Abstract
Communities of benthic macroinvertebrates obtained from a river catchment impacted by point source pollution were investigated from March 2013 to April 2014. The main purpose of this study was to describe the taxon richness of benthic macroinvertebrate communities obtained at upstream and downstream of a wastewater treatment works and combined sewer overflows, assess water quality and to determine major physicochemical variables that affect the distribution and richness of benthic macroinvertebrate communities. The biotic index Whalley Hawkes, Paisley and Trigg revealed distinction between the sites upstream of the wastewater treatment works from the downstream sites. Between and within sampling locations, the dominance of certain taxonomic groups was recorded including Oligochaete, Baetidae and Chironomidae. Predominant in the river were insects which constituted 72% of the overall benthic invertebrate communities followed by Oligochaeta (28%). Distribution of benthic macroinvertebrate communities was not associated with sewage variables but related more with altitude, catchment area, slope and discharge. Altitudes range from 31 to 140 m. The sites at higher altitude had better quality compared to the lower sites and therefore suggests that altitude could provide a spatial proxy for anticipated future ecological changes in the river over time. Classification of the river based on the European Union’s Water Framework Directive indicated that the river had moderate ecological status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The relationship between an ecological metric and environmental variables may inform the source of stress or response experienced by an ecological indicator [1]. These relationships have been demonstrated between benthic macroinvertebrate communities and physicochemical variables in various studies [2,3,4]. In the case of urban rivers, the relationships could reveal the effect of multiple stressors collective termed “urban stream syndrome” [5] or by pollution. Environmental variables including conductivity, discharge, catchment area and nutrient concentrations have been shown to influence the structure and types of benthic macroinvertebrate communities found along a longitudinal stream profile with changes in altitude and slope [6, 7].
Macroinvertebrates have many benefits as good indicators of water quality because they are diverse, ubiquitous, easily collected, and sensitive to a range of environmental and chemical stressors. The Whalley, Hawkes, Paisley and Trigg (WHPT) which is the European Union’s Water Framework Directive (EU WFD) biotic index, described as one of the most well-developed biomonitoring tools in the world [8], was used to classify the study sites on the basis of pollution sensitive/tolerant benthic macroinvertebrate communities [9]. WHPT scores are classified on the basis of their occurrence (presence–absence) and assigned a log abundance-weighted score in one of four categories (1–9, 10–99, 100–999 and > 999 individuals). The ecological quality ratio (EQR) provides an EU WFD classification status for a site. In order to achieve the standard Good Ecological Status for surface waters, both biological and physicochemical variables measured would have to be “good”.
Previous records showed that this study catchment has not met the “Good Ecological Status” required by the European Union’s Water Framework Directive (UK Environment Agency records, 2017) especially due to high phosphate-P concentration [14]. Furthermore, previous studies focused on particular information including identification of seasonal changes of benthic macroinvertebrates [10], assessed the impact of combined sewer overflows on water chemistry [11] or described the challenges of point and diffuse pollution sources on the river in particular nutrient enrichment [12, 13]. The major challenge in the catchment was attributed to intermittent discharge from combined sewer overflows (CSOs) and effluent released from wastewater treatment works (WwTW). Negative public perception of CSOs helped to intensify the need for the UK Environmental Regulators (UK Environment Agency) to monitor and to effectively maintain the infrastructure through the waste water management companies (Irwell Catchment Partnership Group notes). Currently, efforts to reduce intermittent discharges from combined sewer overflows and effluent from wastewater treatment works are investigated by the UK water companies in order to reduce sewage pollution.
The present study examines the relationship between the benthic macroinvertebrate communities and physicochemical variables in order to determine the factors which influence the distribution and richness of the communities. Descriptive studies of this type are important because a major goal of community ecology is to explain why communities and environmental variables change systematically along a gradient including pollution. The objectives of the study were to characterise the distribution and assemblage structures of benthic macroinvertebrates between upstream and downstream of wastewater treatment works and combined sewer overflows over different altitudes ranging from 31 to 140 m, to assess and compare water quality results with the EU Water Framework Directive standards and to determine major physicochemical variables that affect the distribution of benthic macroinvertebrates. The results of this study could contribute to a better understanding of the distribution of freshwater macroinvertebrates in rivers impacted by combined sewer overflows and wastewater treatment works and to inform and focus areas in need of effective management.
