Abstract
We explored the relative clinical efficacy of intensity-modulated radiation therapy (IMRT) in patients with oropharyngeal cancer (OPC) compared with three-dimensional conformal radiation therapy (3D-CRT). Seventy-four OPC patients treated with definitive IMRT or 3D-CRT between May 2010 and December 2018 were analyzed. Of these, 42 patients were treated with IMRT and 32 with 3D-CRT. We compared clinical benefits and complications. Particular attention was focused on osteoradionecrosis (ORN), which is the most problematic radiation late adverse event, and evaluated the irradiated mandibular volumes. There was no significant difference in the 3-year overall survival (OS), progression free survival (PFS) and locoregional control (LRC) rates between the two groups. However, late Grade 2 xerostomia was lower in the IMRT groups. Four patients (19.0%) developed Grade 3, while no patients developed ORN in the IMRT group (P = 0.003). A comparison of the mandible volumes between the two groups showed that the IMRT group had lower mandibular volumes than the 3D-CRT group in the high-dose range of V40, V50, V60 and V70. In addition, a comparison of the mandibular volumes between ORN and non-ORN showed a significant difference in the V60 and V70. ORN incidence was higher in V60 ≥ 25% and V70 ≥ 15%. A comparison of IMRT and 3D-CRT showed no significant difference in outcomes, however, IMRT led to a significant reduction in late xerostomia and ORN in OPC patients. Reducing of the mandibular V60 and V70 by IMRT seemed to reduce the risk of ORN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Japan, the incidence rate of Oropharyngeal cancer (OPC) is 1.8 cases per 100,000 individuals, with 2,277 reported cases in 2019 [1]. When compared to the past decade, there is a gradual upward trend in incidence rates. In recent years, oropharyngeal cancer caused by Human papillomavirus (HPV) infection has been exhibiting an upward trend in Japan. Since the year 2000, approximately 50% of oropharyngeal cancer cases have been reported as HPV-positive, with HPV type 16 constituting 90% of these cases [2]. Surgery and radiation therapy (RT) have traditionally been the standard treatments for OPC [3, 4]. Based on data from November 2022, the 5-year overall survival (OS) for OPC is reported to be 61.3% [5].
Intensity-modulated radiation therapy (IMRT) was developed in the last decade of the twentieth century, and is a system of radiation treatment planning and delivery that allows for more optimal radiation dose distributions [6]. In Japan, IMRT technology was introduced into the medical field around 2000. By 2008, it gained insurance coverage for the treatment of brain tumors, head and neck cancers, and prostate cancer. Subsequently, in 2005, only 170 (22.2%) of the 765 linear accelerators in Japan had IMRT capability, but in 2011, 412 (49.2%) of the 836 linear accelerators in Japan had IMRT capability [7].
Recently, with the implementation of IMRT, a reduction in the incidence of osteoradionecrosis (ORN) has been reported in head and neck cancer treatment [8, 9]. ORN is the most problematic late radiation adverse event, characterized by soft tissue ulceration and exposure of necrotic bone. Despite medical intervention, ORN is refractory and results in long-term deterioration of quality of life (QOL). In a review of ORN and IMRT, De Felice et al. [9] reported a decrease in the incidence of ORN compared to before the introduction of IMRT. However, few studies have compared the outcomes of IMRT and three-dimensional conformal radiation therapy (3D-CRT) for OPC in Japan, and even fewer reports have evaluated ORN. Given the above, we believe it is important to evaluate the clinical efficacy and complications of OPC in Japan by comparing IMRT and 3D-CRT, with a particular focus on ORN and the irradiated mandibular volumes.
Materials and Methods
Patients
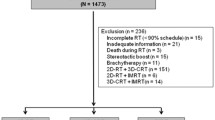
The records of 110 patients treated consecutively with definitive RT for OPC between May 2010 and December 2018 were reviewed in single-institution. Eligibility criteria were as follows: biopsy-proven squamous cell carcinoma, no distant metastases, Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0–2 and written informed consent before the treatment. Thirty-six patients were excluded from the analysis because of combined treatment with both 3D-CRT and IMRT (n = 22), death from acute myocardial infarction before the end of RT (n = 1), discontinuation due to systemic disease progression during RT (n = 1), dropout from RT (n = 1), unknown HPV status (n = 8), or other reasons (n = 3). The remaining 74 patients were included in this analysis.
