldwide through frequent and prolonged bleaching events [1,2,3,4,5]. The synergistic effects of increased temperature, irradiance, and often, reduced water flow that can cause mass bleaching and coral mortality are particularly pronounced during El Niño Southern Oscillation (ENSO) years [6, 7]. The 1997–1998 and 2014–2016 global bleaching events coincided with these strong ENSO years, and the 2014–2016 event reported the highest seawater temperatures on record [3, 8]. As seawater temperatures steadily increase and as ENSO years bring sudden spikes in temperature, it is predicted that bleaching events may become an annual occurrence that could prevent full recovery due to shortened intervals of recovery time between events [2, 4, 9].
Coral bleaching occurs when elevated seawater temperatures cause the breakdown of the relationship between the coral-animal and their symbiotic intracellular algal partners [2, 8, 10]. As corals lose their algal symbionts, or “bleach”, the color of the colony begins to lighten until the tissues are transparent and the skeleton becomes visibly white [7, 10, 11]. Simultaneously, the effects of solar irradiance exacerbate and accelerate tissue damage [6, 12, 13]. Coral colony mortality may occur during the absence of these algal partners, due to nutritional impairment, if elevated temperature persists and normal conditions do not return [6, 14].
The Hawaiian Archipelago did not experience widespread bleaching events until 1996 in the main Hawaiian Islands, and 2002 and 2004 in the Northwestern Hawaiian Islands (NWHI) [1, 8, 15]. However, during the 2014–2015 ENSO year, the entire Hawaiian Archipelago experienced unprecedented bleaching and mortality [8]. Over half the corals on the Hawaiʻi Island exhibited signs of paling and/or bleaching [16] and mass mortality of over 2.4 km of the Montipora dominated reef on Lisianski Atoll in Papahānaumokuākea in the NWHI occurred [17]. During these mass bleaching events (i.e., 2014, 2015), numerous management agencies and research groups expended extensive time and effort quantifying the severity of coral bleaching and subsequent recovery or mortality of corals in Hawaiʻi [2, 3, 8, 16, 17]. These efforts highlighted the need for an inexpensive, non-invasive, and rapid visual tool that allows comparability across user-groups and among all reefs across the Hawaiian Archipelago to monitor reef condition [8, 17, 18].
Since color has been shown to correlate with coral condition, its use as a viable indicator or proxy for health is a practical method of determining coral condition over a large scale. This provides a simplified and effective field method of determining bleaching severity by comparing different shades of color over time among individual coral colonies [5, 19]. In the early 2000s, Siebeck et al. [5] evaluated the usefulness of an observer-based reference card for the assessment of coral bleaching tailored to the common coral colors on Australian reefs. They demonstrated that measurements of coral health, including chlorophyll a (chl a) concentration and symbiont abundance, can be associated with color and give greater precision to traditional subjective assessment of bleaching states. In a simulated “natural” bleaching event, they found significantly fewer symbionts and lower chl a content in pale corals when compared to pigmented corals. This bleaching experiment was used to develop a color card with symbiont and chl a concentrations corresponding to four main hues of Australian corals with six variations of brightness and saturation within hues.
The Australian Coral Watch Coral Health Chart (https://coralwatch.org) has been successfully used as a tool for assessing coral health in over 25 published scientific studies [20] by experienced and non-experienced researchers on reefs across Kenya [21], the Indo-Pacific Islands [22], the Caribbean Islands [23], and the Persian Gulf [19]. Furthermore, Oladi et al. [19] found incomparability of colors with the Australian Coral Health Chart in “healthy” looking Persian Gulf corals due to variation in pigmentation and zooxanthellae characteristics. The success of this chart as a coral health assessment tool and the recognition that pigmentation among corals can vary by region [24] provided the impetus to develop a similar tool that is more representative of coral pigmentation within the Hawaiian Archipelago.
The methods and approach for developing the Hawaiian Koʻa (coral) Card, outlined in this paper, was modeled after the Australian Coral Watch Coral Health Chart [18]. This card will be a valuable, inexpensive, rapid, and non-invasive tool that can be used to assess and report on coral bleaching severity in Hawaiʻi by members of all local communities, state federal and local agencies, non-governmental organizations, scientists, and citizen scientists. The data collected through the use of the Hawaiian Ko‘a Card will substantially increase the capacity for coral reef management through applied research. By understanding differences in areas at the extremes of the bleaching range, management can begin to develop strategies to slow declines. Widespread use of the Hawaiian Koʻa Card for the assessment of bleaching severity will help detect areas of refuge that are more resilient to climate change and concentrate management efforts. In manipulative lab and field experiments, this tool can provide researchers with a quantified method of comparison across temporal and spatial scales. Provision of education and resources also fosters habitat protection through community driven management. Greater understanding and better management are possible with the combined efforts of all parties using a common tool to describe the condition of coral reefs across the state. This simple tool requires minimal instruction and empowers users, providing a vested interest in reef recovery in times of localized and global bleaching events.
2 Methods
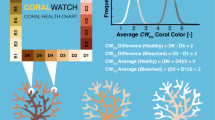
Experimentation, creation, and validation of the Hawaiian Koʻa Card occurred in two phases: Phase I: card development (Phase I) and card validation (Phase II) (Fig. 1).
