Abstract
Transparency of VO2 thin films in the visible region is an important aspect of study for its use in smart window applications. In this regard, we experimentally validate the theoretical prediction regarding the influence of scandium (Sc) doping on modulating the optical spectrum of VO2. Sc-doped nano-crystalline VO2, bulk and thin films were synthesised using a two-step process involving a rapid non-equilibrium solution combustion process and ultrasonic nebulised spray pyrolysis of aqueous combustion mixture respectively; followed by a reduction step. The presence of Sc was determined by X-ray photoelectron spectroscopy where a peak observed at 530.06 eV is attributed to Sc–O interaction. Raman spectra showed a shift in 611 cm−1 peak position to a lower wavenumber due to tensile strain arising from Sc doping. Sc-doped VO2 thin films showed semiconductor to metal transition of four orders of magnitude change in resistance. Sc-doping increased the band gap from 1.76 eV for undoped to 2.02 eV with 2.0 at. % of doping thereby making the films more transparent in the visible region of the optical spectrum. This shift in the band gap of VO2 was consistent with the theoretical reports. Thus, Sc is a potential dopant for modulating the optical spectrum of VO2 for its application in smart windows.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vanadium dioxide (VO2) is a transition metal oxide which finds use in various applications like thermochromic devices [1], micro-bolometer [2], IR-photodetector [3], metamaterial [4, 5], smart windows [6, 7], thermal switching [8], batteries [9, 10], memristic devices [11], THz modulation devices [12] etc. It is been extensively studied due to its ability to undergo a first order phase transition accompanied by semiconductor-to-metal transition (SMT) at 68 °C (T\(_{SMT}\)). During the phase transition, a change in crystal structure from monoclinic (M1) phase (P2\(_1\)/c) to tetragonal (R) phase (P4\(_2\)/mnm) is observed which leads to change in the V–V bond length [13]. During the SMT transition, VO2 shows a change in electrical resistivity, high resistance (T < T\(_{SMT}\)) to low resistance (T > T\(_{SMT}\)) as well as in the optical response with a slight hysteresis which is attributed to the stress involved due to structural change and the change in latent heat [14]. VO2 acts as IR transparent at T < T\(_{SMT}\) and acts as IR reflector at T > T\(_{SMT}\) [15]. Due to this property, it is been for used as a coating material on windows called as smart windows [6, 16]. Various methods like sol–gel [17], pulsed laser deposition [18,19,20], chemical vapour deposition [21], sputtering [22, 23] etc. have been employed for fabrication of doped as well as undoped VO2 thin films.
There are few limitations to use VO2 for smart window applications: (a) High T\(_{SMT}\), which has been overcome by adding dopants in vanadium site [17, 24,25,26,27,28,29,30] and the minimum value for T\(_{SMT}\) has been observed to be ~ 25 °C, when W is doped into the system. Many reasons have been reported where the choice of dopant depends on its effect on the crystal structure. This influence of dopant on the crystal structure decides the reduction of transition temperature(T\(_{SMT}\)). It is reported in the literature that, when the structure of doped VO2 is close to that of tetragonal phase, a reduction in T\(_{SMT}\) will be observed. This is attributed to reduction in \(\beta\)-angle [31]. (b) The reduced transmission in visible range after coating VO2 on the glass resists its use as a smart window material [27, 32]. The dopants added either reduce the T\(_{SMT}\) or manipulate the optical spectrum. When W, Mo is doped into VO2, T\(_{SMT}\) decreases (~ 25 °C) but the optical band gap is very low thereby making the film to be dark in colour. When dopants like Mg, F [27, 33, 34] etc., are doped the band gap increases making the film transmit more light, but these dopants do not induce any change in T\(_{SMT}\). In order to come up with a better solution, co-doped system have been introduced [28] which enhances the visible transmittance but not up to the extent that it can be commercially used. To use VO2 as an efficient smart window material, both the problems need to be overcome at the same time.