The staging of OPC was based on the TNM system of the Union for International Cancer Control (UICC) 8th edition which was revised in 2017. Initially, the previous version of the staging system was used, but it was decided to reevaluate the staging system in the process of conducting this study. Since HPV status is prognostically relevant and important for staging in the UICC 8th edition, patients without HPV status were excluded from the study. Prior to initiation of the treatment, all patients provided a comprehensive medical history and underwent a physical examination and dental evaluation. The staging evaluation included an endoscopic evaluation, palpation of the neck, a complete blood count, computed tomography (CT), positron emission tomography (PET)/CT and cervical magnetic resonance imaging (MRI). The majority of these imaging studies were conducted primarily within the month preceding the commencement of treatment.
Chemotherapy
The chemotherapy regimens consisted predominantly of platinum-based medicines, i.e., cisplatin (CDDP), carboplatin (CBDCA), and nedaplatin (CDGP). Patients without sufficient renal function were given CBDCA or CDGP instead of CDDP. The standard regimen of CDDP consisted of 80 mg/m2 every four weeks intravenously concurrently combined with RT. Toxicity considerations, preexisting medical conditions, and patient preference resulted in the use of S-1 or cetuximab. When the patient had double cancer of esophageal cancer and OPC, we chose the regimens of 5-fluorouracil (5-FU)/ CDDP. No cases underwent induction chemotherapy (ICT).
Radiotherapy
At our institution, we traditionally used 3D-CRT for head and neck cancer. All patients were treated with a sequential-boost (SQB) method consisting of whole-neck radiotherapy to 40 Gy, followed by a boost to the primary tumor and metastatic lymph nodes to a total dose of 70 Gy. When we started performing IMRT in July 2010, whole-neck radiotherapy was irradiated with 3D-CRT, and the SQB plan was irradiated with IMRT. Since January 2015, all patients with OPC have been irradiated exclusively with IMRT, from whole-neck radiotherapy to the boost plan.
Patients were immobilized in a supine treatment position in a custom-made head-and-neck mask. CT-based simulation was performed in all patients. The XiO version 5.10 (CMS, St. Louis, MO, USA) treatment planning system was used to create an individualized radiation therapy plan for 3D-CRT patients, and Eclipse planning version 13.6 (Varian Medical Systems, Palo Alto, CA, USA) was used for treatment planning for IMRT patients. In some cases, the MRI and the PET/CT findings were used as a guide for the contours of tumor volumes on workstations.
The gross tumor volume (GTV) was defined by a clinical examination and diagnostic imaging using modalities such as CT, MRI and/or FDG-PET. The clinical target volume 1 (CTV1) included the GTV primary with an expansion of 5 mm, and the GTV node with an expansion of 5 mm. The planning target volume 1 (PTV1) was defined as CTV1 with a 5 mm margin in all dimensions. The CTV prophylactic (CTV2) was designed to include the lymph nodes at Levels II-V and VII according to nodal regions as defined by the Radiation Therapy Oncology Group (RTOG) consensus guidelines [10]. In cases with base of tongue disease or lymphatic involvement on Level II, Level Ib was included in the CTV prophylactic. In patients with positive nodes, the supraclavicular region was also included. PTV2 was defined as CTV2 with a 5 mm margin in every direction. Avoidance structures, such as the spinal cord, brainstem, parotid glands, oral cavity, and larynx, were delineated at the time of treatment planning.
In 3D-CRT, the planned dose at the isocenter was prescribed as 40–41.4 Gy/20–23 fractions to the sum of the PTV1 and PTV2, followed by 30 Gy/15 fractions to PTV1 with a total dose of 70–71.4 Gy/35–38 fractions (median 71.4 Gy). 3D-CRT treatments were delivered with an ONCOR Impression plus medical accelerator (Siemens Healthineers, Erlangen, Germany). The conventional technique with parallel-opposed lateral fields and a low-anterior-neck field matched below the primary site, or lateral opposed fields with a field-in-field technique, was used. In IMRT, a planned dose of 40–41.4 Gy/20–23 fractions were prescribed for 95% of the volume of the sum of PTV1 and PTV2, and then boosted to PTV1 for a total dose of 66–70.2 Gy/33–39 fractions (median 70.2 Gy). For organs at risk, treatment planning goals include a maximum dose to the spinal cord < 50 Gy, a mean dose to the parotid dose < 26 Gy, and a maximum dose to the brainstem < 54 Gy. We decided to reduce the oral cavity and larynx doses as much as possible without compromising the irradiation of the target. At this point, we had not established dose constraints to the mandible. IMRT treatments were delivered using the Volumetric Modulated Arc Therapy (VMAT) technique with Varian accelerators (Varian Medial Systems). The X-rays beam energies used in 3D-CRT and IMRT were 6–10 MV.