2.1 Phase I: card development
2.1.1 Collection and acclimation
To assure the widest spatial coverage of ecologically important coral species represented on the Hawaiian Koʻa Card, coral species selection criteria was based on the Hawai‘i Coral Reef Assessment and Monitoring Program’s (CRAMP) determination of the species with the highest total percent cover throughout the Main Hawaiian Islands [25] and combined statewide data from other monitoring efforts [26]. Six of the most prominent coral species (n = colonies; Montipora capitata (n = 10), Montipora flabellata (n = 6), Pocillopora meandrina (n = 11), Porites compressa (n = 10), Porites evermanni (n = 8), Porites lobata (n = 12)) were collected from four locations on the east and north facing shores of Oʻahu, Hawaiʻi (Kāneʻohe Bay, Waimānalo, Punaluʻu, and Kualoa). Individual colonies (n) were fragmented into ten replicate fragments (~ 2–5 cm) and secured to ceramic plugs using cyanoacrylate gel to reduce variations in response due to genetic differences. Fragments were allowed to acclimate to mesocosm conditions in ambient seawater (23–24 °C) for 14 days prior to experimentation. All corals were kept in continuous flow-through 170 L3 mesocosms (n = 3) are under full solar radiation that provided a continual supply of unfiltered seawater pumped directly from Kāneʻohe Bay, Hawaiʻi. Therefore, conditions in the mesocosms are realistic and follow natural cycles on the adjacent reef flat. Flow was maintained at a rate of 4 L min−1 resulting in a full volume turnover rate of ~ 45 min. Environmental conditions during the acclimation period are shown in Table 1.
2.1.2 Experimental setup: laboratory bleaching
Flow-through mesocosm temperatures were gradually increased, using titanium heaters (Finnex 800 W), above winter ambient temperatures of 23–24 °C to a peak midday temperature of 32 °C over a four-week period (0.25–0.5 °C per day). Prior to heating, a fragment from each colony was selected as a baseline color to represent each colony from each of the six species (6 February 2017). Once heating commenced, coral fragments were monitored daily and selected based on visual changes in color by the same observer (K. Bahr) to maintain consistency. Selected fragments were measured for photosynthetic efficiency (quantum yield, Fv/FM) and photographed prior to flash freezing in liquid nitrogen for later processing for symbiont counts and chlorophyll a (chl a) analysis.
2.1.3 Coral health measurements: photosynthetic efficiency, symbiont density, and chlorophyll concentration
Rapid light curves (RLCs) using the saturating pulse method were conducted with a Diving-PAM (Heinz Walz GmbH, Germany) on each coral fragment to measure photosynthetic efficiency. Photosynthetically active radiation (PAR) is defined as the wavelengths of light (400–700 nm) utilized by plants for photosynthesis. During this experiment, PAR values were set at 0, 56, 95, 165, 225, 350, 480, 537, 585 µmol photons m−2 s−1 to allow for photosystem saturation in actinic light (PAR) to occur at the sixth or seventh measurement in all species. Once a maximum electron transport rate (ETR) value was reached, the increasing PAR values resulted in a plateau or decrease in ETR, commonly assumed to be a sign of photoinhibition.
The Diving-PAM was connected to a PC with WinControl-3.25 version software and fitted with a blue light-emitting probe (470 nm, LED, 0.05 µmol photons m−2 s−1, 5 Hz) set to intensity level 8, which is too low to induce fluorescence when used as a measuring light. System parameters of the Diving-PAM were evaluated for each species and set at the following consensus values: measuring light intensity = 8, signal damping = 2, gain = 6, saturating light pulse intensity = 8, saturating light pulse width = 1, actinic light intensity = 4, and actinic light width = 0:30. Rubber surgical tubing was attached to the probe end with a 1 mm protrusion beyond the probe’s measuring surface. This allowed consistent measurements at a fixed distance from the coral surface. The fluorescence offset was regularly reset in seawater with the Auto-Zero function.
Selected coral fragments were removed from the outdoor seawater tables, placed in a 2-gallon aquarium and transported to a darkroom for acclimation (~ 40 min). Corals were aerated with battery-powered aerators and kept in total darkness for 20–30 min prior to measurements. Individual corals were moved from the acclimation tank to a measurement tank for each RLC to prevent photosystems in non-target fragments from reacting to the strong saturating light pulse and the increasing actinic light emitted from the PAM during measurements. RLCs were performed at three unique points at a 90° angle to each coral fragment. The first probe location was selected in the dark with subsequent locations as far from the previous as possible. This was necessary to avoid photosystem activation in areas adjacent to the probe. During the 90s from first saturating pulse to last, the probe was carefully held in position to avoid any changes in probe coverage. The mean value of Fv/FM for the three measurements were used for analysis.
Fragments were subsequently photographed, flash-frozen in liquid nitrogen, and stored at − 20 °C. Coral tissue was removed from the skeletons for symbiont and chlorophyll analyses. Symbiont density was determined for each fragment through replicate cell counts using a gridded hemocytometer. Chlorophyll concentration (a, c, and total) level was determined using a spectrophotometer and equations described in Jeffrey and Humphrey [27]. These measurements were standardized to coral skeletal surface area, which were calculated according to the wax dipping technique described in Stimson and Kinzie [28]. Changes in coral health parameters (i.e., symbiont density, chlorophyll a concentration, photosynthetic efficiency) due to elevated temperatures were analyzed using linear and curvilinear regression models (JMP Pro 13).
