Abstract
The results of microstructural examinations of reaction products formed at liquid copper/graphene-coated monocrystalline SiC interface are presented. Samples were prepared during a wettability test performed under a vacuum at T = 1100 °C kept constant for 30 min by capillary purification-sessile drop method. Careful analyses of the microstructure and chemical composition were carried out at the interfaces by high resolution scanning electron microscopy combined with local analysis of chemical composition, Raman spectroscopy and computed tomography. The detailed structural investigations showed that in both systems, at the drop/substrate interface, the substrate (SiC) was dissolved and the zone of reaction products was composed of alternately arranged dark and bright layers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The production of composites (MMCs) reinforced by a two-dimensional phase, e.g. graphene is very challenging. The intercalation of graphene as reinforcement via liquid-assisted processes but without using alloying additions is one of the alternatives more considered mainly in view of the phenomenon known in the literature under the name of “wetting transparency of graphene”. This phenomenon, already observed and described for low-temperature liquids like water and aqueous solutions, consists in the fact that the value of the contact angle between the liquid and the graphene-coated substrate material is the same as for the uncoated substrate [1,2,3]. The first attempt to experimentally investigate the wettability of a graphene-coated metal substrate (Cu) by liquid tin (Sn/CGn/Cu) [4] could not provide clear evidences for the existence of the phenomenon of “wetting transparency of graphene” because, despite significant inhibition of wetting kinetics in the system, strong chemical interactions and formation of intermetallic phases (Cu3Sn, Cu5Sn6) at the phase boundary between Cu and Sn were observed. Specifically, examinations at the Sn/CGn/Cu interface microstructure clearly put in evidence diffusion phenomena of Sn atoms allowed by discontinuities in the graphene layer, mainly produced by thermos-mechanical stresses at high temperature (stresses). The second attempt to experimentally investigate the “wetting transparency of graphene” was reported by Homa et al. [5] for the Cu/CGn/SiCsc (SiC singlecrystal) system. In particular, the behavior of a liquid Cu drop just dispensed on the surface of a monocrystalline SiC substrate coated by graphene (CGn/SiCsc), was similar on CGn/SiCsc and SiCsc substrates: several attempts to detach the drop of Cu from the CGn/SiCsc and SiCs substrates by raising the graphite capillary showed strong adhesion of the droplet to the substrate. Similar behavior of Cu drop occurred in the case of on CGn/SiCsc and SiCsc substrates: several attempts to detach the drop of Cu from the CGn/SiCsc and SiCsc substrates by raising the graphite capillary showed strong adhesion of the droplet to the substrate. On the contrary, during the interaction between liquid Cu drop and graphite substrate the Cu drop was easily detached and no reaction products or combinations of these products were detected. The results of structural analyses suggested that the presence of discontinuities in the graphene layer provoked by thermo-mechanical stresses occurred at high temperature, inhibit, but does not completely prevent, the chemical interaction between the liquid droplet of Cu and SiCsc. This effect was more pronounced for the Cu drops which were deposited on the CGn/SiCsc substrate, structurally and chemically changed by previous contact with liquid Cu [5]. The behavior of the Cu drop in few attempts to detach it from the graphene-coated SiC substrate by rising up a graphite capillary showed a strong adhesion of the liquid Cu to the SiC. Similar behavior of the Cu drop took place with graphene-free SiC substrate while with graphite substrate, the Cu drop was easily detached without raising the substrate. These observations can be considered as indirect experimental evidence of the wetting transparency of graphene layer for liquid Cu.
Based on structural and chemical composition examinations, the paper attempts to interpret the phenomena observed during wetting tests in the Cu/CGn/SiCsc system [5].
2 Preparation of Cu/SiCsc and Cu/CGn/SiCsc Samples and microstructural analyses
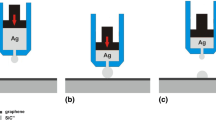
The Cu/SiCsc and Cu/CGn/SiCsc systems were produced during high temperature wettability tests by squeezing out from a capillary a liquid Cu drop at T = 1100 °C, subsequently deposited onto SiCsc and CGn/SiCsc substrate. The experimental set-up and procedure are detailed elsewhere [5], as well as the contact angle behaviors as a function of time observed within wettability tests [5].