Boost plans were re-planned when weight loss was significant. We have not differentiated based on HPV status in terms of dose and target volume.
Definition of ORN
ORN was defined as slow-healing radiation-induced ischemic necrosis of bone with associated soft tissue necrosis, in the absence of local primary tumor necrosis, recurrence, or metastatic disease, with bone exposed through the skin or mucosa persisting for more than 3 months [11]. We suspected ORN based on the clinical presentation (pain, the ulceration of the soft tissue, the exposure of necrotic bone, trismus, tooth movement); when CT/MRI showed bone destruction and denied recurrence of the lesion, we ultimately diagnosed ORN. The ORN was graded by Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [12] as follows: Grade 1, minimal bone exposure with conservative management only; Grade 2, minor debridement received; Grade 3, hyperbaric oxygen needed; Grade 4, major surgery required.
Endpoints and Statistical Analysis
Follow-up evaluations after the treatment were typically performed at intervals of one month for one year, at intervals of three months from one to three years post-treatment and at intervals of six months from three to five years post-treatment. When necessary, upper gastrointestinal fiberscopy and CT were performed at least annually during the follow-up.
Characteristics for both radiation treatment groups were compared using either a T-test or Fisher’s exact test.
The OS was measured from the start date of any treatment at our hospital to the date of the last follow-up or death from any cause. The progression free survival (PFS) was measured from the start date of any treatment at our hospital until the date of disease progression or death. The locoregional control (LRC) was defined as local and/or regional progression as an event. The OS, PFS and LRC rates were calculated using Kaplan–Meier estimates [13].
A multivariate analysis (MVA) for OS, PFS and LRC rates was performed using Cox's proportional hazards regression analysis, with age (> 60 vs. ≤ 60), HPV status (positive vs. negative), overall stage (I–II vs. III–IV), concurrent chemotherapy (CCCH) (yes vs. no), and radiation technique (IMRT vs. 3D-CRT) considered as explanatory variables. P < 0.05 was considered as significant.
Acute adverse events were defined as symptoms observed during radiotherapy and up to eight weeks after treatment. Late adverse events were defined as symptoms observed after that time. Adverse events were classified according to the CTCAE version 5.0 [12]. Fisher’ s exact test was used for comparisons between two groups.
We compared the parotid gland dose and the mandibular doses between the two groups. Mandibular dose-volume histograms (DVHs) were extracted. T-test was used to compare the mandibular volumes that received > 10, 20, 30, 40, 50, 60 and 70 Gy (V10, V25, V30, V40, V50, V60 and V70, respectively). Fisher’s exact test was used to examine the difference in ORN incidence by dosimetric parameters. In addition, for patients with ORN, we investigated the difference in the mandibular volumes produced by different irradiation methods. Furthermore, a MVA for ORN was executed using Cox's proportional hazards regression analysis, with T-stage and N-stage as explanatory variables.
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (version 2.13.0; The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander (version 1.6–3) that was designed to add statistical functions frequently used in biostatistics [14]. P < 0.05 was considered as significant.
Results
Patient and Treatment Characteristics
The median follow-up period was 48 months (range, 7–86) for IMRT patients and 66 months (range, 13–120) for 3D-CRT patients. The characteristics of the 74 eligible patients are shown in Table 1. The number of HPV-positive patients was higher in the IMRT group than in the 3D-CRT group; conversely, in the 3D-CRT group, there were more patients with T3–4, and the overall stages were more advanced than in the IMRT group. CCRT was performed in 38 of 42 patients (90.5%) in the IMRT group and all patients (100%) in the 3D-CRT. Most patients (32 of 38 patients [84.2%] in the IMRT group and 29 of 32 patients [90.6%] in the 3D-CRT group) received platinum-based chemotherapy regimens. Of these, CDDP was the most commonly used, (in 28 patients for IMRT and 27 patients for 3D-CRT). Three patients in the IMRT group received cetuximab. One patient for IMRT and two patients for 3D-CRT received S-1. Two patients in the IMRT group and one patient in the 3D-CRT group with esophageal double cancer received 5-fluorouracil (5-FU)/ CDDP (FP).
