Abstract
Present study involves the simple, rapid, non-toxic and in vitro method of extracellular silver nanoparticles synthesis using Entomopathogenic fungus (Beauveria bassiana). The development of silver nanoparticle in fungal supernatant was confirmed by the absorbance peak at 450 nm in UV–Vis spectrophotometer. Further, presence of AgNPs and its crystal lattice was confirmed by EDS and XRD, respectively. TEM micrograph confirmed the presence of differently shaped (triangular, circular, hexagonal) nanoparticles with size ranging from 10 to 50 nm. Variable shape and size of fungal assisted AgNps was also confirmed in SEM study. The optimal pH and temperature for biosynthesis of nanoparticles was found to be 6.0 and 25 °C, respectively. The continuous effects of AgNPs against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus in time dependent manner was confirmed in growth kinetic studies. During 36 h of growth study, maximum reduction in O.D560 was found in E. coli (67.2%) followed by P. aeruginosa (63.3%) and S. aureus (56.8%) at 30 °C. The MIC values of fungal assisted AgNPs against E. coli, P. aeruginosa and S. aureus was found to be 2.5, 3 and 4.5 ppm, respectively. The MIC values of Ciprofloxacin was observed to be 0.5, 0.5 and 0.7 ppm, whereas MICs of AgNPs + Ciprofloxacin showed 0.4, 0.4, 0.5 ppm against E. coli, P. aeruginosa and S. aureus, respectively, clearly highlighting the synergistic effect of AgNPs in combination with Ciprofloxacin. In the view of challenges for developing antimicrobial nanoparticles of variable shape and size by various other methods, tuning nanoparticles synthesis via fungi can be a wonderful approach to resolve existing hurdles.
Similar content being viewed by others
1 Introduction
The term “nano” is derived from a Greek word which means “dwarf” [1, 2]. Synthesis of nanoparticles has gained attention due to their applications in multiple fields such as antifungal agents, antibacterial agents, biosensor, bioremediation, anticancer, drug delivery, etc. [3,4,5,6,7,8,9,10]. In addition to this, unique properties of nanoparticles such as size, shape, surface to volume ratio, high catalytic activity, etc. make them a unique candidate for multiple applications described above. The synthesis of nanoparticles via biological means has proved to be the better mode as compared to chemical and physical methods [1]. Chemical methods involve toxic chemicals and may generate hazardous or toxic by-product during synthesis, while physical methods have low efficiency and involves high consumption of energy to maintain optimal pressure and temperature during the synthesis process [11, 12]. On the other hand, biological methods for the synthesis of silver nanoparticles provides many advantages i.e., environmental friendly, non-toxic and easy to scale up for large-scale synthesis [13,14,15]. Researcher across the world utilized biological entities such as plant (leafs, flower, seeds), bacteria, algae and fungi for the production of different type of metallic nanoparticles such as gold, silver, cadmium, iron, etc. and studied their applications in the field of medical, bioremediation, drug delivery etc. [16,17,18,19].
Among other metal nanoparticles, synthesis of silver nanoparticles (AgNPs) via biological mean gain wide acceleration due to their profound applications in medical application, textile dye removal, wastewater treatment, biological sensors etc. [16, 20,21,22]. Synthesis of AgNPs via fungi gain more attention because they produce large amount of biomass in a short period of time under lab conditions and produce large amount of extracellular protein that help in synthesis of nanoparticles [23, 24]. Dhanaraj et al. [17] observed the AgNPs synthesis potential of fungi Aspergillus niger and observed significant anti-bacterial properties of AgNPs against Staphylococcus aureus, Klebsiella pneumonia, Escherichia coli, and Salmonella typhi. In another study, Verma et al. [25] investigated the antimicrobial potential of AgNPs synthesized through Aspergillu sclavatus against Candida albicans, Pseudomonas fluorescens and Escherichia coli. Results clearly indicated anti-microbial activity with average minimum inhibitory concentration of 5.83 µg mL−1 against above microbial pathogens. However, the literature discussing about synthesis of AgNPs using entomopathogenic fungus and antimicrobial potential of synthesized nanoparticles is lacking [26, 27]. Most of the studies investigates larvicidal and insecticidal properties of these fungi [28]. Synthesis of AgNPs using entomopathogenic fungus provides additional advantages as they are non-pathogenic and insecticidal in nature. Earlier reports have highlighted the insecticidal potential of B. basssiana (entomopathogenic fungi) AgNPs. As per our findings, only one report has studied the antimicrobial efficacy of B. bassiana AgNPs against Staphylococcus aureus and Escherichia coli using well diffusion assay [29].
