Abstract
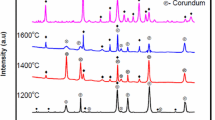
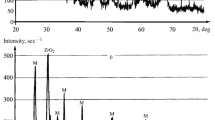
To process mullite ceramics, diphasic aluminosilicate gel was synthesized from inorganic salts by employing the sol-gel route. The process of mullitization was studied by FTIR, DTA and SEM analysis. The particle size of the mullite ceramics was found to be in the nanometer range. The gel was calcined at 800°C. To the calcined gel, oxides of titanium and vanadium were mixed separately. The powder masses were compacted at 100 MPa pressure and then sintered at different elevated temperatures. The mechanical and microstructural properties of the doped samples were studied, and it was found that both the oxides influenced the process of mullitization positively.




Similar content being viewed by others
References
Aksay, I.A., Pask, J.A.: Stable and metastable equilibria in system SiO2-Al2O3.J. Eur. Ceram. Soc. 58 (1975) 507–512.
Klug, F.J., Prochazka, S.: Doremus, R.H. Alumina-silica phase diagram in the mullite region. J. Am. Ceram. Soc. 70 (1987) 750–759.
Schneider, H., Eberhard, E.: Thermal expansion of mullite.J. Am. Ceram. Soc. 73 (1990) 2073–2076.
Hynes, A.P., Doremus, R.H.: High-temperature compressive creep of polycrystalline mullite. J. Am. Ceram. Soc. 74 (1991) 2469–2475.
Kollenberg, W., Schneider, H.: Microhardness of mullite at temperatures to 1000 °C. J. Am. Ceram. Soc. 72 (1989) 1739–1740.
Aksay, A., Dabbs, D.M., Sarikaya, M.: Mullite for structural, electronic and optical applications. J. Am. Ceram. Soc. 74 (1991)2343–2358.
Skoog, A.J., Moore, R. E.: Refractory of the past for the future: mullite and its use as a bonding phase. Am. Ceram. Soc. Bull. 67 (1988)1180–1185.
Ramakrishnan, V., Goo, E., Roldan, J.M., Giess, E.A.: Microstructure of mullite ceramics used for substrate and packaging applications. J. Mater. Sci. 27 (1992) 6127–6130.
Mazel, F., Gonon, M., Fantozzi, G.: Manufacture of mullite substrates from andalusite for the development of thin film solar cells. J. Eur. Ceram. Soc. 22 (2002) 453–461.
Shinohara, N., Dabs, D.M., Aksay, I.A.: Infrared transparent mullite through densification of monolithic gels at 1250 °C. Proc. SPIE—Int. Soc. Opt. Eng. 83 (1986) 19–24.
Cividanes, L.S., Campos, T.M.B., Rodrigues, L.A., Brunelli, D.D.: Thim, G.P. Review of mullite synthesis routes by solgel method. J. Sol-Gel Sci. Technol. 55 (2010) 111–125.
Padmaja, P., Anilkumar, G.M., Warrier, K.G.K.: Characterization of stoichiometric sol-gel mullite by fourier transform infrared spectroscopy. Int. J. Inorg. Mater.3(2001) 693–698.
Yu, J., Shi, J., Yuan, Q., Yang, Z., Chen, Y.: Effect of composition on the sintering and microstructure of diphasic mullite gels, Ceram. Int. 26 (2000) 255–263.
Campos, A.L., Silva, N.T., Melo, F.C.L., Oliveira, M.A.S., Thim, G.P.: Crystallization kinetics of orthorhombic mullite from diphasic gels. J. Non-Cryst. Solids. 304 (2002) 19–24.
Buljan, I., Kosanovic, C., Kralj, D.: Novel synthesis of nanosized mullite from aluminosilicate precursors. J. Alloy. Comp. 509 (2011) 8256–8261.
Roy, J., Maitra, S.: Synthesis and Characterization of Sol-Gel-Derived Chemical Mullite. J. Ceram. Sci. Tech. 5 (2014) 57–62.
Murkhy, M. K., Hummel, F. A.: X-ray study of the solid solution of TiO2, Fe2O3, and Cr2O3 in mullite (3Al2O3•2SiO2). J. Am. Ceram. Soc. 43 (1960) 267–273.
Baudin, C., Moya, J. S.: Influence of titanium dioxide on the sintering and microstructural evolution of mullite. J. Am. Ceram.Soc. 67 (1984) c–134–136.
Naga, S. M., El-Maghraby, A.: Preparation and characterization of porous fibrous mullite bodies with TiO2. Mater. Charact. 62 (2011) 174–180.
Zhang, J., Wu, H., Zhang, S., Yu, J., Xiao, H.: Anisotropic grain growth in diphasic-gel-derived vanadium pentoxide doped mullite. J. Cryst. Growth. 364 (2013) 11–15.
Roy, J., Bandyopadhyay. N., Das, S., Maitra, S.: Effect of TiO2 on the formation of mullite ceramics from diphasic Al2O3-SiO2 gel. Interceram 03–04 (2010) 213–217.
Roy, J., Bandyopadhyay. N., Das, S., Maitra, S.: Role of V2O5 on the formation of chemical mullite from aluminosilicate precursor. Ceram. Int. 36 (2010) 1603–1608.
Roy, J., Bandyopadhyay. N., Das, S., Maitra, S.: Studies on the formation of mullite from diphasic Al2O3-SiO2 Gel by Fourier Transform Infrared Spectroscopy. Iran. J. Chem. Chem. Eng. 30 (2011) 65–71.
Okada, K.: Activation energy of mullitization from various starting materials, J. Eur. Ceram. Soc. 28 (2008) 377–382.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, J., Maitra, S. Effect of Titanium and Vanadium on Nanomullite Derived from Diphasic Precursor Gel. Interceram. - Int. Ceram. Rev. 67, 22–29 (2018). https://doi.org/10.1007/s42411-018-0036-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42411-018-0036-x