Sun et al. [35], depending upon the results obtained from DFT calculations suggests that of all the different transition metal that can be used for doping, scandium (Sc) is the best dopant. Doping Sc into VO2 reduces the \(\beta\)-angle so that the structure is closer to tetragonal phase of VO2 thereby reducing the \(T_{SMT}\) as well as it enhances the optical spectrum as the interaction between Sc\(^{3+}\) and O\(^{2-}\) is weaker than V\(^{4+}\) and O\(^{2-}\), thereby will induce a blue shift in the absorption spectrum [35].
Herein, we validate these theoretical prediction experimentally. We have synthesised bulk of scandium doped V2O5 using rapid solution combustion synthesis (SCS) followed by a reduction step to get scandium doped VO2. Thin films were deposited using simple, cost-effective method i.e., Ultrasonic Nebulised Spray Pyrolysis of Aqueous Combustion Mixture (UNSPACM), first to get phase pure Sc-doped V2O5 thin films on quartz substrate, followed by reduction step to get VO2. Bulk and thin film samples were further characterized to determine the effect of Sc doping on T\(_{SMT}\) and optical spectrum.
2 Experimental section
2.1 Synthesis of bulk materials
For synthesis of bulk material, solution combustion synthesis method was employed. Aqueous combustion mixture (ACM) was prepared using ammonium metavanadate (NH4VO3), scandium nitrate (Sc(NO3)3) as metal source (Oxidizer, O) and urea (CH4N2O) as the fuel (F). Stoichiometric amounts of the precursors were dissolved in dilute nitric acid and water calculated using Eq. 1 so that the oxidiser to fuel ratio is equal to 1 [36],
where x is the amount of scandium doped into the system as mentioned in Table 1. The schematics is shown in Fig. 1.
ACM was kept in a furnace pre-heated at 500 °C. The yellow bulk Sc-doped V2O5 obtained from self-propagating combustion reaction was ground and subjected to reduction treatment in N2 atmosphere saturated with hydrocarbon at 550 °C for 30 min [37]. In order to compare the optical results with W- and Mo-doped VO2, the bulk samples for 2.0 at. % W doped and 1.8 at. % Mo doped were also synthesised. 2.0 at. % W doping and 1.8 at. % Mo doping was considered as these are the highest dopant levels for W- and Mo-doping respectively, that leads to reduction in T\(_{SMT}\) [24, 38].
2.2 Synthesis of thin films
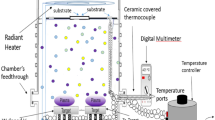
Scandium doped vanadium dioxide thin films was synthesised via. UNSPACM using a home built setup [39], schematics for which is shown in Fig. 1. Sc-doped V2O5 was synthesized on a quartz substrate. The ultrasonically nebulised mist, containing the redox mixture, was carried to the hot substrate by N2 gas flowing at 1000 sccm. The micrometer sized droplets instantaneously pyrolysed on encountering with the quartz substrate. The substrate maintained at 600 °C. The films were synthesised at high temperature to get a crystalline phase of V2O5. The films were deposited for 10 min. The films thus obtained were reduced by treating the product in N\(_2\) atmosphere saturated with hydrocarbon [40] at 550 °C for 2.5 h. The sample codes are listed in Table 1.
2.3 Characterization
Phase confirmation was done on both bulk and thin film samples by XRD using PANALYTICAL with Cu-K\(\alpha\) as the X-ray radiation (\(\lambda =\)1.5418 \({\AA}\)). Raman measurements were done to confirm the phase formation using Horiba JobinYvon HR-Raman-123microPL spectrometer with a green laser having a wavelength of 523 nm. The surface morphology was determined using a F50 Inspect Field Emission SEM and Carl-Ziess scanning electron microscope, accelerating voltage of 25 kV. Transmission electron microscope, JEOL 2100F (TEM) was used at an accelerating voltage of 200 kV to determine the morphology of bulk samples. To determine SMT for bulk samples, DSC measurements were carried out in METTLER-TOLEDO DSC1 system. For thin films, electrical measurements were carried out on a DC probe station equipped with an ATT thermal controller coupled with a B1500A semiconductor device analyser. UV Visible measurements were done using Perkin-Elmer spectrophotometer (Lambda 750) for determination of band gap. FTIR measurements were done as a function of temperature for the thin films using Agilent Carry 600 Fourier Transform infrared spectrometer in reflectance mode. XPS measurements were done using Axis Ultra DLD from Kratos to study the influence of doping on the structural environment using Al-K\(\alpha\) X-ray source. The peak fitting was done using Fityk software.