The present study investigated the extracellular AgNPs synthesis using Beauveria bassiana (entomopathogenic fungus) along with their antimicrobial potential. The antimicrobial activity of silver nanoparticles was compared with the antibiotic and AgNPs-antibiotic combination. The present report is the first investigation on the kinetic study of B. bassiana synthesized AgNPs against gram positive and negative bacteria at different time intervals with additional studies on AgNPs and antibiotic combination. Further, AgNPs were characterised via Transmission electron microscopy (TEM) equipped Energy dispersive spectrometer (EDS), Scanning electron microscopy (SEM), X-ray diffraction (XRD) and zeta potential.
2 Materials and methods
2.1 Micro-organisms and growth conditions
In the present work, Beauveria bassiana was obtained from Institute of Microbial Technology (Chandigarh, India). The strain was stored at 4 °C on potato dextrose agar slant. Prior to experiments, culture was reactivated on potato dextrose agar at 25 °C for 72 h [29]. Culture of Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus stored at 4 °C on nutrient agar slant. Prior to experiments, fresh culture of each bacterial strain was prepared on nutrient broth at 37 °C.
2.2 Extracellular synthesis of silver nanoparticles
Spores from freshly prepared slants of B. bassiana was inoculated to a liquid composite media 100 mL (NH4NO3, 0.5 g L−1; MgSO4·7H2O, 0.1 g L−1; K2HPO4, 0.5 g L−1; NaCl, 1 g L−1; Glucose, 10 g L−1; Yeast extract, 2.5 g L−1) in an Erlenmeyer flask (250 mL) [31]. The flask was incubated at 30 °C at 150 rpm for 72 h (Orbitek, India). The biomass was harvested after 72 h of growth by using Whatman No. 1 filter paper. After harvesting the biomass, extensive washing with distilled water was performed to remove any media components from the biomass. Ten gram of above wet biomass was taken into an Erlenmeyer flask (250 mL) containing 100 mL distilled water and flask was incubated for 48 h at 25 °C and 150 rpm. After 48 h, the filtrate from the flask was obtained by passing flask content through Whatman No. 1 filter paper. The filtrate was mixed with 1 mM silver nitrate (AgNO3) in the ratio of 1:9 (v/v) to obtain the desired reaction mixture. Afterward, reaction mixture was incubated at 25 °C in dark for 30 min at 100 rpm. Control (without AgNO3) was incubated under the same conditions.
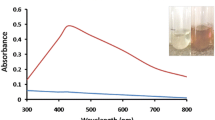
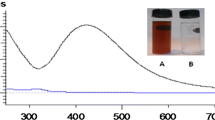
Colour change of the above reaction mixture was the first indicator of nanoparticle synthesis. The reaction mixture with silver nanoparticles (AgNPs) turned brown in colour and it was stored under ambient dark conditions for further analysis and experiments.
2.3 Optimization of physio-chemical parameters for AgNPs synthesis
Effect of temperature and pH on the synthesis of AgNPs was investigated by varying the the temperature (15–40 °C) and pH (3–9). To obtained the desired pH value of fungal extract NaOH and HCl was used prior to the addition of silver nitrate. Sample of 1 mL was withdrawn after 30 min and the absorbance was measured at 450 nm.
2.4 Characterization of AgNPs
2.4.1 Ultraviolent–visible spectroscopy
The Ultraviolet–visible spectroscopy analysis of freshly prepared AgNPs was obtained using the Agilent spectrophotometer with water as control. Samples from reaction mixture for UV–Vis spectra were withdrawn at a regular interval of 10 min for overall reaction time of 30 min. The spectra were recorded in a wavelength ranging from 300 to 1000 nm at a resolution of 0.5 nm.
