Abstract
Agricultural diffuse pollution is a major environmental problem causing eutrophication of water bodies. Despite the problem is widely acknowledged, there has been relatively few major advances in mitigating the problem. We studied the effectiveness of biopolymer-based (tannin, starch, chitosan) natural coagulants/flocculants in treatment of two different agricultural wastewaters that differed in their level of phosphorus pollution and turbidity. We used jar-tests to test the effectiveness of the biopolymer coagulants in reducing water turbidity, total phosphorus, and total organic carbon (TOC) from the wastewaters. In more polluted water (total phosphorus: 300 µg/L, turbidity: 130 FNU, TOC: 30 mg/L), all tested biopolymers performed well. The best reductions for different biopolymer coagulants were 64–95%, 80–98% and 14–27%, for total phosphorus, turbidity and TOC, respectively. Tannin and chitosan coagulants performed the best at doses of 5–10 mL/L, whereas starch coagulants had the best performance at 1–2 mL/L doses. Tannin and chitosan coagulants performed clearly better than the starch coagulants. In less polluted water (total phosphorus: 74 µg/L, turbidity: 3.9 FNU, TOC: 21 mg/L), chitosan and starch coagulants did not produce flocs at any of the tested doses. Tannin coagulant performed the best at doses of 5–8 mL/L, where reductions were 70%, 82%, and 22%, for total phosphorus, turbidity and TOC, respectively. The great reductions of phosphorus and turbidity suggests that biopolymer coagulants could be applied in treatment of agricultural water pollution. The high phosphorus retention in the biodegradable biopolymer sludge suggests that the sludge can be readily used as a phosphorus fertilizer, which would aid the recycling of nutrients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Agricultural soils erode by wind and rain and the sediments and nutrients end up by surface flow to watercourses. Agricultural pollution is a major environmental problem causing nutrient and organic pollution, increase in turbidity, excessive sedimentation, and biodiversity loss in aquatic ecosystems [1,2,3,4]. Productivity in many aquatic ecosystems, especially in freshwaters, is often limited by phosphorus, although nitrogen limitation is not uncommon [5]. Thus, reducing phosphorus loading from agriculture is often the key to limit excessive eutrophication [6]. Despite the pervasiveness of the problem, there has been relatively few major advances in mitigation of agricultural pollution and agriculture continues to be one of the most significant polluters of waterbodies [7]. In addition, the degradation of agricultural soils and predicted global phosphorus crisis [8] places urgent needs to find ways to retain and recycle the nutrients lost from the soils.
Iron- and aluminum salts and synthetic polymers (e.g. polyacrylamides) have been traditionally applied for removal of phosphorus, suspended solids and other contaminants from wastewaters by coagulation/flocculation process [9,10,11]. However, the metal content and potential toxicity of these coagulants makes the recycling of the created sludge and retained phosphorus difficult in agriculture. Biopolymers (e.g. cellulose, chitin, tannin, starch) are polymeric biomolecules produced by living organisms. Several biopolymers can be chemically refined to work as cationic or anionic coagulation/flocculation agents for wastewater treatment [11, 12]. Compared to traditional synthetic polymers, the advantages of biopolymers are their lack of metals, biodegradability and non-toxicity, which makes the recycling of retained nutrients easier [10, 11, 13]. Several studies have tested the coagulation and flocculation properties of biopolymers to organic [14,15,16,17,18] and inorganic [19,20,21,22,23,24] contaminants and at different levels of pollution, pH and dosage. Especially, the coagulation and flocculation properties of chitosan are rather extensively studied [13,14,15,16,17,18,19,20,21,22,23,24,25,26]. However, the vast majority of these experimental studies have simultaneously tested only one biopolymer and used synthetic and homogenous wastewater in the experiments [17, 19,20,21,22, 27]. Only few studies exist on the effectiveness of biopolymer coagulants in treatment of polluted natural surface waters [28].
In this study, we used jar-testing to evaluate and compare the efficiency of tannin, starch and chitosan coagulants in phosphorus, turbidity and total organic carbon (TOC) removal from agricultural wastewaters. To our knowledge, this is the first study to test the applicability of biopolymer coagulants in treatment of natural agricultural wastewaters from diffuse agricultural pollution.