3 Results and discussions
3.1 Bulk samples
3.1.1 Structural characterization
Doping VO2 is a better way to reduce the T\(_{SMT}\) and is mostly due to the alteration in the crystal structure. DFT calculations suggests that whenever the crystal structure is near to tetragonal phase, a reduction in transition temperature is observed [35].
In order to determine the crystal structure, X-Ray diffraction studies were done. As seen in Fig. 2a, the peaks perfectly match with JCPDS file no: 01-077-2498 for V2O5, showing an orthorhombic crystal system with space group, Pmmn. The peaks match with JCPDS file no: 00-19-1398 for VO2 (Fig. 2b) showing a monoclinic crystal system with space group P2\(_1\)/c. The BV sample is crystalline in nature. The crystalline nature reduces with increasing doping concentration of scandium in VO2 bulk sample (Fig. 2b). During synthesis of V2O5 by SCS, though the ignition temperature is maintained at 500 °C, it is reported that at the microscopic scale the temperature can be as high as 1500 °C. This is achieved at an optimum equivalence ratio of fuel (F, urea) and oxidizer (O, metal nitrate) (defined as oxidizer to fuel ratio; O/F) of unity [41, 42]. Due to this effect, the crystallites are well formed, leading to sharp peaks in the XRD pattern. However, the reduction step to get phase pure VO2, is carried out at 550 °C. This temperature is probably too low to develop well defined crystallites, thereby resulting in broader, or low crystallinity as seen from XRD pattern.
3.1.2 Morphology: SEM
Figure S1 (supplementary information) show the SEM images of the doped and undoped VO2 formed before reduction process. The powder samples shows porous morphology which is due to the release of gases as explained from Eq. 1. Figure 3 shows the SEM images of the doped and undoped VO2 formed after reduction process. The surface morphology shows presence of agglomerated sheets (Fig. 3a–c), which are porous. Figure 3d shows the presence of porous agglomerated particles for RBVS6.25 sample.
For all the samples a small needle like projection was seen coming out of the surface. TEM of RBVS2.0 shows sheet morphology as seen in Fig. 4a. The polycrystalline nature of the sample is confirmed by the electron diffraction pattern (Fig. 4a). The presence of scandium is confirmed by the EDS data (Fig. 4b).
3.1.3 DSC on bulk samples
Phase transition for bulk samples was determined by DSC. The measurement was carried out for both heating and cooling from − 80 to 130 °C for these samples. DSC curves are shown in Fig. 5. The data is shown only for 10 to 100 °C. VO2 sample showed a transition at 68 °C with a heating onset at 60 °C whereas a for cooling the onset was around 62 °C and the peak was observed at 60 °C thereby leading to a hysteresis. As the dopant concentration was increased into the system, broader transition was observed which is probably due to the lower crystallinity as seen from Fig. 2b. For better understanding of the T\(_{SMT}\), electrical measurement of thin films was considered. This is discussed in Sect. 3.2.4.
3.1.4 Optical measurements: band gap determination
Scandium doping into VO2 was supposed to induce a blue-shift in the absorption spectrum [35] as suggested by the DFT calculation. To validate that UV measurement was done on the powder samples by diffuse reflectance spectroscopy (DRS). Using Tauc’s plot i.e., \((\alpha h \nu )^n\) vs \(h\nu\) plot (Fig. 6a) where \(\alpha\) = 2.303 log (Absorbance (A)), band gap was determined for the powder samples. n was considered to be 2 as VO2 is a direct band gap semiconductor.