2 Materials and methods
2.1 Study area
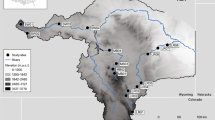
The River Medlock has a catchment area of 57.5 km2 and the average yearly flow rate is 0.82 m3 s−1 (CEH, 2017). The River Medlock (See Fig. 1) is 22 km in length and rises in the Pennine hills to the Northeast of Oldham in Greater Manchester (National Grid Reference (NGR): SD 95308 05431). It passes through a steep-sided wooded region for 10 km before entering a largely urbanised area of Manchester city centre (NGR: SJ 85781 97858).The surveyed reach of the river has a continuously operational wastewater treatment works (WwTW) at Failsworth (NGR: SJ 89674 99800), has about 30 combined sewer overflows and numerous storm water overflows (EA, personal communication 2013) and an unknown number of surface water drains.
Due to site access and sampling safety, five sites (S1–S5) along an altitude of 31–140 m were selected upstream and downstream of the major wastewater treatment plant and combined sewer overflows. The physical variable, altitude was used by the EU’s WFD standard as a proxy for nitrate-N concentration in European rivers and for intensity of land use. Sites S1 and S2 were classified as upland and higher altitudes (> 107–140 m) in the catchment and are located above the catchment’s major waste treatment works while sites S3–S5 are lowland and below the treatment works with altitudes ranging from 31 to 79 m (Table 1). The substrate characteristics at the river were similar but higher percentage was recorded for stones and sand between the study sites (Appendix Table 4).
2.2 Sampling benthic invertebrates data
Monthly samples were obtained at each study location in the catchment between March 2013 and April 2014 in order to obtain an accurate representation of total biodiversity [15] and to detect the impact of pollution. Samples were collected from each location with surface area 50 m2 by using a 1 mm mesh. In a 3-min kick net sampling [16], an additional 1-min visual search was carried out to collect benthic invertebrates under stones that could have been missed through kick sampling [17].
2.3 Physicochemical variables
Physicochemical variables including temperature, pH, Dissolved oxygen (DO), conductivity and spot samples were obtained each month for the duration of sampling. Spot samples obtained for suspended solids (mg/L); Biochemical Oxygen Demand (mg/L); NO3-N (nitrate-N), NH3-N (ammonia-N) and PO4-P (phosphate-P) were analyzed by using the Standard Committee of Analysts Publications [18]. pH, temperature (°C), dissolved oxygen (% saturation) and conductivity (µS cm−1) were obtained by using a pre-calibrated hand-held multiparameter water quality meter (YSi 556 Multi probe system YSI, Yellow Springs, Ohio, USA). River discharge (m3s−1) was determined by using the Area Ratio (AR) method [19]. Other physical variables including catchment area, altitude and slope were determined by information obtained from ArcGIS Geoprocessing toolbox [20].
2.4 Data analysis
Ordination of benthic macroinvertebrate community data was performed by using Non-Metric Multidimensional Scaling (NMDS) based on Bray–Curtis similarity index. The data was normalised by fourth-root transformation in order to down weigh the influence of dominant species. The stress value was used to assess the representations of NMDS solutions according to [21]. Thus, a stress value of < 0.05 gives an excellent representation with no misinterpretation. The discrimination between sites was tested using Analysis of Similarity (ANOSIM) test with R ranging from − 1 to + 1 at α = 0.05. Similarity Percentages (SIMPER) analysis, based on Bray–Curtis dissimilarity metric was used to calculate the contribution of individual species to similarity or dissimilarity between sites. Species-abundance data were log (x + 1) transformed prior to SIMPER analysis and the cut off for low contributions was placed at 90%. Physicochemical variables were analysed using the Principal Component Analysis (PCA) in order to determine the dominant variables across the study catchment with presentations under two principal components (PCs). The biological and environmental (BIOENV) analysis was used to determine which variable(s) affected benthic invertebrate communities’ abundance and distribution. BIOENV analysis was based on weighed Spearman rank correlation coefficient (ρ) between the physicochemical variables and benthic invertebrate communities. Both environmental and biological variables were transformed and normalised to allow comparison at the same scale. The rank with the largest ρ was taken to identify the best match with the benthic macroinvertebrate communities.