2.4.2 Zeta potential-dynamic light scattering
Zeta potential was used to determine the charge that exists between the AgNPs using Nano ZS90 Zeta sizer (Malvern Instruments) equipped with He–Ne laser (633 nm, 5 Mw).
2.4.3 Transmission electron microscopy and energy dispersive spectrometer
To determine the size and the shape of the AgNPs, Transmission electron microscopy was performed. The sample for TEM was prepared by dropping the solution of AgNPs on the carbon side of carbon-copper grid. After drying for 30 min, grid was placed on the sample holder and micro-graphs were obtained for size and shape analysis. For TEM images, sample was analysed via EOL JEM-1400. On the other hand, Energy dispersive micrograph was obtained by Bruker-ASX (Model QuanTax 200) was used.
2.4.4 Scanning electron microscopy
The nanoparticle sample was lyophilized and characterized further to study the morphological features using scanning electron microscopy (ZEISS EVO 50).
2.4.5 X-ray diffraction (XRD) measurement
XRD (X-ray diffraction) was conducted for the nanoparticles using Cuka radiation (k = 1.5406 Å) via X’ Pert PRO Philips Analytical-PW 3040/60. The sample was examined at 40 kV with 30 mA at a 2 h angle pattern and was scanned in the region of 25°–80°. The micro-graph obtained after scanning was compared with the Joint committee on Power Diffraction Standards Library to establish the crystalline structure of nanoparticles.
2.4.6 Antimicrobial activity of AgNPs and antibiotic
Three different species of bacteria (Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus) were tested for their susceptibility to the antimicrobial properties of fungal derived AgNPs in term of growth kinetic and Minimum inhibitory concentration (MIC). Effect of AgNPs on growth kinetic of bacterial strain was evaluated in 100 mL sterilized nutrient broth containing 1 ppm of AgNPs and inoculated with one mL bacterial strain (106 CFU/mL). The media was incubated at 30 °C at 150 rpm. Control without AgNPs was incubated under the same conditions. The flask content (0.2 mL) was withdrawn at a regular interval of 2 h for 36 h and optical density of the sample was measured at 560 nm to obtained growth curve. To determine the minimum inhibitory concentrations (MICs) for all three bacteria against AgNPs, antibiotic (Ciprofloxacin) and AgNPs-Antibiotic combination, nutrient broth was added with different concentration of AgNPs (0.5–10 ppm) and antibiotic(0.5–10 ppm), followed by bacterial inoculation. To determine the combined effects AgNPs-Ciprofloxacin different concentration of AgNPs and antibiotic ranging from 0.5 to 10 ppm was used in ratio of 1:1. All the experiment was performed on 96-well titre plate which was incubated at 30 °C after inoculation. The plate was evaluated visually for biofilm formation after 36 h. Minimum inhibitory concentration was expressed as the lowest AgNPs concentration at which no visible growth was observed.
3 Results and discussion
3.1 Extracellular synthesis of sliver nanoparticles
When filtrate was mixed with AgNO3 solution (1 mM), the colour of the reaction mixture turned from white to dark brown. No change in colour was observed in control flasks containing only filtrate and the other containing milli-Q water (9 mL) and AgNO3(1 mM). Spectral analysis was done at a regular interval for 10 min and results are illustrated in Fig. 1. The reduction of Ag+1 to Ag0 in the reaction mixture cause colour change in reaction mixture. The maximum absorbance at 450 nm was observed in UV spectra analysis which is characteristic surface plasmon resonance peak of fungus assisted AgNps. As shown in Fig. 1, a clear hike in UV–vis peak with time at a wavelength of 450 nm indicates the continuous production of the AgNPs. It was also observed that the peak becomes narrow after 30 min in comparison to 10 min reading, highlighting the formation of monodispersed AgNps formation.
