Abstract
In recent years, toxic pollutants in industrial wastewater are seriously considered as one of the environmental problems due to the heavy metals present in it. Currently, biosorption has been strongly recommended by researchers as an efficient and economical technique to reduce the amount of heavy metals in the different industrial wastewater sources. Chitosan and sugar bagasse ash are the well-known biosorbents due to their low cost, available and effective in adsorption process. In this study, the composite biosorbent was prepared by mixing chitosan and sugar bagasse ash prepared by microwaving at a ratio of 1:5 (ASB-CBs) in acid condition. Adsorbents were characterized by SEM analysis method and FTIR infrared spectra. The parameters affecting the Pb(II) and Cd (II) adsorption such as pH, adsorption time, dose of adsorbent was studied by running the batch experiments. Research data show that the optimal pH for the biosorption process is 5.0. Experimental data is described by the Freundlich equation. Thermodynamic process is also studied in which the negative free Gibbs energy indicated the metal ions adsorption on the ASB-CBs was spontaneous and endothermic nature.
Similar content being viewed by others
1 Introduction
In recent years, toxic pollutants in industrial wastewater is seriously considered as one of the environmental problems due to the heavy metals present in it. These contaminants are not decomposable easily and seriously to affect human health through the food chain causing various diseases and disorders. Among these contaminants, lead and cadmium are recurrently found together, for example, in the wastewater of textile, dye, metal plating, batteries and paint formulation [1]. In potable water, the amount of acceptable limit of Pb(II) is 0.01 mg L−1 and for Cd (II) is 0.003 mg L−1. These heavy metals may generate long-term harm in human beings, including lung fibrosis, dyspnea, anemia, muscle and joint pains, kidney damage, high blood pressure, etc. [2]. Because of their poisonous nature and high risk of accumulation it presents a serious threat to the environment, it is significant to reduce the amount of Cd (II) and Pb(II) from the wastewater before being directly discharged into the environment. Currently, biosorption has been strongly recommended by researchers as an efficient and economical technique to reduce the amount of heavy metals present in the different industrial wastewater sources [3,4,5,6].
Chitosan is used as an adsorbent to remove heavy metals in many studies due to its low-cost, abundance, and high selectivity against contaminants [7, 8]. Chitosan can effectively adsorb various kinds of particles, including heavy metal ions based on the presence of hydroxyl (–OH) and amino (–NH2) functional groups [9]. In order to augment the acid resistance condition of the chitosan, the chemical crosslink process was conducted with some specific agents such as glutaraldehyde, acid citric and epichlorohydrin [10,11,12]. For this research, to improve the raw chitosan acid stability and its mechanical strength, glutaraldehyde was applied to crosslink it. In contrast, the crosslinked polymer network affected the functional groups on the chitosan surface; which lead to decrease of its adsorption abilities. Currently, sugar bagasse, considered as waste after the extraction sugar juice from crushed cane, has been reported as a potential, low-cost biosorbent based on an agriculture by-product. The constituent of sugar bagasse are hemicellulose and lignin which process many oxygen functional groups, for instance hydroxyl; carboxyl and phenol groups [13, 14]. In this regard, sugar bagasse was prepared into activated sugar bagasse ash via microwave radiation to enhance its adsorption capacity and immobilized on the crosslinked chitosan beads.
The purpose of this research is to evaluate the adsorption abilities of the composite material between glutaraldehyde-crosslinked chitosan and activated sugar bagasse ash (ASB-CBs) for reduction of Cd (II) and Pb(II) from liquid solutions. To evaluate the parameters affecting the biosorption process such as solution pH, contact time, and temperature, the batch experiments were conducted. The adsorption kinetics were analyzed by applying the pseudo-first-order and pseudo-second-order models. The mechanism of metal ions adsorption through the modified crosslinked chitosan beads (ASB-CBs) was also examined for further elucidation this biosorption process.