SIMPER analysis, NMDS, PCA and BIOENV were performed using Primer v 6 [21]. The pollution index Whalley Hawkes Paisley and Trigg [9] was used to determine pollution scores and variation between study sites. The river quality was classified under the EU WFD standards [22].
3 Results
3.1 Water quality status
All variables measured in the catchment indicated good status except biochemical oxygen demand (BOD), phosphate-P, nitrate-N and ammonia-N where higher concentrations were recorded at sites S3–S5. On the basis of the EU WFD requirement for these variables, the river was classified as “moderately polluted” (Table 1).
For benthic macroinvertebrates, repeated measures one-way Analysis Of Variance (ANOVA) test was carried out on Whalley Hawkes Paisley and Trigg (WHPT) scores at the river (Fig. 2). WHPT Average Score Per Taxon (WHPT ASPT) yielded a significant difference between study sites (F2.2, 24 = 4.6, p = 0.018) and the difference was shown to be at S1 (Post hoc Tukey). Observed results of WHPT Number of Taxa (WHPT NTAXA) and WHPT ASPT were compared with predicted (expected) pristine results for the study sites when not impacted by pollution to produce the Environmental Quality Ratio (EQR). The EQR for WHPT NTAXA and ASPT would be 0.8 and 1.0 respectively. In comparing the results of this river with EQR, Table 2 showed that the number of taxa present at S1 (with the highest altitude at the catchment) was “good”, “moderately impacted/polluted” at S2 and “Bad” at sites S3–S5 (lower altitudes). The results showed that S1 had the highest number of taxonomic groups. WHPT ASPT was “moderately impacted/polluted” at all sites except at S4 which was classified as “poor”. Therefore, on the basis of the EU WFD standard, both biological and physicochemical variables have not met the “Good Ecological Status” expected for surface water bodies.
3.2 Community of benthic macroinvertebrates
Sampling each of the five sites resulted in a total of 3753 benthic macroinvertebrate communities representing thirty-two families distributed across three phyla Annelida (worms and leeches), Mollusca (Gastropods and bivalves) and Arthropoda (insects and crustaceans). The dominant phyla was Arthropoda which contributed 23 invertebrate families (i.e. constituting 72% of the total benthic community composition including insects) followed by Annelida which made up 28% including the families of Lumbriculidae and Tubificidae.
NMDS ordination of the abundance data (Fig. 3) in two-dimensional space yielded a clear separation between the river’s study locations (stress value of 0.24). Sites S1 and S2 sat at the top of the biplot while sites S3 to S5 were found at the bottom. One-way nested ANOSIM yielded a significant difference between benthic communities at the study locations (global test R = 0.194, p = 0.001). Pairwise ANOSIM test indicated that the following sites were significantly different: S1 and S3 (global test RS1–S3 = 0.226, PS1–S3 = 0.001); S1 and S4 (global test RS1–S4 = 0.504, PS1, S4 = 0.001); S1 and S2 (global test RS1,S2 = − 0.014, PS1,S2 = 0.56); S1 and S5 (global test RS1, S5 = 0.273, PS1,S5 = 0.004); S2 and S3 (global test RS2,S3 = 0.147, PS2,S3 = 0.007); S2 and S4 (global test RS2,S4 = 0.349, PS2, S4 = 0.001); S2 and S5 (global test RS2, S5 = 0.093, PS2,S5 = 0.17); S3 and S4 (global test RS3, S4 = 0.212, PS3, S4 = 0.004) S3 and S5 (global test RS3, S5 = − 0.01, PS3, S5 = 0.52); S4 and S5 (global test RS4, S5 = 0.002, PS4, S5 = 0.45). The results showed that S1 was significantly (p > 0.05) different from other parts of the river.
