Abstract
We have synthesized here a novel ninhydrin derivative 2,2′-(2-oxopropane-1,3-diyl)bis-(2-hydroxy-1H-indene-1,3(2H)-dione) (1) through a green and facile strategy with the use of acetic acid as catalyst. The structure has been determined by spectral analysis and by single crystal X-ray analysis. This molecule contains two 2-hydroxy-1,3-indanedione moieties fused with 2-oxo-propane at 1 and 3 positions. The molecular structure of the title compound (C21H14O7) has been optimized and the structural parameters have been calculated by DFT/B3LYP method using 6-311++G(d,p) basis set. The fundamental vibrational wave numbers and their intensities were calculated and a good agreement between observed FT-IR spectrum and scaled calculated wavenumbers has been achieved. The electronic properties of the molecule are discussed with the help of the descriptors such as HOMO–LUMO and MEPS. In addition, the molecular docking and NBO analyses are also carried out to get a better insight of the molecule.
Graphical abstract
The major intermolecular interactions in the crystal structure are established through hydroxy group to carbonyl oxygen of the adjacent molecules by means of strong O–H…O hydrogen bonds leading to form a zigzag arrangement running along b-axis.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ninhydrin is a common coloration reagent for amino acids and has been recounted as a useful compound in organic, analytical, forensic sciences and biochemical [1]. Ninhydrin was first synthesized in 1910 by Ruhemann [2], after that, various ninhydrin derivatives were synthesized and their chemical properties were investigated [3]. Ninhydrin can be considered as a tricarbonyl compound due to its equilibrium with indane-1,2,3-trione (Scheme 1) [4]. The middle carbon (C-2 position) of this trione is more electrophilic and easily available for nucleophiles. Furthermore, in ninhydrin, acid catalyzes the formation of reactive electrophilic species at C-2 position or make it is an electrophilic centre. Although the reaction of ninhydrin with amino acids and amines were studied by various groups, the reactions of ninhydrin with active methylene nucleophiles are scare [5]. In presence of acid, ninhydrin at C-2 position, form condensation abduct with α-carbon of carbonyls [6,7,8,9]. Previously, we have established a suitable one-pot method for preparing the ninhydrin derivative as 2-Acetonyl-2-hydroxyindan-1,3-dione [6, 10]. In continuation of our work, we are currently exploring the reactions of ninhydrin with ninhydrin derivative containing active methylene group. As part of our current program to synthesized novel ninhydrin derivatives, we have successfully synthesized this titled compound (1) by the reaction of ninhydrin with our previously reported ninhydrin derivative i.e. 2-Hydroxy-2-(2-oxo-propyl)-indane-1,3-dione [5, 6, 10] (Scheme 2). The equilibrium structure of the titled compound is obtained and the intramolecular interaction is studied by quantum theory of atoms in molecule. The vibrational spectral features of this compound have been explained and the spectrum calculated by the DFT/B3LYP method using the 6-311++G(d,p) basis set has been compared with the experimentally recorded FT-IR spectrum. In this regard, the VEDA 4 program has been used to carry out the potential energy distribution (PED) analysis [13, 14]. This work also includes the analysis of HOMO, LUMO and the 3D molecular electrostatic potential surface (MEPS) analysis. We also studied the molecular docking of the title compound in HEME OXYGENASE 1(2ZVU) protein to find its possible pharmacological importance. Based on our literature survey, we found that there are no reports on the title molecule.
2 Experimental
2.1 Materials and physical measurements
Reagent grade ninhydrin was obtained from Sigma-Aldrich and was used as received. All other chemicals and solvents were procured from S. D. Fine Chemicals and Merck, respectively and used without further purification. TLCs were taken on silica gel plates (silica gel 60 F254 on aluminium foil, Merck). Infrared spectrum was recorded on a PerkinElmer Model 1320 spectrometer (KBr disk, 400–4000 cm−1). The 1H NMR and 13C NMR spectra were recorded on a Bruker Avance spectrometer (600 MHz for 1H NMR and 150 MHz for 13C NMR) in DMSO-d6 and chemical shifts are reported in ppm. The following abbreviations are used: m (multiplet), s (singlet), brs (broad singlet). The mass spectrum was recorded on Waters Q-Tof Premier-HAB213 mass spectrometer in ionization mode. Elemental analyses was performed with a Perkin–Elmer 240 analyzer. The melting point was taken on Stuart SMP30 digital melting point apparatus by open capillary method and is uncorrected.