2.1.4 Photographic and color analysis
2.1.4.1 Photographing a coral specimen in the laboratory
To develop sets of relative color schemes for coral specimens for the Hawaiian Ko‘a Card, color changes and range of colors were documented as photographic images in a controlled environment. A digital camera (Canon G16) and an external flash mounted on a stationary stand were used to evenly illuminate and photograph a coral specimen while maintaining a fixed distance and angle. Camera parameters, including shutter speed, aperture, ISO, white balance, and flash settings, were manually set and remained consistent across all images. Coral fragments were placed in aquaria filled with clean seawater during photographing. A commercial underwater color reference card (DGK Color Tools WDKK Waterproof Color Chart) was placed parallel to the hind wall of the aquaria adjacent to the fragment for subsequent color balancing during image development. All images were preserved as raw image files in DNG format to retain full resolution captured by the camera sensor to minimize loss of information. Preserved DNG raw image files were white balanced (90% reflectance) and neutral balanced (gray, 18% reflectance) in Adobe Photoshop CS5 using the color chart captured in each image as reference. The histogram function allowed evaluation of the distribution of red, green, and blue (RGB) values, representing white and neutral, to maintain constant values while avoiding overexposure of images. Images were then converted into TIFF format for subsequent processes of color indexing and selection to establish relationships between colors in photographs and coral fragments.
2.1.4.2 Indexing coral colors
The preserved TIFF image files were used for indexing and selecting representative colors for each coral specimen using the Indexed Color function in Adobe Photoshop CS5. Initially, a representative surface area of each coral fragment in an image was isolated as a separate image layer, excluding shadows. The average surface average sampled was 47.5 ± 2.2 cm2 per colony but varied by species. The 10 most frequent colors appearing in a representative area of each fragment, from each image, were indexed and saved as a color table file with act extension, preserving associated numerical color information including RGB, CMYK (Cyan, Magenta, Yellow, Black), and HSB (hue, saturation, and brightness) values.
2.1.5 Swatch and color wheel production
Area of discoloration (i.e., shaded regions and coral tips) within each photograph were removed prior to color selection. Adobe Photoshop was used to select the ten most common colors per fragment to avoid potential biases in color selection. Color values (RGB, HSB, hex code, and CYMK) were recorded for each color (10 colors for each fragment) for all fragments (total of 5340 colors). All color sets were uploaded into Adobe Creative Cloud Photoshop CC version 19.1.8 and organized by coral species. Color palettes were sorted by species and grouped by sampling date and length of exposure to high temperature. The ten most common colors for each of these fragments were viewed and sorted from darkest to lightest hues (1–9) both visually and using RGB values. From these representative selections, colors were designated to one of four quadrats based on color group. CMYK and hex code values for each color were recorded and provided to the printer along with the finalized design. The Hawaiian Ko‘a Card was designed using Adobe Illustrator CC version 19.0. Colors displayed on the card were selected based on the highest frequency of occurrence within color pallets and representation for the common coral species found within Hawai‘i. A prototype version of the card was produced on waterproof high impact polystyrene sheet plastic in a limited quantity for field and laboratory validation. The final version of the card is printed on ecoplast biodegradable high impact polystyrene plastic (Fig. 2).
2.1.6 Data synthesis and analysis
To determine color numbers for each fragment, the Hawaiian Koʻa Card was photographed in the same lighting as the coral fragments from the Phase 1 simulated bleaching event (n = 534 fragments). The image of the Hawaiian Koʻa Card was placed alongside the photograph of each fragment on the same computer screen to identify the color of each coral fragment post hoc as it related to the colors on the card. The coral color was matched to a number on the Hawaiian Ko‘a Card by four trained observers.
Mean (n = 10 colors per fragment) color values for hue (reflected color), saturation (proportion of grey in the hue), and brightness (relative lightness and darkness) were quantified for each coral fragment. Additionally, mean color values of RGB and CMYK were also calculated for each fragment. Changes in mean color values (HSB, RGB) during the simulated bleaching event were analyzed using linear regressions. Mean color values (HSB, RGB) for each fragment were pooled by Hawaiian Koʻa Card number and compared to the color values displayed on the Hawaiian Koʻa Card. Agreement between the expected color values (from the Hawaiian Koʻa Card) were compared with the mean and SD of the color values obtained from the photographs via Adobe Photoshop.
2.2 PHASE II: card validation
2.2.1 Lab validation: coral health measurements
The laboratory validation was conducted in the same experimental tanks and under similar environmental conditions with the identical species (n = 6) but with fewer coral colonies (n = 3) and fewer fragments (n = 6) than the initial experiment. Only four of the 108 fragments were not used in the health analyses due to mortality. No disease was observed during experimentation. Temperature exposure was similar between experiments. Heating experiments were conducted between 23 October to 14 Nov 2018. As in the previous experiment, temperatures were elevated using titanium heaters (Finnex 800 W) at an increase of 0.25–0.5 °C per day. Corals were assessed daily using the Hawaiian Ko‘a Card and removed for health measurements (i.e., PAM, symbiont density, and chlorophyll density), replicating the initial parameter set used in the initial card development.
2.2.2 Field validation
Field validation of the card colors was conducted on shallow reefs on the island of O‘ahu in December 2018 (Fig. 3). To assure the selection of colors were representative of the majority of common Hawaiian coral species and their color and health condition, sites facing all cardinal and intercardinal directions were selected. Site selection criteria were based on the presence of symbiotic corals and site accessibility. Colors of corals were assessed using the underwater field Hawaiian Ko‘a Card prototype. Eleven sites were surveyed on reefs facing northward (site 1–3), eastward (4–6), southward (7–10), and westward (11 and 12) (Fig. 3). The color assessment was conducted by a pair of snorkelers at each site in depths ranging from 0.75 to 4.5 meters. Each snorkeler identified species and the most common color of a colony by placing the card adjacent to the coral and recording the corresponding number on the card most similar to the coral color. This color assessment was repeated with multiple individual colonies for the 1h survey duration at each site. Additional data, including observer, date, time and percent cloud cover were recorded. Field validation data were used to re-evaluate the prototype selection of representative colors of corals on shallow reefs.