In order to compare the results obtained from NMDS and to provide information on the taxa groups which caused the difference in abundance between study sites, the SIMPER test was applied to analyse benthic macroinvertebrate communities. SIMPER results (Appendix Table 5) showed the highest average dissimilarity (70.08%) to be found between sites S1 and S4 with the top five contributor families including Gammaridae (13.4%), Heptageniidae (10.5%), Chironomidae (10.1%), Baetidae (7.7%) and Lumbriculidae (6.4%). Sites S1 and S5 showed the second highest average dissimilarity (69.63%) with top five contributor families Heptageniidae (12.1%), Gammaridae (10.1), Chironomidae (9.95%), Baetidae (9.84%) and Tubificidae (9.34%). Within each site (Appendix Table 6), the highest similarity of benthic macroinvertebrate community was found at S4 (SIMPER average similarity = 45.1%) with top five contributor families Gammaridae (37.6%), Baetidae (36%), Chironomidae (9.6%), Erpobdellidae (4.5%) and Tipulidae (2.8%). S5 showed the second highest average similarity = 40.4% with top four contributor families including Baetidae (33.9%), Chironomidae (32.6%), Heptageniidae (19.2%) and Tubificidae (6.75%).
3.3 Physicochemical variables
Principal Component 1 (PC1) accounted for 34% of the overall variance and was most heavily weighted on catchment area, nitrate-N, phosphate-P, altitude and slope while PC 2 accounted for 18% of the variance dominated by substrates (Fig. 4a). In order to determine the physicochemical variables directly impacting on the river’s quality, a stepwise backward elimination of highly weighed catchment area, altitude, slope and river substrates was executed. The new PCI showed sites S1 and S2 had high dissolved oxygen (DO) levels and accounted for 26% overall variance while PC2 accounted for 23% of the overall variance and weighted upon discharge, ammonia-N and conductivity at sites S3–S5 (Fig. 4b). This result explains the difference between the sites upstream and downstream of the WwTW i.e. higher altitude (S1 and S2) and downstream, lower altitude (S3-S5).
Principal components of physicochemical variables at the sampling locations. a Variables include temperature, pH, dissolved oxygen, conductivity, nitrate-P, phosphate-P, BOD, suspended solids, ammonia-N, discharge, river substrates, altitude, slope and catchment area. b Variables include temperature, pH, dissolved oxygen, conductivity, nitrate-P, phosphate-P, BOD, suspended solids, ammonia-N and discharge
3.4 Relationship between physicochemical variables and benthic macroinvertebrate assemblages
Catchment area, altitude, conductivity and discharge were the variables that significantly correlated with the benthic macroinvertebrate communities with correlation coefficient (ρ) = 0.274 in BIOENV analysis (Table 3). As nitrate-N and phosphate-P concentrations were not indicated as the major influence on the benthic communities, the results suggest river discharge was the factor that would influence the transport of conductivity enhanced by increasing catchment area, reduced altitude and slope which remain unchanged at the study locations.
4 Discussion
4.1 Study sites and water quality
The sites were selected on the basis of proximity to pollution point sources including the waste water treatment works and the combined sewer overflows. In order to determine the quality status of the river, the results of the WHPT biotic indices and physicochemical variables were compared with the European Union’s water quality standards, the Water Framework Directive (WFD). Each component had “moderate ecological status” which indicated that the river has not met the expected “Good Ecological Status”. The outcome of the results showed a difference between the higher altitudes, upstream (S1 and S2) sites of the main wastewater treatment works and the lower altitude sites downstream (S3–S5). While high phosphate-P concentration has been reported by [13] be a challenge in the study catchment (United Utilities, personal communication, 2019), river classification based on the EU WFD indicators may not indicate other contributory factors such as discharge, slope, altitude and catchment area as shown by the biological and environmental analysis. Therefore, a framework which assesses the river catchment on a case by case basis could help in effective classification and catchment management in the local context [23].