Sc-doping gives a blue shift in the spectrum thereby making it more transparent the reason being that Sc\(^{3+}\) posses no d-electrons. Because of that, Sc\(^{3+}\)–O\(^{2-}\) electrostatic attraction is weaker than W\(^{6+}\)–O\(^{2-}\), Mo\(^{6+}\)–O\(^{2-}\) and V\(^{4+}\)–O\(^{2-}\) thereby increasing the band gap and hence inducing a blue shift [35]. Figure 6b shows that the band gap value increases for scandium doping till 2.0 at. % thus showing a blue shift in the absorption edge, but the band gap decreases when dopant concentration is varied from 2 to 6.25 at. % (theoretical value) thereby showing that the properties are deteriorating when scandium doping concentration increases further.
We can see that as compared to W and Mo, the Sc-doped VO2 shows higher band gap values thereby validating the reason (Fig. 6b). Thus, it can be inferred that 2.0 at. % of scandium in vanadium dioxide system is the best dopant concentration to get maximum blue shift.
3.2 Thin film samples
For smart windows applications thin film of VO2 are usually used. As seen from the UV data for bulk samples (Fig. 6), the optical band gap for bulk samples reduced as the concentration of scandium increased from 2.0 to 6.25 at. %. Thus, for the thin films, synthesis (explained in “Experimental section”) and measurements were limited to 2.0 at. % dopant concentration.
3.2.1 Structural characterization: XRD
The XRD data for V2O5 thin film perfectly match with JCPDS file no: 01-077-2498 (Fig. 7a) showing an orthorhombic crystal system with space group Pmmn and peaks match with JCPDS file no: 00-19-1398 for VO2 (Fig. 7b) showing a monoclinic crystal system with space group P2\(_1\)/c as seen for the powder sample. The V2O5 thin film on the quartz substrate seem to show an orientation about (00l) direction as can be seen from XRD (Fig. 7a). After reduction no such orientation is observed in XRD thereby giving us a polycrystalline VO2 film (Fig. 7b). The broad peak at 2\(\theta\) = 21.525° is due to quartz substrate for thin films (Fig. 7a, b).
3.2.2 Morphology: SEM
Figure 8 shows the SEM of undoped (RTV) as well as Sc-doped thin film (RTVS1.0 and RTVS2.0) obtained after reduction. The films are highly porous in nature due to the release of gases during the pyrolysis process as explained by Eq. 1. A slight change in the morphology was observed with increasing Sc doping in VO2 thin films.
3.2.3 Raman and XPS measurements
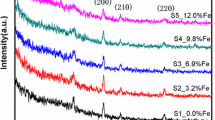
The Raman peaks for thin film (Fig. 9) exactly match with the values reported in the literature as shown in Table S1 (supplementary information). In Raman spectra the lower wavenumber bands (< 400 cm−1) are allocated to V–O–V bending modes; intermediate wavenumber bands (400–800 cm−1) are observed due to V–O–V stretching modes; and higher wavenumber bands (> 800 cm−1), are assigned to V = O stretching modes of distorted octahedra and distorted square-pyramids. The phonon modes in VO2 (M1) are are very complex and are mostly due to the stretching and bending of V–O–V bonds and zigzag chains of V–V [44].The shift in 611 cm−1 peak towards lower wavenumber as seen in Table 2, is observed with doping and is attributed to the alteration in the V–O–V bonds upon doping. To the authors best understanding upon doping Sc into the system there are formation of Sc–O–V bonds which lead to tensile strain into the system thereby shifting the peak to a lower wavenumber.
XPS measurements were done on the undoped and Sc-doped thin film using Al-K\(\alpha\) as the source. The peak position is referenced using C1s (B.E. = 284.8 eV) spectra as reference. O1s and V2p spectra usually fall in the same binding energy (B.E.) region. On deconvoluting the XPS spectra we can find out the oxidation states present in RTV and RTVS2.0. Figure S2 of supporting information shows the deconvoluted V2p and O1s spectra. The peak position, FWHM and area ratio of corresponding oxidation states are tabulated in Table 3. V2p\(_{3/2}\) peak was deconvoluted into three peaks corresponding to V\(^{+5}\), V\(^{+4}\) and V\(^{+3}\) oxidation states after Gaussian background subtraction using Fityk software. From the area ratio for various oxidation state it was observed that upon adding scandium the amount of V\(^{+5}\) and V\(^{+3}\) oxidation state increased (Table 3) thereby suggesting that Sc–O interactions are taking place. This interaction is in good agreement with the Raman data where the shift in wavenumber was attributed to the strain induced due to Sc–O–V bonds formation. This interaction can also be confirmed by O1s spectra [Figure S2 (supplementary information); RTVS2.0] where a peak at 530.06 eV is attributed to Sc–O interaction [45].