Analyses of the microstructure and chemical composition of solidified systems and reaction products formed at the metal/substrate interfaces were carried out using:
Scanning Electron Microscopy (SEM) with local Energy Dispersive X-ray Spectrometry (EDS) analysis (FEI Scios FEG and TM3000 SEM by HITACHI equipped with an EDS by Quantax 70),
Raman Spectroscopy analysis (NTEGRA SPECTRA NT-MDT Europe BV stand in atmospheric air at 25 °C, laser wavelength of 473 nm, power of 50 MW, spot diameter ~ 1 mm, scanning time of individual test point was 30 s,
Computed Tomography (Nanotom 180). CT examinations were performed with the following exposure parameters: voltage—90 kV, current—170 μA, voxel size—4.0 μm/vox. Computer processing and data analysis was carried out in a specialized datos/x-reconstruction using VGStudio Max 2.0 software.
3 Results and discussion
Here the wetting experiment performed on Cu/CGn/SiCsc at T = 1100 °C is summarized. In Fig. 1, the time sequence of the wetting experiment recorded by a HSHR camera is shown. In particular, two samples (from two different freshly squeezed Cu drops with almost identical volume) were produced. The contact angles behaviours as a function of time are described in detail in [5].
After deposition of the first Cu drop on to CGn/SiCsc no wettable behavior was observed and due to the weak adhesion, the drop rolled on the surface and finally settling on the edge of the substrate (Fig. 1a). After 0.15 s from drop deposition, in the place where the first drop touched the substrate, a second Cu drop was squeezed and gently deposited (Fig. 1b) to form the second Cu2nd drop/CGn/SiCsc test system. The second drop was stable and did not change its position during the test. Both the two samples produced were kept under isothermal conditions for 30 min (Fig. 1c), then cooled.
Despite apparently identical test conditions, SEM and CT examinations perfumed on the two solidified Cu/CGn/SiCsc systems (Fig. 2) showed differences in the shape and size of metal drops (Fig. 2a, b). The first drop, with an oval shape, and at the triple line, a black oval “ring” was visible (Fig. 2a). On the contrary, the second drop deposited was spherical-shaped and “seated” on a pyramid with hexagonal base (Fig. 2b), progressively growing up within the experiment (Fig. 1b, c). At the top of the surfaces both drops, few black precipitates, randomly segregated, were observed by SEM/EDS analyses (Fig. 2a, b). By CT- analysis performed on x and z planes (Fig. 2c, d) the presence of a crater formed in the substrate, was revealed for both samples. In addition, carefully analyzing the craters, a two-phase zone of reaction products (bright and dark stripes) was lamellar-structured. The thickness of the reaction layer (~ 0.4 mm) formed under the first drop was irregular. Contrarily, the reaction layer grew up under the second drop was thicker (1 mm) and regular. (Figure 2c, d). In the near-surface area of both drops, black precipitates are visible in the CT images.
In Fig. 3, higher magnified SEM observations focused on both the reaction layers are shown. In both systems, the reaction zone consists in lamellar ranged (bright and dark) layers. Furthermore while in the case of the Cu1st drop/CGn/SiCsc system, the reaction products are flat “ring” shaped (Fig. 3a), in the case of the Cu2nd drop/CGn/SiCsc system, the zone reached a height of about 800 μm (Fig. 3b).
Detailed examinations by SEM/EDS on the two cross-sectioned samples are presented in Figs. 4 and 5. The SEM/EDS results confirmed that in both systems at the drop/substrate interface, the substrate (SiC) was dissolved and the zone of reaction products was composed of alternately arranged dark and bright layers (thickness ≈ 50 μm) (Figs. 4b, 5b). In particular, the dark layer consists of C-precipitates with an average size 2 μm, dispersed in a Cu-Si alloy. By EDS analyses, the chemical composition was 80.5% C, 17.8% Cu, 1.7 Si (Fig. 4c) and 57.8% C, 39.9% Cu, 2.3 Si (Fig. 5c) for the second drop for Cu1st drop/CGn/SiCsc and Cu2nd drop/CGn/SiCsc systems respectively. Due to the limitation of the EDS method, the results obtained show only the tendency to changes in the carbon content. In the bright single-phase layer a Cu-rich Si alloy was detected with dispersed C (Figs. 4c, 5c).