A map of field validation (red) and collection sites (blue) on O‘ahu, Hawai‘i, including: 1. Mokulēʻia, 2. Hale‘iwa, 3. Kuilima, 4. Moku o Lo‘e (Hawai‘i Institute of Marine Biology), 5 Lanikai Beach, 6. Kaiona Beach, 7. Hanauma Bay, 8. Cromwells, 9. Tonggs, 10. Ala Moana Regional Park, 11. Kahe Point, 12. Mākaha 13. Kāne‘ohe Bay 14. Waimānalo 15. Kualoa 16. Hau‘ula. Collections occurred in January 2017 and validation surveys occurred in September 2018.
2.2.3 Observer variation
Field surveys using a range of groups determined the extent of observer bias and error. A group of 20 marine biologists and students used the Hawaiian Koʻa Card to assess the color of nine coral fragments collected from the reef flats of Oʻahu, Hawaiʻi. The assessments were conducted under natural sunlight with corals presented in a black raceway tank (1.5 m × 0.75 m × 0.15 m). The Hawaiian Koʻa Card was submerged underwater next to the coral of interest, while the observer remained above water. The depth of water in which the corals resided in was ~ 0.10 m, minimizing the potential of light and color distoration. While assessments were in progress, internal water flow and aeration to the raceway ceased. Each observer independently determined the majority color of each coral fragment, avoiding tips and cut margins. Observer variation was based on the comparison of the selected color value by each observer to the color value that was selected most frequently for the coral fragment assessed. This will hereafter be referred to as ‘observer precision’. To determine if observer precision is influenced by any extraneous factors, the following was recorded by each observer: age, gender, coral familiarity (never snorkel, snorkel, know coral common names, know coral scientific names), highest level of education (high school, bachelors, advanced degree), and ease of use (easy, medium, difficult). Statistical analyses were performed in R (version i386 3.5.1) using a non-parametric Spearman’s rank correlation.
3 Results
3.1 Phase I: Hawaiian Ko‘a card development
Corals were acclimated to ambient water temperatures (mean ± SE) for 2 weeks (23.6 °C ± 0.04) prior to the temperature increase stage. Water temperatures were heated at 0.25–0.5 °C per day during the temperature increase stage for 20 days, eventually reaching 29.3 ± 0.13 °C. During the Phase I card development, 36 of the 570 fragments in the experiment suffered either partial or full mortality due to prolonged heat stress and were not used in the health analyses. No disease was noted in fragments. Other environmental parameters varied diurnally over the simulated bleaching experiment but remained under optimal conditions for Hawaiian corals (Table 1).
Results of the laboratory experiment show a significant decline in chlorophyll a concentration (linear regression; R2 = 0.398; p = 0.0009), symbiont density (linear regression; R2 = 0.310; p = 0.0049) and photosynthetic efficiency measured as quantum yield (Fv/FM) (polynomial regression; R2 = 0.827; p < 0.0001) with increasing exposure to high temperatures (29.3 ± 0.13 °C) (Fig. 4). Chl a per symbiont remained variable across the exposure period but showed no significant correlation with heat stress (linear regression; R2 = 0.0027; p = 0.2149) (Fig. 4A).
Coral health parameters of chlorophyll a concentration (A), symbiont density (B), chlorophyll a per symbiont (C) and quantum yield (D) with exposure to elevated temperature (days). Data is pooled across species collected during Phase I experiment. Data in A–C are fitted with a linear regression and D is fitted with an exponential curve and error bars are SE of mean
Photographic analysis of mean hue and saturation values of corals did not significantly change during elevated temperature exposure (linear regression: hue R2 = 0.035; p = 0.385; saturation R2 = 0.003; p = 0.7998); however, brightness significantly increased with exposure to high temperatures (linear regression: hue R2 = 0.620; p < 0.0001) (Fig. 5A). Similarly, mean values of RGB for corals significantly increased with prolonged exposure to warming (linear regression: red R2 = 0.586; p < 0.0001; green R2 = 0.628; p < 0.0001; blue R2 = 0.201; p = 0.0278) (Fig. 5B).
HSB (hue, grey square; saturation, black triangle; brightness, white circle) (A) and RGB (red, red circle; green, green circle; blue, blue circle) (B) color scales during simulated warm water event (Phase I: exposure in days). Color scales (HSB and RGB) are pooled across species. Error bars represent SE of the mean
Correlation analysis between mean coral health parameters and mean color scores by exposure time revealed significant negative correlations between chlorophyll a and the RGB color values including red (r = − 0.56), green (r = − 0.60), and the green chromatic coordinate (r = − 0.56) (Table 2). Additionally, strong negative correlations were observed between symbiont density and the green chromatic coordinate (r = − 0.72). Photosynthetic efficiency (quantum yield, Fv/FM) showed strong negative correlations among all the RGB color scores (red, r = − 0.46; green, r = − 0.73; blue, r = − 0.57; green chromatic coordinate, r = − 0.54) and brightness (r = − 0.68) (Table 2).
Moderate levels of agreement were observed between the color values displayed on the Hawaiian Koʻa Card and those quantified from the photographs of individual corals. The highest levels of agreement were in hue (55%), saturation (52%), and brightness (34%) values (Fig. 6A–D). Concurrence between card and coral image values were lower for RGB (35% for all) values (Fig. 6e–h). Five Hawaiian Koʻa Card numbers (i.e., 9, 18, 27, 24, and 34) corresponding to the darkest colors were not observed in tested corals during the Phase I experimentation.