2 Materials and methods
2.1 Materials
The standard solution of Pb(II) and Cd (II) (1000 mg L−1) was provided by Merck Company (Germany). All reagents as well as chitosan used in this research were analytical graded and supplied by Sigma Aldrich. The sugar bagasse used in this work was collected from the Plantation Thanh Long (Tay Ninh, Vietnam). The sugar bagasse was dried, ground and sieved to the size from 0.1 to 0.15 mm for subsequent experiments.
2.2 Preparation of microwave activated sugar bagasse ash
Sugar Bagasse Ash (SBA, 10 g) was mixed with 10 mL of 91% H3PO4 in a porcelain beaker. The mixture was put into microwave model SHARP R-20A1(S)VN for 5 min at 500 W. The product was left to room temperature then centrifuged to wash until pH of solution was at 6.0–6.5. The final result was dried in oven at 80 °C in 3 h to obtain the activated sugar bagasse ash (ASB) which will be used to modified on the crosslinked chitosan beads later.
2.3 Composite biosorbents preparation
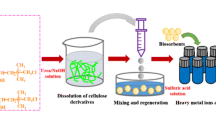
Composite biosorbents were formulated by mixing chitosan powder (5 g) with activated sugar bagasse ash (1 g) dissolved into acetic acid (7%) (240 mL) and left for 16 h. The slurry was poured into an alkali coagulating solution (H2O: methanol: NaOH = 4:5:1, w/w/w) (200 mL), the result are beads that have an average diameter of 3.5 mm. The products (ASB-CBs) were washed away by using distilled water to remove the residual NaOH [15,16,17]. In order to increase the acid resistance ability of the composite adsorbents, the wet ASB-CBs were mixed with a solution of glutaraldehyde (fixed at 1:2) within 16 h. The final beads were rinsed multiple times to remove the residual glutaraldehyde using distilled water. The end result (ASB-CBs) was dehydrated at 80 °C in an oven for 12 h. SEM, FTIR were analyzed to investigate the characteristic of biosorbent.
2.4 Adsorption experiments
The batch adsorption tests were carried out to evaluate the different parameters affected such as biosorbent dose, pH, agitated time, initial concentration of heavy metals on the removal efficiency of metal ions. The affecting of pH parameter was studied with the initiate pH from 3.0 to 8.0. The effect of contact time was carried out at 303 K and 200 rpm. The ASB-CBs (2 g L−1) was stirred with a metal ions solution (100 mL). After been completely mixed by using a shaker incubator (model LM-570RD) at 303 K and 200 rpm, the residual concentration of metal ions was measured using an atomic absorption spectrophotometer (Spectra AA 220, FS Varian). Different concentrations of metal ions ranging from 10 to 250 mg L−1 were examined under the optimal temperature, pH, agitation time and biosorbent dose that have been found in the earlier tests.
The load of a single metal ion adsorbed on the surface of the adsorbent at equilibrium and time, qe and qt respectively, in mg g−1 were estimated based on the equations as follow:
where C0, Ce, and Ct are the initial concentrations of metal ion, at equilibrium and at time t in mg L−1, correspondingly, V represents the solution volume (L), and m symbolizes the adsorbent dose used (g).
3 Results
3.1 Characterization of the biosorbents
The surface morphology of the biosorbents (ASB-CBs) before and after metal ions adsorption was studied using SEM analysis. The SEM images of ASB-CBs before and after metal uptake, as illustrated in Fig. 1a, b, display significant structure changes. The ASB-CBs surfaces are rough, dense and porous texture. However, after contact with Pb(II) and Cd (II), the porous texture disappeared and turn into smooth and tighter structure. The EDX images (Fig. 2a, b) show that Pb(II) and Cd (II) are well-adsorbed onto the composite materials.