The median total dose in the IMRT group was 70.2 Gy (range, 66–70.2 Gy) at the D95 of the PTV. Thirty-six patients (85.7%) received a total dose of 70.2 Gy, 4 (9.5%) received 70 Gy, and 2 (4.8%) received 66 Gy. The median total dose of patients treated with 3D-CRT was 71.4 Gy (range, 70–71.4 Gy) at the isocenter. Fifteen patients (46.9%) received a total dose of 71.4 Gy, 13 (40.6%) received 70.2 Gy, and 4 (12.5%) received 70 Gy.
During treatment, eight patients (two in the IMRT group and six in the 3D-CRT group) required interruption of irradiation for more than seven days.
In the IMRT group, three patients (7.1%) underwent planned neck dissection after definitive RT, but no patient had residual tumor. In contrast, planned neck dissection was performed in 16 patients (50.0%) in the 3D-CRT group, with residual tumors being confirmed histologically in nine.
Treatment Outcomes
Nine out of 42 patients (21.4%) in the IMRT group, and 12 out of 32 patients (37.5%) in the 3D-CRT group had died by the time of this analysis (May 31, 2022). In the IMRT group, two patients died of OPC, one patient died of hematemesis of the internal carotid artery by mucositis, three patients died of double cancer (esophagus, tongue, and malignant lymphoma: one each), and three patients died of other factors. In the 3D-CRT group, five patients died of OPC, one patient died of aspiration pneumonia, five patients died of double cancer (one: esophagus; three: lung; one: malignant lymphoma), and one patient died of other factors.
In the IMRT group, five patients (11.9%) relapsed; these consisted of one patient with relapse at the local site, one with relapse at the regional site, two with relapse at the local and regional sites and one with relapse at the regional sites and with distant metastasis (lung). Of the four patients with locoregional recurrence alone, three underwent salvage surgery including neck dissection and/or resection of the tumor at the primary site, and one received systemic chemotherapy. One patient with distant metastasis in addition to regional sites recurrence received immunotherapy and systemic chemotherapy.
In the 3D-CRT group, 10 patients (31.3%) relapsed; these consisted of two patients with relapse at the local site, five with relapse at the local and regional sites, two with lung metastasis, and one with relapse at the regional sites in addition to distant metastasis (lung). Of the seven patients with locoregional recurrence alone, five underwent salvage surgery including neck dissection and/or resection of the tumor at the primary site, followed by systemic chemotherapy in two cases. The remaining two patients chose best supportive care. One out of the three patients with distant metastases received salvage surgery and systemic chemotherapy, and the other two received systemic chemotherapy.
The 3-year OS was 90.4% (95% confidence interval [CI], 76.4–96.3%) in the IMRT group and 78.1% (95% CI, 59.5–88.0%) in the 3D-CRT group (P = 0.159). The 3-year PFS was 88.0% (95% CI, 73.5–94.8%) for IMRT and 71.7% (95% CI, 52.7–84.2%) for 3D-CRT (P = 0.093). The 3-year LRC was 88.0% (95% CI, 73.5–94.8%) for patients who underwent IMRT and 74.6% (95% CI, 55.5–86.4%) for patients who underwent 3D-CRT (P = 0.163).
Table 2 shows the results of the MVA for OS, PFS and LRC rates. Overall stage and concurrent CHT were significant predictors of OS, PFS and LC rates. On the other hand, there were no statistically significant differences in OS, PFS, and LC among the different radiation techniques.
Acute Adverse Events
We showed the acute toxicities among patients in Table 3. The incidence of acute Grade 2 xerostomia was lower in the IMRT group than in the 3D-CRT group, with Grade 2 xerostomia was detected in 25 (59.5%) and 29 patients (90.6%) in the IMRT and 3D-CRT groups, respectively (P = 0.003). However, there was no significant difference in the incidence of other acute adverse events between the two groups.
Late Adverse Events
There was one patient with Grade 5 mucositis in the IMRT group. The patient was a 52-year-old female with OPC cT4N3M0 (HPV +). The tumor was in full circumferential contact with the internal and external carotid arteries. She was treated with definitive RT and concurrent cetuximab. The patient continued to have prolonged mucositis and a poor oral intake, resulting in a weight loss of -20 kg and poor nutritional status. However, she refused to be hospitalized and could not receive adequate tube feeding or oral protection. Five months after completion of RT, the patient began bleeding from the right external carotid artery and died. One patient in the 3D-CRT group died of aspiration pneumonia. Late Grade 3 dysphasia was detected in 1 (3.1%) and 1 patient (4.8%) in the IMRT and the CRT groups, respectively. No patient required a percutaneous endoscopic gastrostomy (PEG) tube.