Color scale of hue, saturation, and brightness (HSB) (A–D) and red, blue, and green (RGB) values (E–H) for the Hawaiian Koʻa Card numbers (0–35). Color scale values of the Hawaiian Koʻa Card are indicated by bold bars. Mean color scale values from the images of the corals are indicated by markers for HSB scale (hue, grey square; saturation black triangle; brightness, white circle) and RGB channels (red, red circle; blue, blue circle; green, green circle). Mean color scale image values are pooled across species within each Hawaiian Koʻa Card Number and error bars represent SD of the mean
3.2 Phase II: card validation
3.2.1 Lab validation: coral health measurements to mean Hawaiian Koʻa Card values
Color validation experiments were conducted under similar experimental conditions as the card development phase at elevated temperatures (30.7 ± 0.02 °C) for 22 days. Sixteen colors on the Hawaiian Koʻa Card were not observed in corals during Phase II lab experimentation. Of the other 18 colors observed, high level of agreement (66%) was observed in photosynthetic efficiency (Fv/FM) readings between the Phase I and Phase II mean data when pooled by color number (Fig. 7). Mean values of chlorophyll a per symbiont between Phase I and Phase II data slightly agreed (22%) (Fig. 7). Arrangement and grouping of color numbers by redness and brightness showed an increase in photosynthetic efficiency (quantum yield; Fv/FM) in darker colors (Fig. 8).
Coral health parameters of quantum yield (A–D) and chlorophyll a per symbiont (E–H) for Phase I (white circle) and Phase II (black circle) data by Hawaiian Koʻa Card number separated by quadrant of position on the card. Mean coral health response is pooled across species and error bars represent SE of the mean
Coral health parameters of quantum yield (A, B) and chlorophyll a per symbiont (E, D) by Hawaiian Koʻa Card number (left: A, E) and by color number sorted by order (right: B, D) for Phase I (white circle) and Phase II (black circle) data. Mean coral health response is pooled across species and error bars represent SE of the mean
3.2.2 Field validation
A total of 1491 coral colonies were observed across 17 species by 11 observers. Most sampled colonies were observed in locations facing eastward (44%) followed by south (25%), north (22%) and west (8%) facing shores (Fig. 3). These colonies represented 30 of the 35 colors associated with color numbers on the Hawaiian Koʻa Card. The remaining colors were not represented due to lack of high seawater temperatures during the validation period (September 2018). Moreover, bleaching, indicated by #0, was uncommon at field validation sites (0.3%). The most frequently observed colors included #24 (175 colonies, 12% of total observed colonies), #16 (157 colonies, 11%), #14 (135 colonies, 9%), #20 (132 colonies, 9%), #13 (105 colonies, 7%), #13 (103 colonies, 7%), and #7 (101 colonies, 7%) across all locations.
3.2.3 Observer variation
No significant correlation was found between observer precision and observer age, gender, coral familiarity, highest level of education, or perceived ease of use. Despite non-significant results, several observations are worth noting. First, 80% of males that participated in the Hawaiian Koʻa Card trials scored at or below 50% precision. Second, the top 15% of users with the highest precision scores were individuals whose coral familiarity was classified as knowing species names.
Each coral fragment was identified to color by 20 observers. On average, each coral fragment possessed 5 ± 2 observed colors from the Hawaiian Ko‘a Card associated with it. On average, 58.9 ± 15.3% of observers identified with the most frequently observed coral color. There was no clear pattern showing differences in the number of observed colors between coral species.
4 Discussion
Aside from the small contribution of host pigments in some species of corals, coral color is primarily dependent on the abundance of their symbionts (Symbiodinium spp.) and associated chlorophyll levels. Changes in the abundance of symbionts within corals frequently occurs to meet changing environmental conditions [29, 30]; however, extreme changes can lead to the breakdown of the symbiosis between the algal symbionts and the coral and therefore cause color loss. Large scale coral bleaching events are linked to ongoing increases in seawater temperature and can be exacerbated or ameliorated by other environmental drivers (e.g., nutrification, sedimentation, irradiance). With predicted increases in frequency, intensity, and duration of coral bleaching events, it is imperative to develop strategies and tools to mitigate impacts, identify resilience, and direct response efforts. Ongoing monitoring efforts of coral reefs require extensive training, expertise, are not representative of the larger ecosystem, and are frequently costly and labor intensive. To better document and monitor coral response to and recovery from bleaching events, there is a need for reproducible and rapid inexpensive and nondestructive ways to quantify coral health. Additionally, technologies must cover a large area, be rapid and simple, and be widely available at little to no cost. The Hawaiian Ko‘a Card meets these criteria.
The Hawaiian Archipelago is among the most isolated island groups in the world [31]. Due to this extreme isolation and distance from the coral center of diversity, Hawai‘i is coral species depauperate as compared to most other regions. Therefore, the Hawaiian Koʻa Card can be used to assess at least 94% of the total coral cover across the main Hawaiian Islands. Based on current coral species coverage for the Hawaiian Islands [26], the 35 colors on the Hawaiian Koʻa card derived from the six most common Hawaiian species represent a range of color across 17 species that characterize the majority of dominant corals. Here we show that the Hawaiian Koʻa card is an effective, simple, and inexpensive tool that requires little training to monitor coral health. Therefore, this tool can be integrated into local Hawaiian community monitoring groups to increase understanding of coral bleaching and provide a sense of stewardship.