The infrared spectrum analyzes of CBs, ASB-CBs, Pb-adsorbed ASB-CBs and Cd-adsorbed ASB-CBs all had widespread igniting scales at 4000 ÷ 3000 cm−1 indicating functional groups –OH, –NH2, –COO and C–H aromatics. In particular, the presence of strong intensities at 1647.10 cm−1, 1658.67 cm−1 (Fig. 3b) and 1642.31 cm−1, 1639.59 cm−1 has a combined carboxylate functional group NH2 [18]. The 1544.88 cm−1 spectrum corresponds to the quaternary amine group. Peaks at 1317.29 cm−1 and 1380.94 cm−1 in ASB-CBs showed C–H groups. The adsorption peaks have a weak intensity of 669.29 cm−1 and 570.89 cm−1 due to the metal oxide bonding of M–O [19]. Some FTIR spectral absorption bands after adsorption of metal ions decreased the intensity indicating that the shift to low-spectrum bands indicated that there was a linkage between the functional groups and the metal oxide as illustrated in Fig. 3c, d). Changes in the absorption spectra after metal ions adsorption showed the successful preparation of absorbable material. Functional substituent groups on the materials include the –OH, –CH2, –CH3, –COO, –NH2, –COOH, –C–H, –CH2.
3.2 Effect of pH
The pH of the solution significantly impacts the adsorption capacity, Fig. 4 illustrates the effect of the initial pH on the quantity of metal ions adsorbed onto ASB-CBs. The amount of removed metal ions increased with the increasing of pH from 3 to 5. The maximum adsorption capacity was found at pH 5.0 in which Pb(II) biosorbed is 11.79 mg g−1 and Cd (II) is 12.14 mg g−1 at initial metal ions concentration of 25 mg L−1. Nevertheless, as the pH increments over 7.0, the biosorption volume reduces due to the precipitation of the metal hydroxide. As a result, pH 5.0 was chosen as the optimum pH for subsequent experiments. Also, many previous studies report pH 5.0–6.0 as the optimum pH for heavy metals adsorption onto biosorbents [4, 20,21,22].
3.3 The biosorbent dose effect on metal ions biosorption
A significant parameter that highly affects the biosorption capacity is the dose of adsorbent. The adsorption tests were carried out with the range of ASB-CBs between 0.5 and 5 g L−1 under the same conditions, for instance temperature, pH, initial metal ions concentration and agitating time. The Fig. 5 illustrates the adsorption capacity effect of ASB-CBs. By increasing the absorbent dose, the amount of metal ions absorb on ASB-CBs decreases. Due that the adsorption sites have been saturated on the surface of the biosorbent when the dose is incremented, no more adsorption occurs after the equilibrium point [12].
3.4 Kinetic of adsorption process
In order to investigate the adsorption rate and the step which control the rate reaction, such as chemical reaction or mass transfer, kinetic models are used. The pseudo-first-order and the pseudo-second-order kinetic models were employed to determine the data obtained from the tests. The pseudo-first-order kinetic model equation is:
where k1 is the first-order rate constant (1 min−1) and t is the contact time (minutes). The pseudo-second-order kinetic model equation is:
where k2 is the second-order rate constant (g mg−1 min−1).
The value of k1 was estimated by the plot between ln (qe − qt) and t (not shown), and the correlation coefficient (r2) was also found. The plot of (t/qt) versus t (Figs. 6, 7) was used to determine k2 and r2.
The kinetic parameters of the biosorption process for Pb(II) and Cd (II) are listed in Tables 1 and 2, correspondingly. The r2 values in both metal ions adsorption clarified that the experimental data used, fitted with the pseudo-second-order model (r2 > 0.9995). These results validate that the pseudo-second-order model can indicate that the rate-limiting step in metal ions biosorption on the ASB-CBs is the chemisorption.
3.5 Equilibrium modeling
The following equation described the linear form of the Freundlich model [23, 24].
where KF is a constant indicating the relative adsorption capacity of the biosorbent (mg1−(1/n) L1/n g−1), and n is a constant indicating the adsorption intensity.