4.2 Benthic macroinvertebrate communities
Abundance and diversity of benthic macroinvertebrate communities at each study location were investigated alongside other non-sewage indicators including catchment area, altitude, slope and river substrate. Due to geographical proximity (< 60 km2), benthic communities were found to be limited to a few families including Baetidae, Chironomidae, Gammaridae, Tubificidae, Lumbriculidae and Heptageniidae. This study showed that Ephemeroptera dominated the invertebrate composition mainly by Baetidae and though in limited abundance, Heptageniidae was recorded at the sites. This pattern was found in some Asian streams [3]; Leuctridae and Perlodidae were recorded for the presence of Plecoptera while Rhyacophilidae, Hydropsychidae and Limnephilidae composed the Trichoptera. The differences between sites were linked to differences in abundance rather than taxonomically different families. The dominance of Chironomidae and Tubificidae especially at the lower sections of this river have been recorded in the past [10]. These taxa groups have also been found to dominate some UK rivers [24,25,26] in response to organic and metal [27] pollution, and other parts of the world including Papua New Guinea streams [2]. While the WHPT scores (NTAXA and ASPT) indicated a significantly higher score at S1 and achieved good/moderate quality status respectively, the presence and abundance of pollution-tolerant Chironomidae, Tubificidae and Lumbriculidae suggest the impact of other stressors at this site. These taxa groups were also found in the lower altitude locations (S3–S5) and other studies have found them to dominate urban river systems [28, 29]. Therefore, the presence of moderately pollution-sensitive Gammaridae at the lower reaches indicates some improvement in water quality. The combined results from the water quality measurement showed that sites at higher altitude were better in quality as demonstrated by the WHPT scores.
4.3 Physicochemical variables and the community of benthic macroinvertebrates
Non-metric multidimensional scaling of the family-level dataset showed that differences in sample locations influenced the abundance of benthic communities. This was supported by the BIOENV results which indicated that altitude and slope both associated with sub-catchment areas. Also, differences between upstream (higher altitude) and downstream (lower altitude) locations were demonstrated by the higher physicochemical concentrations recorded at sites S3–S5. While this study showed differences in catchment quality for altitudes between 31 and 140 m, other studies showed differences at much higher altitudes e.g. [2, 4, 30, 31]. Lower altitude sites (S3–S5) had higher levels of conductivity, nutrients, ammonia-N and BOD and therefore suggests the impact of increased land use at these sections [22]. The study area is part of the 3301 catchments designated as heavily modified water bodies in England and Wales due to modifications to the hydromorphological characteristics and classified as having Poor Ecological Potential [32].
Variables associated with altitude reveal local environmental factors which determine the structure and distribution of benthic macroinvertebrate communities [3]. A limited composition of benthic macroinvertebrates accounted for a combination of ultimate (altitude, catchment area and slope) and proximate (BOD, phosphate-P, conductivity and discharge) factors. These factors align with studies of benthic macroinvertebrates in Asia countries [3, 4].
Roy et al. [33] and Wang [34] found a relationship between conductivity and benthic macroinvertebrate communities to be related to contamination sources such as urban runoff, sewage outfalls and effluent from point sources. High conductivity levels is associated with high salt concentrations in the more urbanised sections which could impair water quality following increased nutrient enrichment [5]. Benthic macroinvertebrate communities were also shown to be structured by increasing river discharge. Intermittent discharges from combined sewer overflows influence water quality variables [35].
4.4 Nature of the river sampling and drawbacks
Sampling directly from combined sewer overflows could not be determined due to logistics and technical difficulty and this was considered one of the major drawbacks in this study especially as it could impact on benthic macroinvertebrates communities. While the physicochemical variables were correlated with benthic macroinvertebrates at the family levels, this study showed some similarities of families between the study sites. [2] indicated that identification of river benthic macroinvertebrates at species level showed stronger sensitivity to physicochemical variables and when applied on a large scale could also improve understanding of the ecological impacts on biological communities. While surface water sampling was carried out in this study, sampling of the sediment for nutrient concentration could inform the state of the river at conditions of varying flow rates.
5 Conclusion
The composition and distribution of benthic macroinvertebrates in this river catchment was influenced by non-sewage related variables including altitude, conductivity, discharge, catchment area and slope. While these variables do not form part of the EU WFD’s classification requirements for surface waters, they influenced the river’s non-achievement of “Good Ecological Status” for rivers such as the Medlock. The lower altitude sites had higher concentrations of physicochemical variables e.g. phosphorus, conductivity, BOD and ammonia-N, higher composition of benthic macroinvertebrates within sites thereby reducing the overall status of the river. The biotic index WHPT and physicochemical variables showed that sites located at the higher altitude had better water quality. A significant difference between the higher and lower altitudes suggests that selection of sampling points could be executed on the basis of physical variables such as altitude. The results also showed that there were other contributory factors which influenced benthic macroinvertebrate abundance and distribution. This study outcome implied that compliance with “Good ecological Status” for rivers such as this would involve effective management of physical conditions e.g. river flow and collaboration with interest groups. Furthermore, river classification could be addressed on a case by case basis especially if the objective of the strategy is to increase the diversity and composition of the biotic components.