2 Materials and methods
We collected water samples from two study sites that are influenced by agricultural land use and diffuse pollution. The sites are located in municipalities of Ruukki (64°38′, 25°1′) and Tarvaala (62°40, 25°19′) in Western and Central Finland, respectively. The agriculture in the area is mainly focused on dairy and meat production and the fields are used as a pasture or for growing feed to livestock. In Ruukki, we collected the water samples from drainage ditch (catchment area: 0.18 km2) that drains through pasture fields. In Tarvaala, we took the samples from man-made wetland (catchment area: 1.2 km2) that is used for treatment of agricultural runoff waters. The samples from Ruukki were collected in winter (February) and from Tarvaala in spring (April) of 2018.
The level of agricultural pollution was distinctly different between the two sites (Table 1). The phosphorus concentration, turbidity and concentration of various metals, especially iron, were substantially higher in Ruukki drainage ditch than in Tarvaala wetland (Table 1). The soils in Ruukki are acid sulfate soils which are typical in western coast of Finland and have naturally high concentrations of metals [29]. We tested three different biopolymers (tannin, starch and chitosan). The raw materials for tannin were acacia tree bark (Acacia mearnsii), for starch (C6H10O5)n potatoes (Solanum tuberosum) and for chitosan (C6H11NO4)n crustacean (crabs and prawns) shells.
We tested two different tannin and five different starch coagulants that differed in their charge density and chain length (Table 2). Tannin (cationic tannins, HTH and HTG, Haarla Oy) and starch (cationic starches, PrimePHASE 2545, 3545, 3545X10, and CGKT, Chemigate Oy) were chemically refined (see [12, 28], for description of tannin coagulant Tanfloc™ which is a very similar product to HTH and HTG) commercial coagulant products, whereas chitosan was 90% deacetylated food grade chitosan powder (Wellgreen Technology Co.), which is used for multiple purposes.
We tested the effectiveness of the biopolymer coagulants in treating the agricultural waters by using jar-tester (Phipps & Bird, PB-900 series programmable JarTester, Fig. 1) with 1 L beakers. Each beaker contained 1 L of agricultural wastewater and the water temperature during the experiments was around 20 °C. The pH in Ruukki drainage ditch and Tarvaala wetland water samples was near-neutral (6.9 and 6.6, in drainage ditch and wetland, respectively). The performances of several biopolymer coagulants have been observed to be inert to variation in pH [22, 30] or have their best performance in slightly acidic [24, 25, 31] or in neutral conditions [14, 19, 20, 24, 32].
The biopolymers were diluted to 1% active content solution. Tannin and starch were in liquid form and diluted with tap water. Chitosan powder (1 g) was dissolved with 10 mL 0.1 M HCL and heated and stirred with magnetic stirrer for 2 h. Finally, the dissolved chitosan was mixed with 90 mL of tap water to obtain 1% solution. We tested 7 different doses (0, 1, 2, 3, 5, 8, 10 mL/L) of each biopolymer coagulant and each dose was replicated twice. We measured residual total phosphorus concentration, water turbidity and total organic carbon (TOC) and mean value from the two replicates was calculated. After the addition of the biopolymer coagulants, the beakers were stirred at high velocity (200 rotations per minute) for 20 s and then stirring was decreased (40 rotations per minute) for 5 min. Finally, the treated water was allowed to settle for 15 min with no stirring. Water samples were collected with pipette from upper part of the beaker. The water quality variables were analyzed by using standard methods in FINAS accredited laboratory of SYKE (https://www.finas.fi/Documents/T003_M38_2018.pdf).
To drainage ditch waters of Ruukki, we tested the HTH and HTG tannin coagulants, PrimePHASE 2545, 3545X10 and CGKT starch coagulants and chitosan. In these tests, and in preliminary testing with both drainage ditch and wetland water, the different tannin and starch coagulants performed very similarly and thus we used only one tannin (HTG), one starch (PP3545) and chitosan to test the performance in the water of Tarvaala wetland. The initial water quality (0-dose treatment) in testing of each biopolymer coagulant varied to a some extent (Tables 3 and 4), especially in terms of total phosphorus concentration in drainage ditch water samples (Table 3), but this did not cause appreciable effect on coagulant performance and the results were comparable.