2.2 Synthesis of the title compound 1
A mixture of ninhydrin (1.78 g, 10 mmol) and 2-Hydroxy-2-(2-oxo-propyl)-indane-1,3-dione (2.18 g, 10 mmol) were stirred and refluxed in 50 ml of acetic acid for 8 h. The conversion was monitored by TLCs and after completion of the reaction; the reaction mixture was dried on rotary evaporator at low pressure. The crude product was crystallized with chloroform-alcohol (1:1 v/v) to give the translucent crystals of titled compound 1. Yield 2.31 g (60%); m.p. 150 °C, Rf = 0.27 (hexane/acetone, 1:1); IR (KBr): νmax = 3421, 2913, 2850, 1750, 1707, 1599, 1385, 1317, 1294, 1266 cm−1 (Fig. S2). 1H NMR (600 MHz, DMSO-d6, δ): = 3.34 (s, 2H, > CH2), 3.36 (s, 2H, > CH2), 6.69 (brs, 2H, OH), 7.86–7.90 (m, 8H, ArH) ppm (Fig. S3). 13C NMR and DEPT-135 (150 MHz, DMSO-d6, δ): 45.59 (2 X CH2), 73.20 (2 X -C–OH), 122.95 (4 X CH), 135.82 (4 X CH), 139.57 (4 X C), 197.93 (4 X C = O), 205.63 (C=O) ppm (Fig. S4, S5). MS (EI): m/z 378.0696 [M+] ((Fig. S7); Elemental anal. Calcd. for C21H14O7 (378.06): C, 66.67; H, 3.73; O, 29.60%; Found: C, 66.54; H, 3.67; O, 29.57%.
2.3 Computational methods
The molecular structure of titled compound (1) is modeled by the Gaussview program package 5.0.8 [15] and shown in Fig. 1 of the SI. All quantum chemical calculations of the title compound are carried out on an Intel Core I3(TM) CPU/4.20 GHz laptop computer using Gaussian 09 program package, invoking gradient geometry optimization and employing the B3LYP/6-311++G (d,p) levels of theory to predict the molecular structure and vibrational wave numbers [16].
2.4 X-ray structural studies
The crystal data for 1 has been collected on a Bruker APEX-II CCD diffractometer equipped with a graphite monochromator and Mo − Kα (λ = 0.71073 Å, 140 K) radiation. The program SMART1 was used for collecting frames of data, indexing reflections, and determining lattice parameters; SAINT [17] for integration of the intensity of reflections and scaling; SADABS [18] for absorption correction; and SHELXTL [19, 20] for space group and structure determination and least-squares refinements on F2. The crystal structure was solved and refined by full-matrix least-squares methods against F2 by using the program SHELXL-2014 [21] and Olex-2 software [22]. All the non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogens positions were fixed at calculated positions and refined isotropically. Lattice parameters, data collection and refinement parameters are summarized in Table 1 and selected bond distances and bond angles are given in Table S2 of supplementary data. Crystallographic data for the structure reported in this paper have been deposited with the Cambridge Crystallographic Data Center number, CCDC 1831708. Copies of this information may be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223 336 033; e-mail: deposit@ ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk).
3 Results and discussion
3.1 Chemistry
The C-2 of indane-1,2,3-trione (ninhydrin) is much more electrophilic and this is why it reacts readily with nucleophiles. In presence of acid, it protonates the hydroxyl group in ninhydrin that causes elimination of water molecule to produce a reactive electrophilic species; a C-2 carbocation [7]. In our previous study, we reported the synthesis of 2-Hydroxy-2-(2-oxo-propyl)-indane-1,3-dione in excellent yield [6]. Title molecule 1 was synthesized in 60% yield by the reaction of ninhydrin with 2-Hydroxy-2-(2-oxo-propyl)-indane-1,3-dione [6] in acetic acid medium. 2-Hydroxy-2-(2-oxo-propyl)-indane-1,3-dione in acetic acid form a carbon based nulcleophile which attacks on electrophilic C-2 position of ninhydrin to form condensation product (Scheme 1) [8, 9]. In this reaction, a new C–C bond is formed between C-2 of ninhydrin and methyl carbon of 2-oxo-propyl in 2-Hydroxy-2-(2-oxo-propyl)-indane-1,3-dione.