At 2 years post-treatment, the incidence of late Grade 2 xerostomia was significantly lower in the IMRT group than the 3D-CRT group: 6 (19.4%) and 15 patients (71.4%) in the IMRT and 3D-CRT groups, respectively (P = 0.003). The dose to the parotid gland was significantly lower in the IMRT group than in the 3D-CRT, both ipsilaterally and contralaterally; the mean radiation doses to the ipsilateral and contralateral parotid glands for IMRT vs. 3D-CRT were 33.69 Gy (range, 19.25–53.08 Gy) vs. 69.09 Gy (range, 45.69–72.56 Gy) (P < 0.001) and 23.48 Gy (range, 11.68–39.99 Gy) vs. 43.8 Gy (range, 28.58–60.44 Gy) (P < 0.001), respectively.
Four patients (19.0%) developed Grade 3 ORN, one patient (4.8%) developed Grade 2 ORN, and one patient developed Grade 3 laryngeal necrosis in the 3D-CRT group, while no patients developed ORN and laryngeal necrosis in the IMRT group. There was a significant difference in the incidence of Grade 3 ORN between the two groups (P = 0.003). The mean radiation doses to the mandible for IMRT vs. 3D-CRT were 33.90 Gy (range, 23.24–43.27 Gy) vs. 39.75 Gy (range, 31.15–46.05 Gy) (P = 0.003). The max radiation doses to the mandible for IMRT vs. 3D-CRT were 68.63 Gy (range, 53.77–72.75 Gy) vs. 74.34 Gy (range, 68.77–77.99 Gy) (P = 0.001). Figure 1 shows the results of compared the mean percentage of volumes of the mandible receiving > 10, 20, 30, 40, 50, 60 and 70 Gy (V10, V25, V30, V40, V50, V60 and V70, respectively) between the IMRT and 3D-CRT groups. The low-dose range of the mandible (V10 and V20) was higher in the IMRT group, whereas the high-dose range of the mandible (V40, V50, V60 and V70) was higher in the 3D-CRT group than in the IMRT group, with both differences being statistically significant. We also compared the relative volumes of the mandible between the ORN and the non-ORN groups. The result was shown in Fig. 2. The mandibular V60 and V70 values were significantly higher in the ORN group than in the non-ORN group.
Mean percent of volume of mandible receiving between 10 and 70 Gy (IMRT vs. 3D-CRT). Each error bar is constructed using a 95% confidence interval of the mean. The low doses range of the mandible (V10, 20) was higher in the IMRT group, on the other hand the high doses range of the mandible (V40, V50, V60 and V70) were higher in the 3D-CRT group. IMRT intensity-modulated radiation therapy; 3D-CRT three-dimensional conventional radiation therapy
Moreover, the ORN incidence was 16.7% for the mandibular V60 ≥ 25% and 2.1% for V60 < 25% (P = 0.040), and, 23.1% for the mandibular V70 ≥ 15% and 3.4% for V70 < 15% (P = 0.038) (Table 4).
Clinical Characteristics of Patients with ORN
Table 5 shows the five patients in the 3D-CRT group who developed ORN, including four with Grade 3 and one with Grade 2. ORN was located on the ipsilateral side of the gross disease in all cases. The four patients had no cervical lymph node metastasis. The median time to develop ORN was 54 months (range, 32–96 months). The mandibular V70 values in patient 1 and 3 were in the high 30% range. Patient 2 continued smoking after RT and developed diabetes mellitus (DM) after RT. Patient 4 had a poor oral status and had to undergo extractions after RT. Patients 1–5 were treated with antibiotics and hyperbaric oxygen therapy (HBO). However, the discomfort continued, and the condition was not completely improved.
These five patients of ORN were re-planned with IMRT, and the volumes of the mandible receiving > 10, 20, 30, 40, 50, 60 and 70 Gy (V10, V25, V30, V40, V50, V60 and V70, respectively) were compared. The results were shown in Fig. 3. IMRT irradiation was found to significantly decrease the mandibular V40, V50, V60, and V70 values. Figure 4 shows a patient with right soft palate carcinoma, cT1N0M0, in which the isodose line of 70 Gy was observed bilateral mandible by irradiation using 3D-CRT, which was reduced when irradiated using IMRT.