3.2 Characterization of AgNPs
Figure 2 represent the TEM micrographs for the AgNPs synthesized from the fungal filtrate. Different shapes of the AgNPs such as triangular, circular, hexagonal etc. with size ranging from 10 to 50 nm were observed in the TEM micrograph. The size and the shape of the nanoparticle can be further optimized by varying the concentration of filtrate and AgNO3 in the reaction mixture, incubation time, pH, aeration, salt concentration, type of method used and temperature of the reaction mixture [32]. The shape of the AgNPs significantly influences its antimicrobial properties. Pal et al. [33] investigated the effects of differently shaped AgNPs against gram negative Escherichia coli. It was concluded that triangular shaped AgNPs were more effective as compared to circular and rod shape nanoparticles [33]. In another study [34] similar high antimicrobial activity against Escherichia coli, was observed with triangular AgNPs (chemically synthesised) as compared to spherical AgNPs. Further, elemental analysis of AgNPs was confirmed by Energy dispersive spectrometer (EDS) micrograph. Presence of peaks at approximately 3 keV in Energy dispersive spectrometer micrograph confirmed the presence of AgNPs. In addition to this, significant peak of carbon and copper were also appeared in the Energy dispersive spectrometer micrograph. Moreover, morphological features of fungus assisted nanoparticles was also visualised in scanning electron micrograph which showed variable shaped and sized nanoparticles with few clump formations as shown in Fig. 3a. Further, in order to study the crystalline nature of developed nanoparticles, XRD was performed, highlighting significant peaks at (111), (200), (220), (311), (420) (Fig. 3b) confirming face centered cubic (fcc) structures of nanoparticles as per the Joint Committee on Powder Diffraction Standards (JCPDS) file no: ICDD-PDF2, PA,USA, 2007.
3.3 Zeta potential of AgNPs
The zeta potential value of − 22 mV with a single and sharp peak was observed for AgNPs (Fig. 4). It was observed that negative charge exits between the biologically synthesized AgNPs. The negative or positive charge of AgNPs suspension, cause repulsion of AgNPs and reduce their ability or tendency to form aggregate, proving the stability of AgNPs. The negative charge of AgNPs might be due to the polyphenolic components released by the fungus B. bassiana in extract used for AgNPs synthesis. Polyphenolic compounds are released as secondary metabolites by the multiple fungus during growth [31, 35]. Polyphenolic components take part in capping during AgNPs synthesis [36]. Similar negative value of − 17.19 mV was observed for AgNPs synthesized from fungus Trichoderma harzianium [37]. In another study by Prakasham et al. [38], a negative zeta potential of − 8.5 mV was observed for nanoparticles synthesized using Streptomyces albidoflavis.
3.4 Optimization of physio-chemical parameters for AgNPs synthesis
It was observed that maximum synthesis of AgNPs take place at 30 °C followed by 25 °C and 35 °C (Fig. 5). Further, it was examined that synthesis efficiency was reduced by 57.8% and 39.06% at 15 °C and 40 °C, respectively. Similar reduction in synthesis efficiency at higher temperature was reported by Dhanaraj et al. [17]. It was observed that optimal pH for AgNPs synthesis was 6, same as the pH of the fungal supernatant (Fig. 5). Further, up to 66.9% reduction in biosynthesis of AgNPs was observed with acidic supernatant. The possible reason of low conversion efficiency at acidic pH is the slow rate of nucleation process for the formation of nano crystal leading to large nanoparticles [39].
3.5 Antimicrobial activity of AgNPs (MIC and growth kinetic)
Figure 6 illustrate the growth kinetic of microbes Escherichia coli (gram negative), Pseudomonas aeruginosa (gram negative) and Staphylococcus aureus (gram positive) in control (C) and in presence of AgNPs. The growth curve clearly indicates that AgNPs have inhibitory effect on the growth of each microbe investigated during the experiments. It was observed that lag phase for all three microbes increase from 3–4 h (in control) to 7–8 h (in presence of AgNPs). Maximum reduction in optical density at 560 nm was observed for Escherichia coli (67.2%) followed by Pseudomonas aeruginosa (63.3%) and Staphylococcus aureus (56.8%), during 36 h of growth period at 30 °C. These finding clearly indicates that the presence of AgNPs in the growth media deter the reproductive capability of microbes. Low reduction of growth in case of gram positive bacteria might be due to the presence of thick peptidoglycan layer [40]. Moreover, presence of negatively charged teichoic acids in peptidoglycan layer may result in repulsion of AgNPs, hence reducing the overall toxicity of the synthesized AgNPs against Staphylococcus aureus. A clear increase in antimicrobial potential of nanoparticles was recorded in different time scale studies, highlighting the continuous interactions of fungal assisted AgNPs with selected microbes. Chauhan et al. [41] observed various events of bacterial-AgNps interactions i.e., integration of AgNps with bacterial membrane, membrane disintegration and finally complete damage of bacterial cells.