3 Results
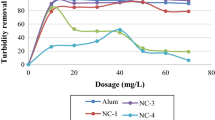
In the agricultural wastewater of Ruukki drainage ditch HTH, HTG (tannins) and chitosan performed very similarly (Fig. 2). For HTH the best reductions of total phosphorus (95%, from 320 to 22 µg/L) and turbidity (98%, from 145 to 2.8 FNU) were achieved at dose of 10 mL/L. However, at doses of 2–3 mL/L the phosphorus and turbidity reductions were already close to maximum reductions (86–90% for phosphorus and 95–96% for turbidity) and increasing the coagulant dose did not improve the residual water quality appreciably (Fig. 2). HTG had similar reductions at dose of 10 mL/L for phosphorus (90%, from 180 to 20 µg/L) and turbidity (97%, from 68 to 2.4 FNU) as HTH but almost equal residual water quality was achieved already at doses of 2–3 mL/L (81–87% for phosphorus and 93–96% for turbidity) (Fig. 2). Chitosan had the best reduction of phosphorus (89%, from 180 to 19 µg/L) and turbidity (97%, from 68 to 2.3 FNU) at doses of 8 and 5 mL/L, respectively. Similarly to tannins, the reductions were close to maximum values already at doses of 2–3 mL/L (83–88% for phosphorus and 93–96% for turbidity).
Total organic carbon (TOC) did not reduce relatively as much as turbidity and total phosphorus. Maximum reductions were achieved at 3 mL/L for HTH (26%, from 33 to 24 mg/L) and HTG (15%, from 26 to 22 mg/L) and 5 mL/L for chitosan (27%, from 26 to 19 mg/L) (Fig. 2).
Starch coagulants had clearly different performance compared tannin and chitosan. In general, the best performance was achieved with 1 mL/L dose in Ruukki drainage ditch water, except for PP3435x10 that reached the best performance at dose of 2 mL/L (Fig. 2). The best reductions of phosphorus were 64% (from 230 to 84 µg/L), 80% (from 320 to 64 µg/L) and 67% (from 230 to 76 µg/L) for PP2545, PP3454x10 and CGKT, respectively. The best reductions of turbidity were 80% (from 148 to 31 FNU), 87% (from 145 to 19 FNU), 82% (from 149 to 28 FNU) and of TOC 14% (from 32 to 28 mg/L), 20% (from 33 to 26 mg/L), and 19% (from 32 to 26 mg/L) for PP2545, PP3454x10 and CGKT, respectively. At larger doses the performance was significantly poorer and TOC substantially increased (Fig. 2).
The starch (PP3545) coagulant and chitosan did not produce flocs in less polluted Tarvaala wetland water and the residual water quality was deteriorated, especially with starch (Fig. 3). Tannin coagulant (HTG) only formed flocs at doses of 5–8 mL/L and at 10 mL/L the solution restabilized and residual water quality decreased (Fig. 3). The best performance with HTG was achieved at dose of 5 mL/L, where total phosphorus concentration reduced 70% (from 71 to 22 µg/L), turbidity 82% (from 7.5 to 1.4 FNU) and TOC 22% (from 18 to 14 mg/L) (Fig. 3).
4 Discussion
Agricultural water pollution and loss of nutrients, especially phosphorus, is a major environmental problem that urgently needs sustainable solutions. Chemical treatment of agricultural effluents with traditional metal salts or polyacrylamids is a potential option to reduce nutrient and other pollutant loading. However, the metal residuals in treated water may adversely affect the receiving water bodies and recycling of the retained nutrients is difficult due to the metal content and non-biodegradability of the sludge. We found that metal-free and biodegradable biopolymer coagulants were very effective in reducing total phosphorus concentration and turbidity and to some extent the organic carbon concentration, but the effectiveness was dependent on optimal dose, the initial pollution level and type of biopolymer coagulant.