In Fig. 6, high resolution SEM analyses (150,000 ×) performed on the black precipitates are presented.
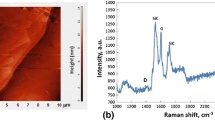
C-flakes forming “cones” were revealed with an average size of 1 μm (Fig. 6). Voids between C-flakes precipitate/Cu-Si phase were observed. By Raman spectroscopy performed on the surface of black ring in the Cu1st drop/CGn/SiCsc reaction layer the characteristic spectrum of graphene was revealed (Fig. 7) with well-distinguished D (1375 cm−1), G (1610 cm−1) and 2D (2727 cm−1) peaks [6, 7].
The results of structural examinations carried out on the Cu/CGn/SiCsc system confirmed the conclusions drawn from earlier wettability tests [5], according to which the presence of graphene layered on the SiC substrate inhibited but not completely prevented the chemical interaction between liquid Cu drop and substrate. The appearance of discontinuities (by thermos-mechanical stress) in graphene changes the unreactive Cu/CGn/SiCsc system into the reactive Cu/SiC system. In the examined systems this was evidenced by: (1) differences in the shape of the zone of reaction products and (2) differences in the thickness of the zone of reaction products. In the case of the Cu2nd drop/CGn/SiCsc system, the characteristic hexagonal shape of the zone of reaction products (Fig. 1b) previously observed by [8] was the result of a direct interaction between liquid Cu and α-SiC monocrystal of the (0001) orientation. Thus, at the point of contact between the liquid Cu drop and the surface of a monocrystalline substrate, there was not effective barrier against diffusion of the reacting phases (Cu and SiC). In the case of the Cu1st drop/CGn/SiCsc system, the final microstructure of reaction layers (irregular in shape and thickness) reveals occurring diffusion phenomena, but the kinetics was limited by the presence of graphene at the Cu/SiC interface. The presence of area of preserved primary graphene might be the explanation of the different values in the right (edge of the substrate) and left contact angles (affected by the drop rolling after the drop deposition) value observed within the experiments [5] and the difference in the thickness of reaction layer/crater grew up under the drop. The amount of liquid Cu penetrating the few discontinuities in the graphene in 30 min, was not enough to confer at the growing reaction product a “perfect” hexagonal shape. In both examined systems, the mechanism of the formation of the reaction product layer was similar and could be explained with the help of a binary Cu-Si phase diagram and a ternary Cu–Si–C phase diagram [9].
At the test temperature of 1100 °C, in the liquid state, Si and Cu exhibit an unlimited solubility with respect to each other, and therefore the single crystal of SiC is unstable in contact with liquid Cu. Due to the strong interaction at the Cu/SiCsc interface, SiC is dissolved promoting the formation a Cu-Si alloy and free C. For both tested systems, however, the holding time (30 min) was sufficient to generate the formation of a layered zone of reaction products (alternately arranged bright and dark layers). It means that the holding time of 30 min was sufficient to achieve thermodynamic equilibrium among the phases. According to Rado et al. [9], the mechanism of the formation of such layers in the Cu/SiC system is a multi-step process involving: (1) dissolution of SiC by Cu to form Cu-Si; (2) precipitation of a layer of C at the Cu/SiC interface; (3) separation of the layer of C from the Cu/SiC phase boundary (critical thickness reached and stress accumulation); (4) Cu reaction with pure SiC substrate.
The examined systems displayed differences in the thickness and shape of reaction product layer. In the Cu1st drop/CGn/SiCsc system, the reaction layer was thinner than in the Cu2nd drop/CGn/SiC system. This phenomenon was related to the presence of graphene on the surface of SiC. Liquid Cu did not have free access to the substrate, and its penetration occurred locally through discontinuities in graphene, as also indicated by the inhomogeneous thickness of the zone of reaction products (Fig. 1c) and its flat ring-like shape. The scheme of interaction in the Cu1st drop/CGn/SiCsc system is shown in Fig. 8.