Further, maximum MIC value for AgNPs by Staphylococcus aureus indicate the resistance provided by the peptidoglycan layer and teichoic acid [40]. The MIC value for antibiotic was found to be 0.5, 0.5 and 0.7 ppm for Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus, respectively (Table 1). Further, it was observed that when AgNPs was used along with antibiotic, a very low concentration was able to inhibit the total growth of each bacterium. The MIC value for AgNPs-Ciprofloxacin conjugate was found to be 0.4, 0.4 and 0.5 ppm for Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus, respectively, showing the synergistic effect of AgNPs and antibiotic when present simultaneously. Salar et al [42], observed the similar synergistic effect of AgNPs-Streptomycin combination against Staphylococcus sp. with 18.37% increase in overall activity when present in combination as compared to antibiotic alone. Further, Smekalova et al. [43] indicate additive, synergistic and indifferent effect of AgNPs-antibiotic combination. For example, AgNPs along with amoxycillin produce additive, indifferent and synergistic effect against S. aureus, E. coli and Actinobacillus pleuropneumoniae, respectively. Whereas similar AgNPs were showed synergistic effect with gentamycin against S. aureus, E. coli and Actinobacillus pleuropneumoniae. Hence, an increase in the potency of AgNPs was observed, if used in combination with antibiotics highlighting synergistic or additive effects depending on the type of antibiotic as well as type of bacterial pathogen used in the experiments.
4 Conclusion
The present study clearly demonstrated the potential of selected Entomopathogenic fungus, B. bassiana for development of extracellular silver nanoparticles (AgNPs). Various analytical tools were used for characterisation of synthesised AgNPs i.e., UV–vis spectrophotometer, Zeta-DLS, TEM, SEM, XRD etc. The techniques used for characterisation showed synthesis of AgNPs in fungal supernatant with variable shapes (triangular, spherical, hexagonal) and size range of 10–50 nm, the synthesized nanoparticles were found to be stable under fungal solution (Zeta: − 22 mV). The fungal assisted AgNPs showed time dependent increase in antimicrobial efficacy against S. aureus, P. aeruginosa and E. Coli, moreover synergistic effect was also observed with antibiotic, Ciprofloxacin against all the bacterial strains. Further, as development of AgNPs was performed with eco-friendly approach, it can be utilized in various sectors where safety is the primary concern i.e., environment and healthcare.
References
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomed Nanotechnol Biol Med 6:257–262
Sanguiñedo P, María Fratila R, Belén Estevez M, Martínez De La Fuente J, Grazú V, Alborés S, Biosynthesis E (2018) Iss. 2 of silver nanoparticles using fungi and their antibacterial activity. Nano Biomed Eng 10:165–173
Lü T, Qi D, Zhang D, Zhang C, Zhao H (2019) Correction to: one-step synthesis of versatile magnetic nanoparticles for efficiently removing emulsified oil droplets and cationic and anionic heavy metal ions from the aqueous environment. Environ Sci Pollut Res 26:23207
Gandhi AD, Murugan K, Umamahesh K, Babujanarthanam R, Kavitha P, Selvi A (2019) Lichen Parmelia sulcata mediated synthesis of gold nanoparticles: an eco-friendly tool against Anopheles stephensi and Aedes aegypti. Environ Sci Pollut Res 26:23886–23898
Nagarajan D, Venkatanarasimhan S (2019) Copper(II) oxide nanoparticles coated cellulose sponge—an effective heterogeneous catalyst for the reduction of toxic organic dyes. Environ Sci Pollut Res 26:22958–22970
Zaki SA, Eltarahony MM, Abd-El-Haleem DA (2019) Disinfection of water and wastewater by biosynthesized magnetite and zerovalent iron nanoparticles via NAP-NAR enzymes of Proteus mirabilis 10B. Environ Sci Pollut Res 26:23661–23678