References
Allan JD (2004) Landscapes and riverscapes: the influence of land use on stream ecosystems. Annu Rev Ecol 35:257–284
Dudgeon D (2012) Responses of benthic macroinvertebrate communities to altitude and geology in tributaries of the Sepik River (Papua New Guinea): the influence of taxonomic resolution on the detection of environmental gradients. Freshw Biol 57:1794–1812
Jun YC, Kim NY, Kim SH, Park YS, Kong DS, Hwang SJ (2016) Spatial distribution of benthic macroinvertebrate assemblages in relation to environmental variables in Korean nationwide streams. Water (Switzerland) 8(1):1–20
Kim JY, Kim PJ, Hwang SJ, Lee JK, Lee SW, Park CH, Moon JS, Kong DS (2017) Korean stream types based on benthic macroinvertebrate communities according to stream size and altitude. J Freshw Ecol 32(1):741–759
Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP (2005) The urban stream syndrome: current knowledge and the search for a cure. J North Am Benthol Soc 24(3):706
Baumgartner SD, Robinson CT (2017) Changes in macroinvertebrate trophic structure along a land-use gradient within a lowland stream network. Aquat Sci 79(2):407–418
Sandin L, Johnson R (2004) Local, landscape and regional factors structuring benthic macroinvertebrate assemblages in Swedish streams. Landsc Ecol 19(5):501–515
Wilkes MA, Mckenzie M, Murphy JF, Chadd RP (2017) Assessing the mechanistic basis for fine sediment biomonitoring: Inconsistencies among the literature, traits and indices. River Res Appl 33(10):1618–1629
Water Framework Directive – United Kingdom Advisory Group (WFD-UKTAG) (2014) UKTAG river assessment method benthic invertebrate fauna invertebrates (General degradation): Whalley, Hawkes, Paisley & Trigg (WHPT) metric in River Invertebrate Classification Tool (RICT). ISBN: 978-1-906934-49-1
Frost S, Chiu MTL, Thomas MP (1976) Seasonal changes of invertebrate populations in the polluted River Medlock. Environ Pollut 11(3):223–242
Rees A, White KN (1993) Impact of combined sewer overflows on the water quality of an urban watercourse. Regul Rivers Res Manag 8:83–94
Tyson JM, Foster V (1996) Valuing environmental benefits from river quality improvements. Water Sci Tech 33(9):285–293
James P, Atkinson S, Barlow D, Bates A, Comyn F, Duddy M, Dutton D, Fraser J, Horsfall W, Hothersall A, Hutchinson A, Lowry K, Moore A, Rothwell J, Schofiled M, Smith A, Surtees A, Taylor D, Tollitt B, Towers C, Tzoulas K, Whitaker G, Wright A, Causer K (2012) The Irwell catchment pilot: the rivers return. The Environment Agency, Warrington, UK
UK Technical Advisory Group on Water Framework Directive (UKTAG) (2013) Updated recommendations on phosphorus standards for rivers. River Basin Management (2015–2021) Final Report, UK. http://www.wfduk.org/sites/default/files/Media/Environmental%20standards/UKTAG%20Environmental%20Standards%20Phase%203%20Final%20Report%2004112013.pdf
Armitage PD, Hawczak A, Blackburn JH (2012) Tyre track pools and puddles: anthropogenic contributors to aquatic biodiversity. Limnologica 42(4):254–263
Metzeling L, Chessman B, Hardwick R, Wong V (2003) Rapid assessments of rivers using macroinvertebrates: the role experience, and comparison with quantitative methods. Hydrobiologia 510:39–52
Murray-Bligh JAD (1997) Procedure for collecting and analysing macroinvertebrate samples for RIVPACS. Joint publication by the Institute of Freshwater Ecology and the Environment Agency, pp 162. http://www.eu-star.at/pdf/RivpacsMacroinvertebrateSamplingProtocol.pdf
Environment Agency (2011) Standing Committee Of Analysts (SCA library): methods for the examination of waters and associated material—Index of methods for the examination of waters and associated materials 1976–2011 blue book 236. http://www.standingcommitteeofanalysts.co.uk