Analysis of Raman spectra carried out in the zone of reaction products, in particular on single cone-shaped carbon precipitates, already observed by Rodo et al. [9] clearly revealed the presence of graphene. At the interface of the C/Cu-Si system, the discontinuities and voids observed provide a possible explanation to the decrease in thermal conductivity observed in Cu/SiC composites [9], so far attributed to the presence of Si in Cu. The absence of an interface between these two dissimilar and well distinguished phases (not connected) of C-phase metal matrix (i.e. “graphene cone”/Cu-Si interface) observed in this study is probably limits the transport of electrons, and despite the fact that graphene according to the literature has a thermal conductivity of 4000 W/mK [10], its presence in Cu-SiC systems can not compensate for the influence of Si.
4 Conclusion
The results of microstructural examinations of reaction products formed at liquid copper/graphene-coated monocrystalline SiC interface showed that the presence of graphene layered on the SiC substrate inhibited but not completely prevented the chemical interaction between liquid Cu drop and substrate. The appearance of discontinuities (by thermos-mechanical stress) in graphene changes the unreactive Cu/CGn/SiCsc system into the reactive Cu/SiC system. The mechanism of the formation of product layers can be described: (1) dissolution of SiC by Cu to form Cu-Si; (2) precipitation of a layer of C at the Cu/SiC interface; (3) separation of the layer of C from the Cu/SiC phase boundary (critical thickness reached and stress accumulation); (4) Cu reaction with pure SiC substrate.
References
Rafiee J, Rafiee MA, Yu ZZ, Koratkar N (2010) Superhydrophobic to superhydrophilic wetting control in graphene films. Adv Mater 22:2151–2154
Rafiee J, Mi X, Gullapalli H, Thomas AV, Yavari F, Shi Y, Ajayan PM, Koratkar NA (2012) Wetting transparency of graphene. Nat Mater 11:217–222
Du F, Huang J, Duan H, Xiong C, Wang J (2018) Wetting transparency of supported graphene is regulated by polarities of liquids and substrates. Appl Surf Sci 45:249–255
Sobczak N, Sobczak JJ, Kudyba A, Homa M, Bruzda G, Grobelny M, Kalisz M, Strobl K, Singhal R, Monville M (2014) Wetting transparency of graphene deposited on copper in contact with liquid tin. Trans Found Res Inst LIV 3:3–11
Homa M, Sobczak N, Sobczak JJ, Kudyba A, Bruzda G, Nowak R, Giuranno D, Pietrzak K, Chmielewski M, Strupiński W (2018) Interaction between graphene-coated SiC single crystal and liquid copper. J Mater Perform 27(5):2317–2329
Gajewski K, Kopiec D, Moczała M, Piotrowicz A, Zielony M, Wielgoszewski G, Gotszalk T, Strupiński W (2015) Scanning probe microscopy investigations of the electrical properties of chemical vapor deposited graphene grown on a 6H-SiC substrate. Micron 68:17–22
Grodecki K (2013) Raman spectroscopy of graphene. Electron Mater 41(1):47
Nogi BK, Ogino K (1998) Characteristic wettability of SiC by liquid pure Cu. Trans Jpn Inst Met 29(10):805–811
Rado C, Drevet B, Eusthatopoulos N (2000) The role of compound formation in reactive wetting: the Cu/SiC system. Acta Mater 48:4483–4491
Balandin AA (2011) Thermal properties of graphene, carbon nanotubes and nanostructured carbon materials. Nat Mater 10:569–581
Acknowledgements
The authors are grateful to the National Centre for Research and Development for financial support (Project No. GRAF-TECH/NCBR/10/29/2013 “Novel graphene reinforced metal matrix composites for power and electronic industry”). The research was co-funded FRI (Poland) and UCF (USA) in frame of Polish-USA collaboration (Project No. 6010/00). Dr. Ł Boroń (structural characterization), Msc. I. Krzak (computed tomography) and Dr. R. Nowak (Raman spectra) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in connection with the works reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Homa, M. Microstructural characterization of reaction products in Cu/Graphene/SiC system. SN Appl. Sci. 1, 1584 (2019). https://doi.org/10.1007/s42452-019-1571-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1571-4