Mazumder JA, Perwez M, Noori R, Sardar M (2019) Development of sustainable and reusable silver nanoparticle-coated glass for the treatment of contaminated water. Environ Sci Pollut Res 26:23070–23081
Venkatesan EP, Kandhasamy A, Sivalingam A, Kumar AS, Ramalingam K, Joshua PJT, Balasubramanian D (2019) Performance and emission reduction characteristics of cerium oxide nanoparticle-water emulsion biofuel in diesel engine with modified coated piston. Environ Sci Pollut Res 26:1–10
Abd El-Maksoud EM, Lebda MA, Hashem AE, Taha NM, Kamel MA (2019) Ginkgo biloba mitigates silver nanoparticles-induced hepatotoxicity in Wistar rats via improvement of mitochondrial biogenesis and antioxidant status. Environ Sci Pollut Res 26:1–11
Bhowmik PN, Barman P, Ahmed MA (2019) Iron-polyphenol complex nanoparticles for removal of greenhouse gas emission from bitumen and formation of paraffins. Environ Sci Pollut Res 26:1–8
Bhainsa KC, D’Souza SF (2006) Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B Biointerfaces 47:160–164
Sharma D, Kanchi S, Bisetty K (2015) Biogenic synthesis of nanoparticles: a review. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.11.002
Kuppusamy P, Yusoff MM, Maniam GP, Govindan N (2016) Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications: an updated report. Saudi Pharm J 24:473–484
Agarwal H, Venkat Kumar S, Rajeshkumar S (2017) A review on green synthesis of zinc oxide nanoparticles: an eco-friendly approach. Resour Technol 3:406–413
Ahmed MS, Soundhararajan R, Akther T, Kashif M, Khan J, Waseem M, Srinivasan H (2019) Biogenic AgNPs synthesized via endophytic bacteria and its biological applications. Environ Sci Pollut Res 26:1–8
Molnár Z, Bódai V, Szakacs G, Erdélyi B, Fogarassy Z, Sáfrán G, Varga T, Kónya Z, Tóth-Szeles E, Szűcs R, Lagzi I (2018) Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci Rep 8:3943
Dhanaraj S, Bharathiraja P, Dhandapani R, Subbaiya R, Kathireshan AK (2018) Biosynthesis and characterization of silver nanoparticles from Aspergillus niger and its antibacterial activity. Res J Pharm Technol 11:5282–5286
Xue B, He D, Gao S, Wang D, Yokoyama K, Wang L (2016) Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal activity against genera of Candida, Aspergillus and Fusarium. Int J Nanomed 11:1899–1906
Paul NS, Yadav RP (2015) Biosynthesis of silver nanoparticles using plant seeds and their antimicrobial activity. Asian J Biomed Pharm Sci 5:26
Popli D, Anil V, Subramanyam AB, Rao SN, Rai RV, Govindappa M (2018) Endophyte fungi, Cladosporium species-mediated synthesis of silver nanoparticles possessing in vitro antioxidant, anti-diabetic and anti-Alzheimer activity. Artif Cells Nanomed Biotechnol 46:676–683
Saravanan M, Arokiyaraj S, Lakshmi T, Pugazhendhi A (2018) Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb Pathog 117:68–72
Arisha AH, Ahmed MM, Kamel MA, Attia YA, Hussein MMA (2019) Morin ameliorates the testicular apoptosis, oxidative stress, and impact on blood-testis barrier induced by photo-extracellularly synthesized silver nanoparticles. Environ Sci Pollut Res 117:1–14
Barbosa AC, Silva LP, Ferraz CM, Tobias FL, de Araújo JV, Loureiro B, Braga GM, Veloso FB, de Freitas Soares FE, Fronza M, Braga FR (2019) Nematicidal activity of silver nanoparticles from the fungus Duddingtonia flagrans. Int J Nanomed 14:2341–2348
Neethu S, Midhun SJ, Radhakrishnan EK, Jyothis M (2018) Green synthesized silver nanoparticles by marine endophytic fungus Penicillium polonicum and its antibacterial efficacy against biofilm forming, multidrug-resistant Acinetobacter baumanii. Microb Pathog 116:263–272
Verma VC, Kharwar RN, Gange AC (2010) Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine 5:33–40