4.1 Performance of biopolymer coagulants in highly polluted water
In the highly polluted Ruukki drainage ditch water (total phosphorus: 300 µg/L, turbidity: 130 FNU, TOC: 30 mg/L) the tannin and chitosan coagulants performed equally well and up to 95 and 98% reductions were achieved for total phosphorus and turbidity, respectively. The highest reductions were achieved at dose of 10 mL/L, however dose of 3 mL/L gave almost equal results. The phosphorus and turbidity reductions were at the higher end of spectrum compared to results in previous studies with biopolymer coagulants [25, 31, 32]. Moreover, the turbidity reductions were very high compared to previous experiments done with polluted river surface water, where around 65% reductions in turbidity were achieved with tannin [28]. Strikingly, use of tannin and chitosan coagulants in Ruukki drainage ditch water did not cause restabilization, charge reversal or deterioration of floc formation even at the highest doses, suggesting that the treated water contained plenty of charged colloid and fine solid particles for attachment and particle-polymer aggregate formation. A similar results have been observed in turbidity and suspended solid removal from various wastewaters with tannin [30], chitosan [20] starch coagulants [32].
In contrast to tannin and chitosan coagulants, the starch coagulants had clearly different performance in Ruukki drainage ditch water. The best reductions of total phosphorus, turbidity and TOC were achieved at around 1–2 mL/L doses. At optimum doses the best reductions with starch coagulants were 80% and 82% for total phosphorus and turbidity, respectively. At larger doses the performance was clearly poorer and the solution restabilized, although only TOC increased from the initial level. The reduction curves suggests that charge neutralization was the main mechanism controlling coagulation and higher than optimum dosing caused charge reversal and decreased the efficiency of coagulation [24].There has been only limited number of previous studies on the performance of starch based biopolymer coagulants in wastewater treatment [32]. Teh et al. [32] found that up to 88.4% reduction in total suspended solids could be achieved with rice starch in treatment of palm oil mill effluents, and 48.0% reduction in total phosphorus, which is substantially less than in our experiment. The difference in performance compared to tannins and chitosan are likely not related to differences in charge density or molecular chain length because some of the starch coagulants were similar in their charge density and chain length compared to chitosan. On the other hand, charge density or chain length variation did not cause major differences in performance within the different starch and tannin coagulant types. Thus, the reasons are probably related to other differences in molecular structure of the coagulants. Teh et al. [32] reported that an unmodified potato starch gave the poorest performance among different unmodified starches in treatment of agro-industrial wastewaters. The authors suggested that high amount of covalently bonded and negatively charged phosphate monoester groups in potato starches, reduce the coagulation of negatively charged particles [32]. However, the mechanistic reasons for the difference in performance between biopolymer coagulants should be addressed in more detailed studies.
Total organic carbon reductions (TOC) were around 20% for tannin, starch and chitosan coagulants in treatment of Ruukki drainage ditch water. Majority of the organic carbon in these waters is due to humic substances that largely cause the brownish-yellow background coloration [33]. In previous studies, 98–100% reductions of humic substances, measured in water color, were achieved with chitosan [14, 15]. However, only 7% reductions in TOC were achieved in treatment of debarking plant wastewaters with chitosan [16]. It was obvious by visual observation and turbidity measures that the water clarity increased substantially and the concentration of humic substances likely reduced significantly. Thus, the rather modest decrease in organic carbon suggest that some portion of the biopolymer coagulant remains unreacted in the water (increases organic carbon concentration) and that decrease in humic organic carbon is likely to be larger than the reduction of total organic carbon alone suggests.
4.2 Performance of biopolymer coagulants in less polluted water
The starch coagulant and chitosan did not produce flocs in less polluted Tarvaala wetland water (total phosphorus: 74 µg/L, turbidity: 3.9 FNU, TOC: 21 mg/L) and the residual water quality was substantially poorer than the initial water quality, especially with starch. Tannin coagulant (HTG) formed flocs only at doses of 5–8 mL/L and at 10 mL/L the solution restabilized and residual water quality strongly deteriorated. The optimum dose was 5 mL/L, where 70%, 82% and 22% reductions were achieved for total phosphorus, turbidity and TOC, respectively.
The higher concentration of nutrients, turbidity and various metals is likely the reason for better performance of coagulants in wastewater of Ruukki drainage ditch compared to water from Tarvaala wetland. The higher concentration of charged suspended and colloidal particles improves the coagulation and floc formation process by enhancing interparticle contacts [21, 27]. This is also supported by the strict range of optimum dose with the tannin coagulant in the less polluted water. Overdosing the tannin biopolymer likely resulted charge reversal (high formation of positively charged tannin-particle aggregates) and electrostatic repulsion, which inhibited the floc formation. The performance of starch and chitosan could have been improved by altering the pH of treated water. Especially, chitosan is a more cationic in acidic water due to the presence of amine groups and some studies report substantially better removal of impurities in more acidic water compared to the near-neutral pH [24, 25, 31]. However, it was not our interest to manipulate the treated water by any other means than addition of the coagulants.