Coral color has been previously documented as a proxy for coral health [5, 32, 33]. Results of our simulated bleaching experiment determined significant decline in coral health parameters, chlorophyll a concentration, symbiont density, and photosynthetic efficiency (Fv/FM), with prolonged exposure to high temperatures. In conjunction with the observed decreases in coral health parameters, we found significant increases in red and green color values obtained from photographic analysis of coral color. Previous work has shown chlorophyll density to be highly correlated with the red channel intensity [34].
Hawaiian Koʻa Card validation trials show high levels of agreement between measured (Phase II) and expected (Phase I) results for photosynthetic efficiency measurements. Less agreement was observed in chlorophyll a per symbiont measurements, likely due to differences in coping strategies among coral species during heat stress. As our results established measurements of fluorescence yields in corals have been known to be a reliable indicator of holobiont stress. Decline in yields were detected prior to occurrence of visible signs (e.g., paling) [24]. Field validation data confirmed the selection of representative colors of Hawaiian shallow reef corals. The common colors selected represent the majority of the coral community encompassing over 90% of corals.
Issues encountered during card development included high color variation within species and non-uniform coral colors that led to color selection uncertainty. Even among healthy corals, species color variability occurs. This is contingent upon location, environmental conditions, and genetic variability. Confounding variables can affect the relationship between color and health condition factors. These include inter- and intraspecific species differences and host accessory pigments. Fluorescent- and chromo-proteins are pigments within the coral. As symbiont loss progresses these proteins become more visible. Bright purples, and pinks that are not associated with symbiont loss, but signify a natural progression of bleaching in some species, are represented in the Hawaiian Ko‘a Card at the lighter end of each quadrant. Blue pigments are not represented in the final selection due to a lack of high representation in experimental corals. Differences in time of day, cloud cover, or other environmental factors that may affect color selection during field validations were not tested in this study.
An additional limitation regarding the application of the Hawaiian Ko‘a Card is a lack of data confirming comparable efficiency of utilization at deeper depths. Field validations for this project were conducted in relatively shallow areas (0.75–4.50 m). Scientific studies using the Australian Coral Watch Coral Health Chart on in situ coral colonies were conducted at a depth range of 1–6 m [21, 22]. However, it is reported that hundreds of recreational divers include CoralWatch monitoring as part of their dive training and regular dive trips, with recreational SCUBA-diving groups listed as one of the program’s most frequent contributors [18, 20]. Water absorbs distinct wavelengths of light at different levels, and the varying degrees of attenuation for different wavelengths affect the visibility of colors underwater [35,36,37,38]. Loss of colors occur underwater at depth in the order they appear in the color spectrum, with red disappearing first, having the lowest energy and the longest wavelength. While there is currently a lack of research validating the use of these coral health charts at deeper depths, additional outreach with community dive groups that has been initiated, show the accompanying use of an underwater strobe or light give sufficient results. We suggest that the card be used with an accompanying underwater strobe or light at a depth of > 5 m.
The Hawaiian Ko‘a Card is an excellent tool for community engagement, educational training, research applications, and development of management and conservation responses and strategies. The resultant outcomes and benefits are extensive. It offers a significant progression from the current subjective observations of the state of bleaching and creates potential for rapid, large-scale assessment of changing coral condition. Management capabilities can be improved through the statewide evaluation of coral health to establish baselines and subsequent declines, define focus areas to concentrate management efforts, identify regions of resilience, and evaluate the efficacy of management strategies. Detecting refuges resistant to bleaching can assist research efforts in determining associated environmental or conservation factors. Incorporation of this methodology in experimental research can facilitate in coral condition comparisons between treatments. Education and outreach in the use of this card will instruct students and the public about the effects of climate change on coral reefs, providing information on problems and offering a viable solution.
Hawai‘i has a long history of effective traditional resource management [39] and a more recent history of marine resource declines. There are major differences between traditional and contemporary management, but there are also similar elements and ways of incorporating past management practices that are vastly applicable today [40]. Under traditional management approaches, resources users embraced responsibility and stewardship of their marine resources. Present management approaches and practices lack this concept. This creates a structure of exploitation with individual advantage at the expense of the resources. Although contemporary management is largely top-down, influence by the communities have been growing through local active participation and community-based resource management. The Community-Based Subsistence Fishing Area at Hā‘ena, Kaua‘i is a prime example of the ability of a community to create social and environmental change. This demonstrates the State of Hawai‘i’s openness to restoring some aspects of responsibility, accountability, and enforcement to the community. This also highlights the State’s adaptive management ability in the light of social, political, economic, or biological changes. With increasing threats of climate change this capability will be crucial. Incorporating past approaches that have been proven successful must be reexamined and incorporated where applicable. Coupled with historic methods, effective traditional management can be restored in appropriate regions by providing the current tools necessary to succeed. In the past, observation by resource users was the most effective way of determining reef condition. Today, we have lost this ability to detect slight changes in the environment. The Hawaiian Ko‘a Card restores this skill and trains ocean users to detect these differences. The ability to visually experience a transformation in coral color gives individuals and community groups a vested interest in working toward solutions. With appropriate tools and strategies, habitat protection through community driven management can improve. Coral condition can also serve as a proxy for fish condition since they are so closely interrelated, tying this into an ecosystem-based approach [41,42,43].