The structure of compound 1 was assigned based on the IR (Fig. S2), 1H NMR (Fig. S3), 13C NMR (Fig. S4), DEPT-135 (Fig. S5), C-H HSQC (Fig. S6), mass spectra (Fig. S7), elemental analysis and single crystal x-ray analysis. In the IR spectra of compound 1, the OH absorption bands appeared at 3421 cm−1. Weak bands at 3070 cm−1 and 1599 cm−1 confirmed aromatic C–H and C=C stretchings. The band at 2913 cm−1 was assigned to the C-H stretching vibrations of the methylene groups. The intense bands at 1750 and 1707 cm−1 were assigned to the > C=O vibrations in indane rings & condensed 2-oxypropyl moiety. In the 1H NMR spectrum of title molecule, four protons as singlet at δ 3.36 ppm were assigned for two -CH2. This four-proton singlet at δ 3.36 correlates with 13C peak at δ 45.59 ppm in HSQC; which goes negative in dept-135 spectrum and confirmed as two methylenes. Two multiplets integrating for four protons each at δ 7.86–7.88 and 7.88–7.90 ppm clearly show expected aromatic protons on two indane moieties of 1. These two multiplets correlate in HSQC with 13C peaks at δ 122.95 and 135.82 ppm, those gone positive in dept-135 spectrum and confirmed as aromatic -CHs. One broad singlet at δ 6.69 ppm integrating for two protons indicated two OH groups. The desired signal for two tertiary carbons attached with OH; falls on δ 71.16 ppm. The 13C signals at δ 205.63 (C=O) and 197.93 (4C=O) are absent in dept-135 and correlating with no proton in HSQC, that confirm them as five carbonyl carbons. The 13C signal in aromatic at δ 139.57 (4C) is absent in dept-135 and correlating with no proton in HSQC and assigned as aromatic tertiary carbons. Along with all desired peaks, it clearly indicates the formation of condensation product between ninhydrin and 2-Hydroxy-2-(2-oxo-propyl)-indane-1,3-dione.
3.2 Crystal structure analysis
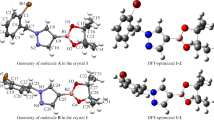
The single crystal X-ray structural study revealed that the compound 1 crystallizes in orthorhombic space group Iba2 with half of molecule in the asymmetric unit. An ORTEP diagram of the compound 1 is shown in Fig. 1, indicating the presence of 2-oxopropane moiety (C‒O: 1.209(2) Å–1.419(2) Å, and C‒C: 1.384(3) Å–1.542(2) Å) which is flanked on both side by the 2-hydroxy-1H-indene-1,3(2H)-dione moiety. The planes of the 2-hydroxy-1H-indene-1,3(2H)-dione moiety and 2-oxopropane moiety are approximately orthogonal to each other (inter-planar angle = 82.29°). The bond lengths and bond angle of the titled compound is present within the expected ranges for ninhydrin derivatives [10]. Figure 2 demonstrates the crystal packing of compound 1 which is stabilized by hydrogen bonding and some weak, non-classical contacts [11, 12] viz. π···π, C–H···π, and lone pair···π. These interactions are intermolecular in nature. The strong hydrogen bonding is observed between O(002)–H···O(003), with interatomic distance of 2.145(3) Å (Fig. 3a) where as the weak non-classical: [1] π···π and O(004)···π contacts are observed between C(00C)···C(00C) and O(004)···C(00B), with interatomic distances of 3.385(2) Å and 3.204 (3) Å, respectively (Fig. 3b), and [2] C–H···π contact is observed between C(00A)–H···C(00D) with interatomic distance of 3.099 (4) Å (Fig. 3c). The major intermolecular interactions in the crystal structure are established through hydroxy group to carbonyl oxygen of the adjacent molecules by means of strong O–H…O hydrogen bonds leading to form a zigzag arrangement running along b-axis, as depicted in Fig. 4. These zigzag arrangements in the single layer are propagates through C(00C)···C(00C) and O(004)···C(00B) contacts to form a 2D supramolecular layers. Each 2D supramolecular layers are further connected through C(00A)–H···C(00D) contacts to form overall crystal lattice.