Mean percent of volume of mandible receiving between 10 and 70 Gy of osteoradionecrosis patients (IMRT vs. 3D-CRT). Each error bar is constructed using a 95% confidence interval of the mean. The 5 patients of ORN were re-planned with IMRT and volume of mandible receiving between 10 and 70 Gy were compared with those of irradiated with 3D-CRT. IMRT irradiation was found to significantly decrease the mandibular V40, V50, V60, and V70 values. IMRT intensity-modulated radiation therapy; 3D-CRT three-dimensional conventional radiation therapy; ORN osteoradionecrosis
The patient was a 75-year-old male with oropharyngeal cancer (rt. soft palate), cT1N0M0. a) Isodose curves of irradiation with 3D-CRT. b) Isodose curves of irradiation with IMRT. c) CT; the region with ORN indicated by the yellow arrows. The region of ORN corresponding to the high dose region. 3D-CRT three-dimensional conventional radiation therapy; IMRT intensity-modulated radiation therapy; CT computed tomography; ORN osteoradionecrosis
Table 6 shows the results of the MVA for ORN. No significant differences in the incidence of ORN were observed based on T-stage (1–2 vs. 3–4) or N-stage (0–1 vs. 2–3).
Discussion
A comparison of IMRT and 3D-CRT showed no significant difference in outcomes, however, IMRT led to a significant reduction in late xerostomia and ORN in OPC patients.
In the domain of radiation oncology, the concept of IMRT emerged during the mid-1990s. By leveraging computer-aided inverse treatment planning and dynamic modulation of radiation beam intensity during treatment, IMRT facilitated the delivery of 3D conformal radiotherapy. This state-of-the-art technology enabled the targeted attenuation of radiation dose to essential normal structures, such as the parotid glands and mandible in cases of HNC, even when these structures were contiguous or enmeshed with tumors [15].
The majority of studies comparing IMRT to CRT for patients with OPC have reported no marked difference in tumor control rates between these two techniques. Alterio et al. [16] conducted a systematic review and meta-analysis of eight studies. They showed that there was no statistical difference between IMRT and 2D/3D-RT in terms of death (SRR = 0.93, 95% CI: 0.83–1.04 with no heterogeneity I2 = 0%) and relapse (SRR = 0.92, 95% CI: 0.83–1.03, with no heterogeneity I2 = 0%). In terms of treatment outcomes, the present study yielded similar results to previous reports. In this study, differences in patient characteristics (HPV status, T-stage, overall stage) were observed between the two groups, which might have introduced some bias in the evaluation of treatment outcomes. However, as shown in Table 2, the results of the MVA demonstrated that there were no significant differences in OS, PFS, and LC based on variations in radiation technique, thereby proving that there was no disparity in treatment outcomes due to differences in irradiation techniques.
The present study revealed differences in patient characteristics between the IMRT and 3D-CRT groups, with variations noted in HPV status, T-stage, and overall stage. Notably, the number of patients who tested positive for HPV has increased in recent years. In Japan, a nationwide study by Hama et al. [2] investigated the HPV prevalence in OPC across 21 institutes participating in the Basic Research Cooperative Group of HNC. The study reported a significant increase in the prevalence of HPV-positive OPC cases from 32% in the 2000s to 50.0% in the 2010s [3]. Our study included patients who commenced radiotherapy after 2014 in the IMRT group, which may have contributed to the higher proportion of HPV-positive patients observed in this group compared to the 3D-CRT group. HPV-positive OPC are distinguished in the UICC 8th edition, revised in 2017, because they are more radiosensitive and chemosensitive than HPV-negative cancers and have a better prognosis [17,18,19]. We suspected that the increased prevalence of HPV positivity in the IMRT group may have contributed to the relatively lower number of Stage IV cases compared to the 3D-CRT group.
Based on a review of previous reports, we concluded that the adverse events observed in our study was within an acceptable range. In the PARSPORT trial [20], a randomized trial conducted in the UK that compared 3D-CRT with IMRT in HNC, xerostomia rates were significantly lower in the IMRT group than in the 3D-CRT group. In the present study, the mean radiation dose to both the ipsilateral and contralateral parotid was also significantly lower in the IMRT group compared to the 3D-CRT group. It has been established that the highest tolerable mean dose to the parotid gland is 26 Gy [21]. According to our outcomes, the parotid gland dose administered by IMRT was found to be tolerable. Furthermore, our findings confirmed that the use of IMRT resulted in a significant reduction in the incidence of late xerostomia.