3.2.4 Electrical characterization
Figure 10a shows resistance for Sc-doped system as a function of temperature for both heating and cooling experiments done on the thin film synthesised using UNSAPCM. The electrical property was determined by an in-plane measurement using a two-probe DC probe station. The transition temperature for undoped (RTV) thin film was observed at 82 °C. This increase in the transition temperature is due to presence of quartz substrate. The thin film showed a four orders of resistance change. The sample did not show any change in the transition temperature rather the thermal hysteresis increased upon scandium doping (2.0 at. %) as can be seen from Fig. 10b.
To further understand the trend, activation energy was calculated for the heating curves of undoped and doped thin films using the Arrhenius equation:
The slope of the plot between ln R(T) versus 1/K\(_B\)T was calculated to determine the activation energy for both semiconducting and metallic phase of Sc-doped VO\(_2\) (Fig. 10c). Figure 10d shows the change in activation energy for both semiconducting and metallic phase of Sc-doped VO\(_2\) as a function of scandium doping. We observe that the activation energy remains constant till 1.0 at. % doping and then decreases as the concentration is further increased to 2.0 at. % in the semiconducting phase. Similar trend was observed in the metallic phase though an increased value of activation energy was observed when compared with the semiconducting phase.
3.2.5 Optical measurements:UV–visible and FTIR
Figure 11 shows that the band gap value increases for scandium doped VO2 thin films which shows a blue shift in the absorption edge as observed for powder samples and the results are in good agreement with the theoretical reasoning.
VO2 upon transition from semiconducting phase to metallic phase shows a change in IR reflectance i.e. shows high reflectance in metallic phase and low reflectance in semiconducting phase as stated earlier [6]. To study the influence of scandium doping on the reflectance, temperature-dependent FTIR measurements were done on the RTV and RTVS2.0 (Figure S3, supplementary information) thin films in reflectance mode. The measurement was carried out after an interval of 5 \(^o\)C with an equilibrating time of 5 minutes before each measurement was carried out.
For undoped VO\(_2\) (RTV), 80 % change in the reflectance was observed at \(\lambda\) = 9.31 \(\upmu\)m and for 2.0 at. % Sc-doped VO\(_2\) (RTVS2.0), 60 % change in the reflectance was observed at \(\lambda\) = 8.48 \(\upmu\)m as seen in Fig. 12a. Figure 12b shows the derivative plot as a function of temperature thus showing the tunability in the spectral range upon doping and hence making it suitable for a metamaterial type of application. In metallic phase, change in surface plasmon is only observed at \(\lambda\) > 2.0 \(\upmu\)m [40]. The dip in the reflectance observed in the range of \(\lambda\) = 2.5–4 \(\upmu\)m is basically due to the interference of atmospheric CO\(_2\) [46]. Dip around \(\lambda\) = 10 \(\upmu\)m is attributed to the substrate (quartz).
4 Conclusions
In this study, we gave experimental evidence for the theoretical prediction on the influence of Sc-doping in modulating the optical spectrum of VO2. We synthesised Sc-doped VO2 (M1) bulk and thin films by SCS and UNSPACM respectively, in two steps. XPS and Raman measurements confirmed the structural influence of Sc doping into VO2 thin films. Lowering of 611 cm−1 peak position to 606 cm−1 in Raman spectra was attributed to tensile strain resulting from Sc doping. A blue shift in the optical spectrum from 1.7 eV (undoped) to 2.02 eV (2.0 at. %) was observed for both bulk as well as thin films which is in agreement with the theoretical report. Sc-doped VO2 showed four orders of magnitude change in resistance and an increased transition width maintaining the T\(_{SMT}\) of semiconductor to metal transition. 60 % change in the IR reflectance was observed at \(\lambda\) = 8.48 \(\upmu\)m with 2.0 at. % of Sc-doping. Sc-doping only altered the optical spectrum of VO\(_2\) contrary to the theoretical prediction where changes in both T\(_{SMT}\) and band gap were reported. Tuning of both optical spectrum and T\(_{SMT}\) of VO\(_2\) may be possible by co-doping with other dopants for smart window applications.