Amerasan D, Nataraj T, Murugan K, Panneerselvam C, Madhiyazhagan P, Nicoletti M, Benelli G (2016) Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: Culicidae). J Pest Sci 89:249–256
Soni N, Prakash S (2012) Efficacy of fungus mediated silver and gold nanoparticles against Aedes aegypti larvae. Parasitol Res 110:175–184
Banu AN, Balasubramanian C (2014) Myco-synthesis of silver nanoparticles using Beauveria bassiana against dengue vector, Aedes aegypti (Diptera: Culicidae). Parasitol Res 113:2869–2877
Prabakaran K, Ragavendran C, Natarajan D (2016) Mycosynthesis of silver nanoparticles from Beauveria bassiana and its larvicidal, antibacterial, and cytotoxic effect on human cervical cancer (HeLa) cells. RSC Adv 6:44972–44986
Gola D, Malik A, Namburath M, Ahammad SZ (2018) Removal of industrial dyes and heavy metals by Beauveria bassiana: FTIR, SEM, TEM and AFM investigations with Pb(II). Environ Sci Pollut Res 25:20486–20496
Gola D, Dey P, Bhattacharya A, Mishra A, Malik A, Namburath M, Ahammad SZ (2016) Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Bioresour Technol 218:388–396
Singh P, Kim YJ, Zhang D, Yang DC (2016) Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol 34:588–599
Pal S, Tak YK, Song JM (2015) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. J Biol Chem 290:1712–1720
Van Dong P, Ha C, Binh L, Kasbohm J (2012) Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int Nano Lett 2:9
Lu Z, Zhu H, Fu P, Wang Y, Zhang Z, Lin H, Liu P, Zhuang Y, Hong K, Zhu W (2010) Cytotoxic polyphenols from the marine-derived fungus Penicillium expansum. J Nat Prod 73:911–914
Vivek R, Thangam R, Muthuchelian K, Gunasekaran P, Kaveri K, Kannan S (2012) Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem 47:2405–2410
Ahluwalia V, Kumar J, Sisodia R, Shakil NA, Walia S (2014) Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind Crops Prod 55:202–206
Prakasham RS, Kumar BS, Kumar YS, Shankar GG (2012) Characterization of silver nanoparticles synthesized by using marine isolate Streptomyces albidoflavus. J Microbiol Biotechnol 22:614–621
Chitra K, Annadurai G (2014) Antibacterial activity of pH-dependent biosynthesized silver nanoparticles against clinical pathogen. Biomed Res Int 2014:725165
Kim SH, Lee HS, Ryu DS, Choi SJ, Lee DS (2011) Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J Microbiol Biotechnol 39:77–85
Chauhan N, Tyagi AK, Kumar P, Malik A (2016) Antibacterial potential of Jatropha curcas synthesized silver nanoparticles against food borne pathogens. Front Microbiol 7:1748
Salar RK, Sharma P, Kumar N (2015) Enhanced antibacterial activity of streptomycin against some human pathogens using green synthesized silver nanoparticles. Resour Technol 1:106–115
Panáček A, Smékalová M, Večeřová R, Bogdanová K, Röderová M, Kolář M, Kilianová M, Hradilová Š, Froning JP, Havrdová M, Prucek R, Zbořil R, Kvítek L (2016) Silver nanoparticles strongly enhance and restore bactericidal activity of inactive antibiotics against multiresistant Enterobacteriaceae. Colloids Surf B Biointerfaces 142:392–399
Acknowledgements
Authors gratefully acknowledge CST, Uttar Pradesh [Grant no: CST/8276 (young scientist scheme) and CST/1586] for funds. We are thankful to the Department of Textile Engineering (IIT Delhi) and CRF facility (IIT Delhi) for their technical support in EDS and TEM analysis. Guidance received by Prof. Anushree Malik, CRDT, IIT Delhi is acknowledgeable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tyagi, S., Tyagi, P.K., Gola, D. et al. Extracellular synthesis of silver nanoparticles using entomopathogenic fungus: characterization and antibacterial potential. SN Appl. Sci. 1, 1545 (2019). https://doi.org/10.1007/s42452-019-1593-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1593-y