Predictive climate change models assist in complex problems and understanding large interrelated systems. These representations can test theories, find solutions, predict scenarios, and explore trends. Increasing our spatial and temporal understanding of bleaching can improve the reliability of these forecasts and validate climate change models. Strong connections of watersheds to reefs exist and are threatened by bleaching impacts which can uncouple the link between them since reefs can be affected independently of the adjacent watershed [44, 45]. Addition of bleaching data into ridge to reef databases will increase our understanding of present and future connectivity and recognition of shifts as climate impacts progress. The products of this research will be used to update supporting materials for resource managers and educators in reef resilience, bleaching occurrence, and socioeconomic impacts of climate change.
To strengthen the project outcome, feedback was received from all the major marine stakeholders and those responsible for marine resources in the State of Hawai‘i, non-governmental agencies, researchers, educators, and community groups, over the three-year project period. These agencies and groups have incorporated the Hawaiian Ko‘a Health Assessment Card into monitoring programs, experimental designs, and educational curriculum.
References
Aeby GS, Kenyon JC, Maragos JE, Potts DC (2003) First record of mass coral bleaching in the Northwestern Hawaiian Islands. Coral Reefs. https://doi.org/10.1007/s00338-003-0309-2
Chung AE, Wedding LM, Meadows A et al (2019) Prioritizing reef resilience through spatial planning following a mass coral bleaching event. Coral Reefs 38:837–850. https://doi.org/10.1007/s00338-019-01812-w
Hughes TP, Anderson KD, Connolly SR et al (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 80-(359):80–83. https://doi.org/10.1126/science.aan8048
Jokiel PL, Brown EK (2004) Global warming, regional trends and inshore environmental conditions influence coral bleaching in Hawaii. Glob Change Biol 10:1627–1641. https://doi.org/10.1111/j.1365-2486.2004.00836.x
Siebeck UE, Marshall NJ, Klüter A, Hoegh-Guldberg O (2006) Monitoring coral bleaching using a colour reference card. Coral Reefs 25:453–460. https://doi.org/10.1007/s00338-006-0123-8
Brown BE (1997) Coral bleaching: causes and consequences. Coral Reefs 16:129–138. https://doi.org/10.1007/s003380050249
Glynn PW (1984) Widespread coral mortality and the 1982–83 El Niño warming event. Environ Conserv 11:133–146. https://doi.org/10.1017/S0376892900013825
Bahr KD, Jokiel PL, Rodgers KS (2015) The 2014 coral bleaching and freshwater flood events in Kane’ohe Bay, Hawai’i. PeerJ. https://doi.org/10.7717/peerj.1136
Hoeke RK, Jokiel PL, Buddemeier RW, Brainard RE (2011) Projected changes to growth and mortality of Hawaiian corals over the next 100 years. PLoS ONE. https://doi.org/10.1371/journal.pone.0018038
Buddemeier RW, Fautin DG (1993) Coral bleaching as an adaptive mechanism. Bioscience 43:320–326. https://doi.org/10.2307/1312064
Douglas AE (2003) Coral bleaching—how and why? Mar Pollut Bull 46(4):385–392
Jokiel P, York R Jr (1982) Solar ultraviolet photobiology of the reef coral Pocillopora damicornis and symbiotic zooxanthellae. Bull Mar Sci 32:301–315
Lesser MP, Farrell JH (2004) Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 23:367–377. https://doi.org/10.1007/s00338-004-0392-z
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–866. https://doi.org/10.1071/MF99078
Hoeke R, Brainard R, Moffitt R, Merrifield M (2006) The role of oceanographic conditions and reef morphology in the 2002 coral bleaching event in the Northwestern Hawaiian Islands. Atoll Res Bull 543:489–503
Kramer KL, Cotton SP, Lamson MR, Walsh WJ (2016) Bleaching and catastrophic mortality of reef-building corals along west Hawai‘i island: findings and future directions. In: Proceedings o 13th international coral reef symposium
Couch CS, Burns JHR, Liu G et al (2017) Mass coral bleaching due to unprecedented marine heatwave in Papahānaumokuākea Marine National Monument (Northwestern Hawaiian Islands). PLoS ONE. https://doi.org/10.1371/journal.pone.0185121
Siebeck UE, Logan D, Marshall NJ (2008) CoralWatch—a flexible coral bleaching monitoring tool for you and your group. In: Proceedings of 11th international coral reef symposium
Oladi M, Shokri MR, Rajabi-Maham H (2017) Application of the coral health chart to determine bleaching status of Acropora downingi in a subtropical coral reef. Ocean Sci J. https://doi.org/10.1007/s12601-017-0025-4
Marshall NJ, Kleine DA, Dean AJ (2012) CoralWatch: education, monitoring, and sustainability through citizen science. Front Ecol Environ 10:332–334
Montano S, Seveso D, Galli P, Obura DO (2010) Assessing coral bleaching and recovery with a colour reference card in Watamu Marine Park, Kenya. Hydrobiologia. https://doi.org/10.1007/s10750-010-0407-4
Coelho VR, Fenner D, Caruso C et al (2017) Shading as a mitigation tool for coral bleaching in three common Indo-Pacific species. J Exp Mar Biol Ecol. https://doi.org/10.1016/j.jembe.2017.09.016
Knipp AL, Pettijohn JC, Jadot C, Hertler H (2020) Contrasting color loss and restoration in survivors of the 2014–2017 coral bleaching event in the Turks and Caicos Islands. SN Appl Sci. https://doi.org/10.1007/s42452-020-2132-6