5 Supplementary information
See Supplementary information for: SEM image of the undoped as well as Sc-doped V2O5 powder samples. Raman modes for V2O5 and VO2 as compared with the literature values; Temperature dependent reflectance plots for undoped (RTV) and 2.0 at. % Sc-doped (RTVS2.0) VO2 thin films and V2p and O1s spectra for RTV and RTVS2.0 thin films that have been deconvoluted using Fityk software.
References
Kang L, Gao Y, Zhang Z, Du J, Cao C, Chen Z, Luo H (2010) Effects of annealing parameters on optical properties of thermochromic VO\(_2\) films prepared in aqueous solution. J Phys Chem C 114:1901–1911
Rajendra Kumar RT, Karunagaran B, Mangalaraj D, Narayandass SK, Manoravi P, Joseph M, Vishnu G (2003) Pulsed laser deposited vanadium oxide thin films for uncooled infrared detectors. Sensor Actuat A Phys 107:62–67
Fu WB, Ma H, Wei Y et al (2017) Preparation and infrared response properties of vanadium dioxide nanowire/carbon nanotube composite film. J Mater Sci 52:7224–7231
Wen Q-Y, Zhang H-W, Yang Q-H, Chen Z, Long Y, Jing Y-L, Lin Y, Zhang P-X (2012) A tunable hybrid metamaterial absorber based on vanadium oxide films. J Phys D Appl Phys 45:235106
Kumar S, Maury F, Bahlawane N (2016) Electrical switching in semiconductor-metal self-assembled VO\(_2\) disordered meta-material coatings. Sci Rep 6:37699
Zhang Z, Gao Y, Chen Z, Du J, Cao C, Kang L, Luo H (2010) Thermochromic VO\(_2\) thin films: solution-based processing, improved optical properties, and lowered phase transformation temperature. Langmuir 26:10738–10744
Lu Z, Li C, Yin Y (2011) Synthesis and thermochromic properties of vanadium dioxide colloidal particles. J Mater Chem 21:14776–14782
Lee CE, Atkins RA, Gibler WN, Taylor HF (1989) Fiber optic application for thermal switching in vanadium dioxide films. Appl Opt 28:4511–4512
Xia X, Chao D, Ng CF, Lin J, Fan Z, Zhang H, Shen ZX, Fan HJ (2015) VO\(_2\) nanoflake arrays for supercapacitor and Li–ion battery electrodes: performance enhancement by hydrogen molybdenum bronze as an efficient shell material. Mater Horiz 2:237–244
Chen L, Ruan Y, Zhang G, Wei Q, Jiang Y, Xiong T, He P, Yang W, Yan M, An Q, Mai L (2019) Ultrastable and high-performance Zn/VO\(_2\) battery based on a reversible single-phase reaction. Chem Mater 31:699–706
Hassein-Bey ALS, Tahi H, Lafane S, Djafer AZA, Hassein-Bey A, Belgroune N (2016) Substrate effect on electrical properties of vanadium oxide thin film for memristive device applications. In: 2016 IEEE international conference on semiconductor electronics (ICSE), pp 240–243
Wu X, Wu Z, Ji C, Zhang H, Su Y, Huang Z, Gou J, Wei X, Wang J, Jiang Y (2016) THz transmittance and electrical properties tuning across IMT in vanadium dioxide films by Al doping. ACS Appl Mater Interfaces 8:11842–11850
Wu C, Feng F, Xie Y (2013) Design of vanadium oxide structures with controllable electrical properties for energy applications. Chem Soc Rev 42:5157–5183
Son J-H, Wei J, Cobden D, Cao G, Xia Y (2010) Hydrothermal synthesis of monoclinic VO\(_2\) micro- and nanocrystals in one step and their use in fabricating inverse opals. Chem Mater 22:3043–3050
Liu M, Hwang HY, Tao H, Strikwerda AC, Fan K, Keiser GR, Sternbach AJ, West KG, Kittiwatanaku S, Jiwei L, Wolf SA, Omenetto FG, Zhang X, Nelson KA, Averitt RD (2012) Terahertz-field-induced insulator-to-metal transition in vanadium dioxide metamaterial. Nature 487:345