3.3 Vibrational analysis
In a nonlinear molecule, number of normal modes of vibrations, are (3 N-6), where N is the number of atoms [23, 24]. The title compound (1) contains 42 atoms and hence exhibits 120 normal modes of vibrations. The Gaussview 5.0.8 program package is used for the assignment of calculated frequencies which provides a 3D view of the vibrational modes VEDA 4 software program is used to calculate the potential energy distribution (PED). An empirical uniform scaling factor of 0.983 up to 1700 cm−1 and 0.958 for above 1700 cm−1 were used to correct the overestimations of the calculated harmonic frequencies [25, 26]. All the vibrational assignments are presented in table S1.
3.3.1 O–H stretching
(O–H) stretching appears generally at 3600–3400 cm−1 [27]. In the FTIR spectrum of the title molecule (Fig S2) a very strong absorption peak is observed at 3421 cm−1 and this mode is calculated at 3501 cm−1 with 100% PED.
3.3.2 (C–H) stretching
The characteristic region for the (C–H) stretching is 3100–2900 cm−1. A weak absorption peak is observed at 2913 cm−1 which is assigned to six calculated frequencies obtained at 3104, 3094, 3081, 3068, 3015 and 2958 cm−1. The (C–H) in plane bending modes are useful for characterization and they appear as strong band in the FTIR spectrum at frequency peaks 1385, 1334, 1317, 1294, 1266 cm−1 and are well assigned well with calculated frequencies.
3.3.3 (C=O) Stretching
This stretching mode makes the skeleton of the compound as rigid and the formation of hydrogen bond checks the charge distribution in the ring and side chains. In the present study this mode is observed with two strong peaks at 1750 & 1707 cm−1 in the FTIR spectrum and are calculated at 1736 & 1621 cm−1 by DFT/B3LYP method. It is in good agreement with the reported literature which is in the range 1750–1630 cm−1 [28].
3.3.4 (C–C) stretching
The semicircle aromatic stretching (C–C) gives rise to characteristics bands in the spectral range 1600–1000 cm−1 [29]. In this study this vibrational mode is calculated in the range 1583–886 cm−1 and spectral peaks for these modes are observed at 1599, 138, 1334, 1266, 1179, 1078 and 969 cm−1 in the FTIR spectrum. The corresponding in plane bending and torsional modes were found to be consistent with the recorded spectral values.
3.3.5 (C–O) stretching
The (C–O) stretching along with bending and torsional modes, are calculated in the range 1115–705 cm−1 and is well matched with literature [29].
3.3.6 Low frequency vibrational modes
In the study of weak intermolecular interactions which generally occurs in enzyme reactions, study lower frequency vibrations play vital role [30]. It is also helpful in the interpretation of the reaction of electromagnetic radiation on biological systems [31]. In the present study, several out of plane modes mixed with torsional modes are calculated in the range 700–50 cm−1 and these are observed in the form of three peaks observed at 692, 567, and 500 cm−1 in the FTIR spectrum.
3.4 Electronic properties
The frontier molecular orbitals (HOMO and LUMO) participate in chemical reactions and their energy gap decides the intensity of the chemical reactivity and stability of a compound [32]. A molecule with small energy band gap is more polarizable and generally possesses high chemical reactivity and low kinetic stability and is termed as soft molecule [33, 34]. In this study the plots of LUMO and HOMO are shown in Fig. 5 and their values are given in table S1. From 2D plot, it is evident that the entire HOMO is spread on the benzene rings and penta rings in increasing order. The 2D plot of LUMO (− 2.7265 eV) of the title molecule shows that LUMO is uniformly distributed on benzene rings and penta rings. Unlike HOMO, small amount of LUMO is also spread on backbone atoms O2, O1, C11 and C6 in decreasing order. The frontier orbital gap is calculated as 2.2271 eV for the title molecule. The molecular electrostatic potential surface (MEPS) is shown in Fig. 6 with color scale ranging from − 4.001 to + 4.001 a.u for the title molecule. MEPS displays the molecular shape, size and electrostatic potential values in terms of color coding which is a useful tool for the correlation between molecular structure and physiochemical properties of the molecules [35, 36, 37].