The values of KF and n found from the graph of ln qe and ln Ce at different temperatures are reported in Tables 3 and 4. The n value indicates the trend of the adsorption process; if n = 1, a linear adsorption occur; if n < 1, it indicates that is a chemical process; if n > 1, it indicates that is a physical process [25]. The obtained correlation coefficient r2 is larger than 0.998, indicating the feasibility of the application of the Freundlich equation. In this research, the value of n is found between 0.7 and 0.8 that is almost equal to 1.0, demonstrating not only chemical but also physical process of metal ions biosorption on the ASB-CBs.
In order to reveal the adsorption behavior on the surface [16, 26, 27], the Langmuir isotherm model was applied. The linearized Langmuir equation is:
where qmax is the maximum quantity of adsorption (mg g−1), and KL is the Langmuir constant (L mg−1).
The values of KL and qm found from the graph Ce/qe and Ce, listed in Tables 3 and 4, compares the experimental data with the ones fitted by the Langmuir equation (Eq. 6) at different temperatures.
The maximum amount of Pb(II) and Cd (II) adsorption are 185.18 mg g−1 and 84.03 mg g−1 at 313 K, respectively. The ASB-CBs in this study show improvement and promising performance for metal ions removal compare with other biosorbents in previous studies.
3.6 Thermodynamic
The Gibbs energy (∆G) (kJ mol−1), the enthalpy change (∆H) (kJ mol−1), and the entropy change (∆S) (J mol−1 K) of an adsorption process can be estimated by [28]
where T is the absolute temperature (K) and R is the universal gas constant (8.314 J mol−1 K−1). The values of ∆H and ∆S can be obtained from the slope and intercept of the graph of ln KL against 1/T based on the van’t Hoff equation
Tables 5 and 6 show the thermodynamic parameters of the adsorption process of Pb(II) and Cd (II) on the ASB-CBs. The negative ∆G values which indicated the metal ions adsorption on the ASB-CBs was spontaneous. The values of ∆H and ∆S were 19.49 kJ mol−1 and 111.1 J mol−1 K−1, correspondingly in case of Pb(II); 15.9 kJ mol−1 and 110.1 J mol−1 K−1 in case of Cd (II). The positive enthalpy change (∆H) reveals the endothermic nature. The entropy change value is positive, indicating increased randomness at the surface of the ASB-CBs during the process [29].
References
Gavrilescu M (2004) Removal of heavy metals from the environment by biosorption. Eng Life Sci 4(3):219–232
Ahmad A, Khatoon A, Mohd-Setapar S-H, Kumar R, Rafatullah M (2016) Chemically oxidized pineapple fruit peel for the biosorption of heavy metals from aqueous solutions. Desalination Water Treat 57:6432–6442
Arief VO, Trilestari K et al (2008) Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: characterization, biosorption parameters and mechanism studies. CLEAN Soil Air Water 36(12):937–962
Bayo J (2012) Kinetic studies for Cd(II) biosorption from treated urban effluents by native grapefruit biomass (Citrus paradisi L.): the competitive effect of Pb(II), Cu(II) and Ni(II). Chem Eng J 191:278–287
Chojnacka K (2010) Biosorption and bioaccumulation: the prospects for practical applications. Environ Int 36(3):299–307
Kapoor A, Viraraghavan T (1995) Fungal biosorption: an alternative treatment option for heavy metal bearing wastewaters—a review. Biores Technol 53(3):195–206
Wan Ngah WS, Teong LC et al (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83(4):1446–1456
Wu F-C, Tseng R-L et al (2010) A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J Environ Manag 91(4):798–806
Vold IMN, Vårum KM et al (2003) Binding of ions to chitosan: selectivity studies. Carbohydr Polym 54(4):471–477
Beppu MM, Vieira RS et al (2007) Crosslinking of chitosan membranes using glutaraldehyde: effect on ion permeability and water absorption. J Membr Sci 301(1–2):126–130
Monteiro OAC Jr, Airoldi C (1999) Some studies of crosslinking chitosan–glutaraldehyde interaction in a homogeneous system. Int J Biol Macromol 26(2–3):119–128