Powell MJ, Quesada-Cabrera R, Taylor A, Teixeira D, Papakonstantinou I, Palgrave RG, Sankar G, Parkin IP (2016) Intelligent multifunctional VO\(_2\)/SiO\(_2\)/TiO\(_2\) coatings for self-cleaning, energy-saving window panels. Chem Mater 28:1369–1376
Xu S, Ma H, Dai S et al (2004) Study on optical and electrical switching properties and phase transition mechanism of Mo\(^{6+}\)-doped vanadium dioxide thin films. J Mater Sci 39:489–493
Bian J, Miao L, Zhao S et al (2015) Vanadium oxide films deposited on sapphire substrate with in situ AlN stress layer: structural, electric, and optical properties. J Mater Sci 50:5709–5714
Bhardwaj D, Singh DK, Krupanidhi SB et al (2020) Fabrication of smooth thin film of vanadium oxides (VO\(_x\)) using pulsed laser deposition. Appl Phys A 126:157
Bhardwaj D, Goswami A, Umarji AM (2018) Synthesis of phase pure vanadium dioxide (VO\(_2\)) thin film by reactive pulsed laser deposition. J Appl Phys 124:135301
Maruyama T, Ikuta Y (1993) Vanadium dioxide thin films prepared by chemical vapour deposition from vanadium(III) acetylacetonate. J Mater Sci 28:5073–5078
Gopalakrishnan G, Ramanathan S (2011) Compositional and metal-insulator transition characteristics of sputtered vanadium oxide thin films on yttria-stabilized zirconia. J Mater Sci 46:5768–5774
Guinneton F, Sauques L, Valmalette JC, Cros F, Gavarri JR (2001) Comparative study between nanocrystalline powder and thin film of vanadium dioxide VO\(_2\): electrical and infrared properties. J Phys Chem Solids 62:1229–1238
Rajeswaran B, Umarji AM (2016) Effect of W addition on the electrical switching of VO\(_2\) thin films. AIP Adv 6:035215
Manning TD, Parkin IP, Pemble ME, Sheel D, Vernardou D (2004) Intelligent window coatings: atmospheric pressure chemical vapor deposition of tungsten-doped vanadium dioxide. Chem Mater 16:744–749
Chen B, Yang D, Charpentier PA, Zeman M (2009) Al\(^{3+}\)-doped vanadium dioxide thin films deposited by PLD. Sol Energy Mater Solar Cells 93:1550–1554
Zhou J, Gao Y, Liu X, Chen Z, Dai L, Cao C, Luo H, Kanahira M, Sun C, Yan L (2013) Mg-doped VO\(_2\) nanoparticles: hydrothermal synthesis, enhanced visible transmittance and decreased metal-insulator transition temperature. Phys Chem Chem Phys 15:7505–7511
Wang N, Liu S, Zeng XT, Magdassi S, Long Y (2015) Mg/W-codoped vanadium dioxide thin films with enhanced visible transmittance and low phase transition temperature. J Mater Chem C 3:6771–6777
Netsianda M, Ngoepe PE, Catlow C, Richard A, Woodley SM (2008) The displacive phase transition of vanadium dioxide and the effect of doping with tungsten. Chem Mater 20:1764–1772
Lv X, Cao Y, Yan L, Li Y, Zhang Y, Song L (2018) Atomic layer deposition of V\(_{1-x}\)Mo\(_x\)O\(_2\) Thin films, largely enhanced luminous transmittance, solar modulation. ACS Appl Mater Interfaces 10:6601–6607
Yuan X, Zhang Y, Abtew TA, Zhang P, Zhang W (2012) VO\(_2\): orbital competition, magnetism, and phase stability. Phys Rev B 86:235103
Gu D, Li Y, Zhou X, Xu Y (2019) Facile fabrication of composite vanadium oxide thin films with enhanced thermochromic properties. ACS Appl Mater Interfaces 11:37617–37625