Archfield SA, Vogel RM (2010) Map correlation method: selection of a reference streamgage to estimate daily streamflow at ungaged catchments. Water Resour Res 46(10):1–15
Jenson SK, Dominque JO (1988) Extracting topographic structure from digital elevation data for geographic information system analysis. Photogram Eng Remote Sens 54(11):1593–1600
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E Ltd Plymouth Marine Laboratory, Plymouth, UK
WFD_UKTAG (2013) UKTAG WFD Environmental Standards River Basin Management 2015–2021. Wfd_Uktag, vol 2013, November 2013
Pinto U, Maheshwari B (2014) A framework for assessing river health in peri-urban landscapes. Ecohydrol Hydrobiol 14(2):121–131
Cao Y, Bark AW, Williams WP (1996) Measuring the responses of macroinvertebrate communities to water pollution: a comparison of multivariate approaches, biotic and diversity indices. Hydrobiologia 341(1):1–19
Wenn CL (2008) Do freshwater macroinvertebrates reflect water quality improvements following the removal of point source pollution from Spen Beck, West Yorkshire. Earth Environ 3:369–406
Nolan PA, Guthrie N (1998) River rehabilitation in an urban environment: examples from the Mersey Basin, North West England. Aquat Conserv Mar Freshw Ecosyst 8(5):685–700
Metcalfe JL (1989) Biological water quality assessment of running waters based on macroinvertebrate communities: history and present status in Europe. Environ Pollut 60(1–2):101–139
Minshall GW (1988) Stream ecosystem theory: a global perspective. J North Am Benthol Soc 7(4):263–288
Guimaraes RM, Facure KG, Pavanin LA, Jacobucci GB (2009) Water quality characterization of urban streams using benthic macroinvertebrate community metrics. Acta Limnol Bras 21(2):217–226
Jun Y-C, Kim N-Y, Kwon S-J, Han S-C, Hwang I-C, Park J-H, Won D-H, Byun M-S, Kong H-Y, Lee J-E, Hwang S-J (2011) Effects of land use on benthic macroinvertebrate communities: comparison of two mountain streams in Korea. Ann Limnol Int J Limnol 47:S35–S49
Soldner M, Stephen I, Ramos L, Angus R, Wells NC, Grosso A, Crane M (2004) Relationship between macroinvertebrate fauna and environmental variables in small streams of the Dominican Republic. Water Res 38(4):863–874
UK Technical Advisory Group on the Water Framework Directive (2006) Criteria and guidance principles for the designation of heavily modified water bodies. https://www.wfduk.org/sites/default/files/Media/Setting%20objectives%20in%20the%20water%20environment/HMWB%20rapid-case%20designation%20tool_Final_010308.pdf
Kefford BJ (1998) The relationship between electrical conductivity and selected macroinvertebrate communities in four river systems of south-west Victoria, Australia. Int J Salt Lake Res 7(2):153–170
Roy AH, Rosemond AD, Paul MJ, Leigh DS, Wallace JB (2003) Stream macroinvertebrate response to catchment urbanization (Georgia, U.S.A.). Freshw Biol 48(2):329–346
Wang J (2014) Combined sewer overflows (CSOs) impact on water quality and environmental ecosystem in the Harlem River. J Environ Prot 5(13):1373
Acknowledgements
The author wishes to thank Dr Kofi Owusu, University of Manchester, UK for producing the map, Fig. 1 and for the anonymous reviewers who provided comments needed to improve this document.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Medupin, C. Distribution of benthic macroinvertebrate communities and assessment of water quality in a small UK river catchment. SN Appl. Sci. 1, 544 (2019). https://doi.org/10.1007/s42452-019-0464-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0464-x