Lesser MP, Gorbunov MY (2001) Diurnal and bathymetric changes in chlorophyll fluorescence yields of reef corals measured in situ with a fast repetition rate fluorometer. Mar Ecol Prog Ser. https://doi.org/10.3354/meps212069
Rodgers KS (2005) Evaluation of nearshore condition and identification of indicators in the main Hawaiian islands. PhD Dissertation, University of Hawaiʻi, Dept. of Geography. Honolulu
Rodgers KS, Jokiel PL, Brown EK et al (2015) Over a decade of change in spatial and temporal dynamics of Hawaiian coral reef communities. Pac Sci 69:1–13. https://doi.org/10.2984/69.1.1
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194. https://doi.org/10.1016/s0015-3796(17)30778-3
Stimson J, Kinzie RA III (1991) Temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus). J Exp Mar Biol Ecol. https://doi.org/10.1016/S0022-0981(05)80006-1
Fitt WK, Cook CB (2001) Photoacclimation and the effect of the symbiotic environment on the photosynthetic response of symbiotic dinoflagellates in the tropical marine hydroid Myrionema amboinense. J Exp Mar Biol Ecol. https://doi.org/10.1016/S0022-0981(00)00302-6
Muller-Parker G (1987) Seasonal variation in light-shade adaptation of natural populations of the symbiotic sea anemone Aiptasia pulchella (Carlgren, 1943) in Hawaii. J Exp Mar Biol Ecol. https://doi.org/10.1016/0022-0981(87)90115-8
Hourigan TF, Reese ES (1987) Mid-ocean isolation and the evolution of Hawaiian reef fishes. Trends Ecol Evol 2:187–191
Edmunds PJ, Gates RD (2003) Has coral bleaching delayed our understanding of fundamental aspects of coral–dinoflagellate symbioses? Bioscience. https://doi.org/10.1641/0006-3568(2003)053%5b0976:hcbdou%5d2.0.co;2
Koren S, Dubinsky Z, Chomsky O (2008) A photographic method for analyzing areal chlorophyll and bleaching status of the coral Stylophora pistillata. In: 11th international coral reef symposium
Winters G, Holzman R, Blekhman A et al (2009) Photographic assessment of coral chlorophyll contents: implications for ecophysiological studies and coral monitoring. J Exp Mar Biol Ecol. https://doi.org/10.1016/j.jembe.2009.09.004
Kumari N, Kumar Sunkaria R (2013) Haze removal and color compensation of underwater image. Int J Comput Appl. https://doi.org/10.5120/11583-6915
Chiang JY, Chen YC (2012) Underwater image enhancement by wavelength compensation and dehazing. IEEE Trans Image Process. https://doi.org/10.1109/TIP.2011.2179666
Trucco E, Olmos-Antillon AT (2006) Self-tuning underwater image restoration. IEEE J Ocean Eng. https://doi.org/10.1109/JOE.2004.836395
Jaffe JS (1990) Computer modeling and the design of optimal underwater imaging systems. IEEE J Ocean Eng. https://doi.org/10.1109/48.50695
Winter KB, Ticktin T, Quazi SA (2020) Biocultural restoration in Hawaiʻi also achieves core conservation goals. Ecol Soc. https://doi.org/10.5751/ES-11388-250126
Jokiel PL, Rodgers KS, Walsh WJ et al (2011) Marine resource management in the Hawaiian Archipelago: the traditional Hawaiian system in relation to the western approach. J Mar Biol. https://doi.org/10.1155/2011/151682
DeMartini EE, Friedlander AM (2004) Spatial patterns of endemism in shallow-water reef fish populations of the Northwestern Hawaiian Islands. Mar Ecol Prog Ser 271:281–296. https://doi.org/10.3354/meps271281
Friedlander AM, Brown EK, Jokiel PL et al (2003) Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs 22:291–305. https://doi.org/10.1007/s00338-003-0317-2
Friedlander AM, Parrish JD (1998) Habitat characteristics affecting fish assemblages on a Hawaiian coral reef. J Exp Mar Biol Ecol 224:1–30. https://doi.org/10.1016/S0022-0981(97)00164-0
Rodgers KS, Jokiel PL, Bird CE, Brown EK (2010) Quantifying the condition of Hawaiian coral reefs. Aquat Conserv Mar Freshw Ecosyst. https://doi.org/10.1002/aqc.1048
Rodgers KS, Kido MH, Jokiel PL et al (2012) Use of integrated landscape indicators to evaluate the health of linked watersheds and coral reef environments in the Hawaiian Islands. Environ Manag. https://doi.org/10.1007/s00267-012-9867-9
Acknowledgements
We would like to thank volunteers, interns, and members of the Coral Reef Ecology Lab for assistance in Phase I, including P. Mino, S. May, F. Fleumeria, J. Joseph, N. Garaganza-Tengan, and K. Cockerille. Phase II validation groups include Windward Community College (M. Onuma) and the HIMB Community Education Program and members of the community providing feedback on ease of use, flexibility, and design. We would like to thank Mike McGowan and the Condit team for their generous support in printing the Hawaiian Koʻa Card. We appreciate the support for this project by the Division of Aquatic Resources, The National Oceanic and Atmospheric Administration, The Nature Conservancy, and other management agencies. The Hawaiian Koʻa Card is dedicated to continuing the research legacy of the founder of the Coral Reef Ecology Lab, Dr. Paul Jokiel.
Funding
Partial funding for this research was provided by the Harold K L Castle Foundation and the Hawai‘i Community Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bahr, K.D., Severino, S.J.L., Tsang, A.O. et al. The Hawaiian Koʻa Card: coral health and bleaching assessment color reference card for Hawaiian corals. SN Appl. Sci. 2, 1706 (2020). https://doi.org/10.1007/s42452-020-03487-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03487-3