Mlyuka NR, Niklasson GA, Granqvist CG (2009) Mg doping of thermochromic VO\(_2\) films enhances the optical transmittance and decreases the metal-insulator transition temperature. Appl Phys Lett 95:171909
Dai L, Chen S, Liu J, Gao Y, Zhou J, Chen Z, Cao C, Luo H, Kanehira M (2013) F-doped VO\(_2\) nanoparticles for thermochromic energy-saving foils with modified color and enhanced solar-heat shielding ability. Phys Chem Chem Phys 15:11723–11729
Sun C, Yan L, Yue B, Liu H, Gao Y (2014) The modulation of metal-insulator transition temperature of vanadium dioxide: a density functional theory study. J Mater Chem C 2:9283–9293
Patil KC, Hegde MS, Rattan T, Aruna ST (2008) Chemistry of nanocrystalline oxide materials: combustion synthesis, properties and applications. World Scientific, Singapore
Rajeswaran B, Umarji AM (2017) Phase evolution and infrared transmittance in monophasic VO\(_2\) synthesized by a rapid non-equilibrium process. Mater Chem Phys 190:219–229
Bharathi R, Umarji AM (2013) Effect of Mo doping on the electrical properties of VO\(_2\) phase. In: International conference on advanced nanomaterials emerging engineering technologies, pp 424–425
Kamble VB, Umarji AM (2013) Gas sensing response analysis of p-type porous chromium oxide thin films. J Mater Chem C 1:8167–8176
Bharathi R, Naorem R, Umarji AM (2015) Metal-insulator transition characteristics of vanadium dioxide thin films synthesized by ultrasonic nebulized spray pyrolysis of an aqueous combustion mixture. J Phys D Appl Phys 48:305103
Sherikar BN, Umarji AM (2013) Synthesis of diopside by solution combustion process using glycine fuel. Int J Mod Phys Conf Ser 22:217–223
Sherikar BN, Umarji AM (2011) Effect of adiabatic flame temperature on nano alumina powders during solution combustion process. Trans Indian Ceram Soc 70:167–172
Wu JM, Liou LB (2011) Room temperature photo-induced phase transitions of VO\(_2\) nanodevices. J Mater Chem 21:5499–5504
Lee S, Ivanov IN, Keum JK, Lee HN (2016) Epitaxial stabilization and phase instability of VO\(_2\) polymorphs. Sci Rep 6:19621
Moulder JF, Stickle WF, Sobol PE, Bomben KD (1992) Handbook of X-ray photoelectron spectroscopy, edited by J Chastain. Perkin-Elmer, Eden Prairie
Pradhan JK, Ramakrishna SA, Rajeswaran B, Umarji AM, Achanta VG, Agarwal AK, Ghosh A (2017) High contrast switchability of VO\(_2\) based metamaterial absorbers with ITO ground plane. Opt Express 25:9116–9121
Acknowledgements
DB thanks CSIR for providing Ph.D. fellowship. Centre for Nanoscience and Engineering (CeNSE) at IISc is acknowledged for providing access to Raman and I-V measurements. The authors thank Prof. S. Anantha Ramakrishna, Department of Physics, IIT Kanpur for providing access to high Temperature FTIR facility. Authors also like to thank Ms. Debadarshini Samantaray and Dr. Prem Wicram Jaschin for helping us to carry out SEM for the thin film and TEM imaging, respectively. The authors would also like to thank DST-FIST for providing funda for XRD facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhardwaj, D., Umarji, A.M. Scandium: An efficient dopant to modulate the optical spectrum of vanadium dioxide (VO2). SN Appl. Sci. 2, 1553 (2020). https://doi.org/10.1007/s42452-020-03332-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03332-7