Overall, the biopolymer-based coagulants showed promising results for treatment of agricultural wastewaters. The optimal conditions in terms of dosage and different mixing programs should be further explored and are likely to be case specific. The chemical coagulation and flocculation mechanisms (e.g. charge neutralization, bridge formation, electrostatic patch, precipitative coagulation) responsible for the differences in performance between the biopolymer coagulants [9, 11] should be addressed by further studies.
5 Conclusions
This study demonstrates that biopolymers are potential coagulants for treating agricultural water pollution. As many biopolymers, such as chitin, can be obtained from waste of industrial production, the use of biodegradable and metal-free biopolymers as coagulants is a promising management option for treatment of agricultural wastewater and in nutrient recycling. However, the performance of biopolymer coagulants in field conditions should be tested to address applicability in practical scale and in variable environmental conditions. The best potential of biopolymer coagulants in treatment of agricultural wastewaters is likely to be in treatment of point source effluents from farms that have high concentrations of nutrients and organic pollution or in treatment of diffuse loading that have a high concentration of pollutants. At low pollutant concentrations the biopolymer dosing needs to be very precise for optimal coagulation and flocculation or otherwise there is a danger of deteriorating the water quality. Thus, very accurate dose adjustment relative to wastewater volume needs to be implemented for treatment in applied settings. The substantial retention of phosphorus in biopolymer coagulants suggests high potential to recycle the retained phosphorus in agriculture. However, the remineralization of phosphorus and the response of crop plants to biopolymer sludge fertilization should be explored in further experiments.
References
Carpenter SR, Caraco NF, Correl DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
Hilton J, O’Hare M, Bowes MJ, Jones JI (2006) How green is my river? A new paradigm of eutrophication in rivers. Sci Total Environ 365:66–83
Turunen J, Muotka T, Vuori KM, Karjalainen SM, Rääpysjärvi J, Sutela T, Aroviita J (2016) Disentangling the responses of boreal stream assemblages to low stressor levels of diffuse pollution and altered channel morphology. Sci Total Environ 544:954–962
Turunen J, Markkula J, Rajakallio M, Aroviita J (2019) Riparian forests mitigate harmful ecological effects of agricultural diffuse pollution in medium-sized streams. Sci Total Environ 649:495–503
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Withers PJA, Haygarth PM (2007) Agriculture, phosphorus and eutrophication: a European perspective. Soil Use Manag 23:1–4
Ekholm P, Rankinen K, Rita H, Räike A, Sjöblom H, Raateland A, Vesikko L, Bernal JEC, Taskinen A (2015) Phosphorus and nitrogen fluxes carried by 21 Finnish agricultural rivers in 1985–2006. Environ Monit Assess 187:216–232
Cordell D, Drangert JO, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Change 19:292–305
Ebeling JM, Sibrell PL, Ogden SR, Summerfelt ST (2003) Evaluation of chemical coagulation-flocculation aids for the removal of suspended solids and phosphorus from intensive recirculating aquaculture effluent discharge. Aquacult Eng 29:23–42
de-Bashan LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as a fertilizer (1997–2003). Water Res 38:4222–4246
Lee CS, Robinson J, Chong MF (2014) A review on application of flocculants in wastewater treatment. Process Saf Environ Prot 92:489–508
Suopajärvi T, Liimatainen H, Hormi O, Niinimäki J (2013) Coagulation–flocculation treatment of municipal wastewater based on anionized nanocelluloses. Chem Eng J 23:59–67
Renault F, Sancey B, Badot PM, Crini G (2009) Chitosan for coagulation/flocculation processes—an eco-friendly approach. Eur Polym J 45:1337–1348
Bratskaya S, Scwarz S, Chervonetsky D (2004) Comparative study of humic acids flocculation with chitosan hydrochloride and chitosan glutamate. Water Res 38:2955–2961