3.5 Molecular docking
The process of molecular docking decides the manner in which two molecules, like a drug (ligand) and a receptor (protein), fit to each other and it inhibits its function and thus behaves effectively as a drug [38]. Initially using Pass10 evaluation professional package we choose the structure which is most resemble with our title molecule and by considering the anticarcenogenic activity we select the gene HEME OXYGENASE 1. The data in pdb format of the target protein has been obtained from the protein data bank (PDB) [39] database with PDBID = (2ZVU). In the present theoretical study the inhibition of HEME OXYGENASE 1(2ZVU) with the title compound (C21H14O7), molecular docking study has been carried out using Hex program 8.0. Figure 7 shows the docked conformation of the protein 2ZVU with the binding site of the target ligand. The docking method is a model which provides the binding affinity of a particular site in terms of the e-value. Larger the negative e-value the better the docking is. In present study total e-value calculated from Hex program is -182.42 for HEME OXYGENASE 1(2ZVU) protein, which indicates that the title compound (C21H14O7) can inhibit the protein HEME OXYGENASE 1(2ZVU).
4 Conclusion
The optimized molecular geometry, vibrational wavenumbers, electronic parameters of the title compound, 2,2′-(2-oxopropane-1,3-diyl)bis-(2-hydroxy-1H-indene-1,3(2H)-dione) have been calculated using DFT B3LYP method adopting 6-311++G(d,p) basis set. A good agreement between experimental and calculated normal modes of vibrations has been achieved. The frontier orbital energy gap is calculated as 2.2271. The results of molecular docking studies speculate that this biologically active molecule (C21H14O7) might serve as a potential candidate for the inhibition to protein “HEME OXYGENASE 1(2ZVU)” thereby indicating its possible pharmacological importance.
References
Joullie MM, Thompson TR, Nemeroff NH (1991) Ninhydrin and ninhydrin analogs syntheses and applications. Tetrahedron 47:8791–8830
Ruhemann S (1910) CXXXII.—Cyclic di- and tri-ketones. J Trans Chem Soc 97:1438–1449
Hansen DB, Madeleine MJ (2005) The development of novel ninhydrin analogues. Chem Soc Rev 34:408–417
Herath AMC, Rajapakse RMG, Karunarathne V, Wicramasinghe A (2006) Electrochemical investigation of superoxide anion scavenging ability of 1, 2, 3-triketohydrindene hydrate in aprotic solvents. Electrochim Acta 51:2890–2897
Kneubuhler S, Carta V, Altomare C, Carotti A, Testa B (1993) Synthesis and monoamine oxidase inhibitory activity of 3-Substituted 5H-Indeno[1,2- c]pyridazines. Helv Chim Acta 76:1956–1963
Ghalib RM, Hashim R, Sulaiman O, Jawad A, Mehdi SH, Bogdanovic GA, Trifunovic SR, Kawaruma F, Ahamed BMK, Majid AMSA (2017) Green synthesis of novel class of benzazocine derivatives, their crystal structures and anticancer activity. Curr Org Synth 14:127–136
Das S, Pramanik A, Fröhlich R, Patra A (2004) Facile acid-catalyzed condensation of 2-hydroxy-2,2′-biindan-1,1′,3,3′-tetrone with phenols, methoxyaromatic systems and enols. Tetrahedron 60:10197–10205
Chakrabarty M, Mukherji A, Arima S, Harigaya Y, Pilet G (2009) Expeditious reaction of ninhydrin with active methylene compounds on montmorillonite K10 clay. Monatsh Chem 140:189–197
Klumpp DA, Fredrick S, Lau S, Jin KK, Bau R, Surya Prakash GK, Olah GA (1999) Acid-catalyzed condensations of ninhydrin with aromatic compounds. preparation of 2,2-Diaryl-1,3-indanediones and 3-(Diarylmethylene)isobenzofuranones. J Org Chem 64:5152–5155
Fun H-K, Quah CK, Parveen M, Ghalib RM, Mehdi SH (2009) 2-Acetonyl-2-hydroxy-indan-1,3-dione. Acta Cryst E 65:o1209
Cossar PJ, Russell CC, McCluskey SN, Pope D, Bernhardt PV, McCluskey A (2018) Crystal structure of ethyl 2,4-dimethyl-1-phenyl-6-thioxo-1,6-dihydropyrimidine-5-carboxylate: the product from the reaction of ethyl 3-aminocrotonate, phenylisothiocyanate and acetic anhydride. J Chem Crystallogr 48:91–95