Suc NV, Ly HTY (2013) Lead (II) removal from aqueous solution by chitosan flake modified with citric acid via crosslinking with glutaraldehyde. J Chem Technol Biotechnol 88:1641–1649
Foo KY, Lee LK et al (2013) Preparation of activated carbon from sugarcane bagasse by microwave assisted activation for the remediation of semi-aerobic landfill leachate. Biores Technol 134:166–172
Hamza IA, Martincigh BS, Ngila JC, Nyamori VO (2013) Adsorption studies of aqueous Pb(II) onto a sugarcane bagasse/multi-walled carbon nanotube composite. Phys Chem Earth 66:157–166
Dalida MLP, Mariano AFV et al (2011) Adsorptive removal of Cu(II) from aqueous solutions using non-crosslinked and crosslinked chitosan-coated bentonite beads. Desalination 275(1–3):154–159
Eser A, Nüket Tirtom V et al (2012) Removal of nickel(II) ions by histidine modified chitosan beads. Chem Eng J 210:590–596
Liu Y-G, Liao T et al (2013) Biosorption of copper(II) from aqueous solution by Bacillus subtilis cells immobilized into chitosan beads. Trans Nonferrous Met Soc China 23(6):1804–1814
Haque MN, Morrison GM et al (2007) Characteristics of arsenic adsorption to sorghum biomass. J Hazard Mat 145(1):30–35
Anandan K, Rajendran V (2011) Morphological and size effects of NiO nanoparticles via solvothermal process and their optical properties. Mat Sci Semicond Process 14(1):43–47
Al-Jariri JS, Khalili F (2010) Adsorption of Zn(II), Pb(II), Cr(III) and Mn(II) from water by Jordanian bentonite. Desalination Water Treat 21(1–3):308–322
Chen A-H, Liu S-C et al (2008) Comparative adsorption of Cu(II), Zn(II), and Pb(II) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin. J Hazard Mater 154(1–3):184–191
Laus R, Costa TG et al (2010) Adsorption and desorption of Cu(II), Cd(II) and Pb(II) ions using chitosan crosslinked with epichlorohydrin-triphosphate as the adsorbent. J Hazard Mater 183(1–3):233–241
Cui L, Wu G et al (2010) Adsorption performance of nickel and cadmium ions onto brewer’s yeast. Can J Chem Eng 88(1):109–115
Göksungur Y, Üren S et al (2005) Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Biores Technol 96(1):103–109
Ngah WSW, Fatinathan S (2006) Chitosan flakes and chitosan-GLA beads for adsorption of p-nitrophenol in aqueous solution. Colloids Surf A 277(1–3):214–222
Popuri SR, Vijaya Y et al (2009) Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Biores Technol 100(1):194–199
Ghaee A, Shariaty-Niassar M et al (2012) Adsorption of copper and nickel ions on macroporous chitosan membrane: equilibrium study. Appl Surf Sci 258(19):7732–7743
Sarı A, Tuzen M et al (2007) Biosorption of Pb(II) and Ni(II) from aqueous solution by lichen (Cladonia furcata) biomass. Biochem Eng J 37(2):151–158
Padmavathy V (2008) Biosorption of nickel(II) ions by baker’s yeast: kinetic, thermodynamic and desorption studies. Biores Technol 99(8):3100–3109
Acknowledgements
I would like to thank the Faculty of Chemical and Food Technology from HCMC University of Technology and Education, I also would like to express my deep gratitude my colleagues and my family for their support in this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We hereby pledge that this study is a true scientific research of my own, conducted under my research group. The references in the manuscript are from scientific papers, books have been verified and published widely. We promise to quote the source clearly in the reference list. The research results in this project are done by the group itself strictly, honestly and not overlapping with other topics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nguyen, L.M., Nguyen, T.T.H. Enhanced heavy metals biosorption using chemically modified chitosan coated microwave activated sugarcane baggage ash composite biosorbents. SN Appl. Sci. 1, 1555 (2019). https://doi.org/10.1007/s42452-019-1607-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1607-9