Bratskaya SY, Avramenko VA, Sukhoverkhov SV, Schwarz S (2002) Flocculation of humic substances and their derivatives with chitosan. Colloid J 64:681–685
Leiviskä T, Sarpola A, Tanskanen J (2012) Removal of lipophilic extractives from debarking wastewater by adsorption on kaolin or enhanced coagulation with chitosan and kaolin. Appl Clay Sci 61:22–28
Roussy J, Van Vooren M, Guibal E (2005) Influence of chitosan characteristics on coagulation and flocculation of organic suspensions. J Appl Polym Sci 98:2070–2079
Wang J-P, Chen Y-Z, Yuan S-J, Sheng G-P, Yu H-Q (2009) Synthesis and characterization of a novel cationic chitosan-based flocculant with a high water-solubility for pulp mill wastewater treatment. Water Res 43:5267–5275
Bina B, Mehdinejad MH, Nikaeen M, Attar HM (2009) Effectiveness of chitosan as natural coagulant aid in treating turbid waters. Iran J Environ Sci Eng 6:247–252
Divakaran R, Pillai VNS (2001) Flocculation of kaolinate suspensions in water by chitosan. Water Res 35:3904–3908
Jadhav MV, Mahajan YS (2013) Investigation of the performance of chitosan as a coagulant for flocculation of local clay suspensions of different turbidities. KSCE J Civ Eng 17:328–334
Li J, Jiao S, Zhong L, Pan J, Ma Q (2013) Optimizing coagulation and flocculation process for kaolinite suspension with chitosan. Colloids Surf A 428:100–110
Roussy J, Van Vooren M, Dempsey BA, Guibal E (2005) Influence of chitosan characteristics on the coagulation and the flocculation of bentonite suspensions. Water Res 39:3247–3258
Szyguła A, Guibal E, Palacín M, Ruiz M, Sastre AM (2009) Removal of an anionic dye (Acid Blue 92) by coagulation-flocculation using chitosan. J Environ Manag 90:2979–2986
Bhalkaran S, Wilsin LD (2016) Investigation of self-assembly processes for chitosan-based coagulant-flocculant systems:a mini-review. Int J Mol Sci 17:1–21
Chi FH, Cheng WP (2006) Use of chitosan as coagulant to treat wastewater from milk processing plant. J Polym Environ 14:411417
Özacar M, Şengil A (2003) Evaluation of tannin biopolymer as a coagulant aid for coagulation of colloidal particles. Colloids Surf A Physicochem Eng Asp 229:85–96
Martín-Sánchez J, Beltrán-Heredia J, Solera-Hernández C (2010) Surface water and wastewater treatment using a new tannin-based coagulant. Pilot plant trials. J Environ Manage 91:2051–2058
Åström M, Björklund A (1995) Impact of acid sulfate soils on stream water geochemistry in western Finland. J Geochem Explor 55:163–170
Beltrán-Heredia J, Martín-Sánchez J (2009) Municipal wastewater treatment by modified tannin flocculant agent. Desalination 249:353–358
Rizzo L, Lofrano G, Belgiorno V (2010) Olive mill and winery wastewaters pre-treatment by coagulation with chitosan. Sep Sci Technol 45:2447–2452
Teh CY, Wu TY, Juan JC (2014) Potential use of rice starch in coagulation–flocculation process of agro-industrial wastewater: treatment performance and flocs characterization. Ecol Eng 71:509–519
Laudon H, Berggren M, Ågren A, Buffam I, Bishop K, Grabs T, Jansson M, Köhler S (2011) Patterns and dynamics of dissolved organic carbon (DOC) in boreal streams: the role of processes, connectivity, and scaling. Ecosystems 14:880–893
Acknowledgements
Open access funding provided by Finnish Environment Institute (SYKE). We warmly thank Chemigate Oy, Haarla Oy and Wellgreen technology Co. for providing us the biopolymers for this study. We thank Tarja Stenman, Samuli Lahtela for providing us the water samples from Tarvaala and Valentine Leroy for assisting in the laboratory. The study was funded by Finnish Ministry of Environment in a funding programme aiming to enhance the state of river basins and marine areas. The study also implements the Government key programme ‘Breakthrough to a circular economy and adoption of clean solutions’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Turunen, J., Karppinen, A. & Ihme, R. Effectiveness of biopolymer coagulants in agricultural wastewater treatment at two contrasting levels of pollution. SN Appl. Sci. 1, 210 (2019). https://doi.org/10.1007/s42452-019-0225-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0225-x