Mendez IJL, Pleizier JS, Wang HB, Wisner JA (2018) The Structure of Cis-2,2′-Azopyridine in the Solid State. J Chem Crystallogr. https://doi.org/10.1007/s10870-018-0737-z
Jamroz MH (2004) Vibrational energy distribution analysis, VEDA 4 program. Warsaw, Poland
Jamroz MH (2013) Vibrational energy distribution analysis (VEDA): scopes and limitations. Spectrochim Acta Mol Biomol Spectrosc 114:220–230
Frisch E, Hratchian HP, Dennington RD II, Keith TA, Millam J, Neilsen B, Holder AJ, Hiscocks J (2009) Gaussview, version 508, vol 235. Gaussian Inc, Wallingford
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JJA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A. 02. Gaussian Inc, Wallingford
SMART & SAINT (2003) Software reference manuals, version 6.45. Bruker Analytical X-ray Systems, Inc., Madison
Sheldrick GM (2002) SADABS a software for empirical absorption correction; version 2.05; program for empirical absorption correction of area detector data. University of Göttingen, Göttingen
SHELXTL (2000) Reference manual, version 6.1. Bruker Analytical Xray Systems Inc., Madison
Sheldrick GM (2001) SHELXTL v.6.12. Bruker AXS Inc., Madison
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Cryst 42:339–341
Silverstein RM, Bassler GC, Morrill TC (1991) Spectrometric identification of organic compounds. John Wiley and sons, Hoboken
Socrates G (2001) Infrared, Raman characteristic group frequencies-tables, charts, 3rd edn. Wiley, New York
Karabacak M, Kurt M, Cinar M, Coruh A (2009) Experimental (UV, NMR, IR and Raman) and theoretical spectroscopic properties of 2-chloro-6-methylaniline. Mol Phys 107(3):253–264
Sundaraganesan N, Ilakiamani S, Saleem H, Wojciechowski PM, Michalska D (2005) FT-Raman and FT-IR spectra, vibrational assignments and density functional studies of 5-bromo-2-nitropyridine. Spectrochim Acta A 61:2995–3001
Biradar NS, Kulkarni VK (1971) Spectroscopic study of tin(IV) complexes with multidentate Schiff bases. J Inorg Nucl Chem 33:3781–3786
Ruddick JNR, Sames JR (1973) Mössbauer and infrared spectroscopic studies of some organotin(IV) schiff base complexes. J Organometal Chem 60:233–246
Colthup NB, Daly LH, Wiberley SE (1964) Introduction to infrared, Raman Spectroscopy. Academic Press, New York, pp 74–315
Chou KC (1984) Biological functions of low-frequency vibrations (phonons). III. Helical structures and microenvironment. Biophys J 45:881–889
Frohlich H (1988) In biological coherence and response to external stimuli. Springer, Berlin
Sklenar H, Jager J (1979) Molecular structure–biological activity relationships on the basis of quantum-chemical calculations. Int J Quantum Chem 16:467–484
Fleming I (1976) In frontier orbitals and organic chemical reactions. Wiley, Hoboken
Sajan D, Joseph L, Vijayan N, Karabacak M (2011) Natural bond orbital analysis, electronic structure, non-linear properties and vibrational spectral analysis of l-histidinium bromide monohydrate: a density functional theory. Spectrochim Acta A 81:85–98
Murray JS, Sen K (1996) In molecular electrostatic potentials: concepts and applications. Elsevier, Amsterdam
Alkorta I, Perez JJ (1996) Molecular polarization potential maps of the nucleic acid bases. Int J Quant Chem 57:123–135
Karabacak M, Cinar M (2012) FT-IR, FT-Raman, UV spectra and DFT calculations on monomeric and dimeric structure of 2-amino-5-bromobenzoic acid. Spectrochim Acta A 86:590–599
Srivastava V, Kumar A, Mishra BN, Siddiqui MI (2008) Molecular docking studies on DMDP derivatives as human DHFR inhibitors. Bioinformation 4:180–188
Available from, http://www.rcsb.org/pdb/explore.do?structureID=2ZVU
Acknowledgements
We would like to acknowledge Faculty of Sciences and Arts-Khulais, University of Jeddah, Jeddah, Kingdom of Saudi Arabia for research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghalib, R.M., Mehdi, S.H., Hasan, T. et al. 2,2′-(2-Oxopropane-1,3-diyl)bis-(2-hydroxy-1H-indene-1,3(2H)-dione): synthesis, crystal, electronic and molecular docking studies. SN Appl. Sci. 1, 351 (2019). https://doi.org/10.1007/s42452-019-0366-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0366-y