In our research, there was a significant difference in the incidence of ORN between the two groups. Reuther et al. [22] reported on the median time to ORN onset. The authors reported that among 830 patients monitored over a 30-year period, the median time for the onset of ORN was 13 months (range, 2–122 months). The median follow-up duration in our study was shorter in the IMRT group than in the 3D-CRT group, at 48 months [range, 7–86] and 66 months [range, 13–120], respectively. However, we believe that these durations are not unreasonable for evaluating the incidence rate of ORN based on the literature by Reuther et al. [22] et al.
In reviewing the literature, it is necessary to consider when cases were treated with RT, as technological advances may have contributed to the lower incidence of ORNs. In a study by Wahl et al. [23], the incidence of ORN decreased from 11.8% before 1968 to 5.4% between 1968 and 1992, and further to approximately 3% after 1997. Tsai et al. [8] reported a trend towards a lower incidence of ORN in patients treated with IMRT compared to 3D-CRT in the 2000–2009 period, with rates of 6% and 13%, respectively. The use of IMRT enables selective dose reduction even when the mandible is adjacent to or surrounded by the tumor, which could have contributed to the observed lower incidence of ORN.
Several studies have assessed dosimetric parameters and the potential for ORN, with a majority reporting a markedly elevated risk of ORN in patients receiving substantial radiation doses to the mandible. Chang et al. [24] found a radiation dose of 70 Gy to be significantly associated with an increased risk of ORN. The MD Anderson Head and Neck Cancer Symptom Working Group showed that majority of ORN cases have mandibular V44 ≥ 42% and V58 ≥ 25% in patients with OPC using RPA analysis in a matched case control study [25]. Kubota et al. [26] demonstrated that mandibular V60 > 14% was identified as a significant risk factor of ORN in HNC. In our study, significant differences were observed in the extent of mandibular V40, V50, V60, and V70 values between the IMRT and 3D-CRT groups, with evidence of a dose reduction in the high-dose range. Notably, when mandible volumes were compared between the ORN and non-ORN groups, significant differences were found in the mandibular V60 and V70 values, suggesting their potential importance in predicting ORN risk. Indeed, ORN incidence was observed to be higher in patients with V60 ≥ 25% and V70 ≥ 15%. When the five patients of ORN were re-planned with IMRT, and significant differences were found in the mandibular V40, V50, V60, and V70 values. Four of the five patients with ORN were T1–3N0, and the extent of cancer involvement was localized. Multiple lymph node metastases would have required massive irradiation of the mandible, but the patients were not at that stage. Table 6 shows the results of MVAon the incidence of ORN, but no significant differences were observed in the T-stage and N-stage. After re-planning with IMRT, the mandibular V60 was decreased significantly from 35.74% to 10.44% and the V70 from 19.78% to 2.92%, particularly in the high-dose range. Consequently, the reduction in mandibular V60 and V70 values due to IMRT irradiation was thought to be responsible for the decrease in ORN incidence.
Several reports have identified risk factors for ORN, including DM, smoking, stage of disease, dental health, extraction of teeth after irradiation, and excessive alcohol [9, 27,28,29,30,31,32], Therefore, the occurrence of ORN could not be attributed solely to the irradiation dose to the mandible. Although the reduction in the mandibular V60 and V70 values with the introduction of IMRT seemed to be related to a significant risk reduction. This is because in cohorts of HNC patients who underwent IMRT, Owosho et al. [33] found that smoking and DM were not significant risk factors for ORN. Due to the widespread adoption of RT using IMRT, other risk factors for ORN have become less significant.
The current study is associated with several limitations that require acknowledgment. Firstly, patients were treated by a uniform group of radiation and surgical oncologists, yet they were not chosen or treated based on a prospective protocol. Unmeasured confounding variables and potential selection biases could not be accounted for in our analysis. Second, there were differences in overall stage, T stage, and HPV status between the two groups, which may have slightly biased the assessment of adverse events. We believe that the recent increase in HPV-positive OPC is also behind this patient background. However, the fact that IMRT reduced rate of xerostomia and ORN is very significant. In particular, in this study, we compared DVHs between the IMRT and 3D-CRT groups and between the ORN and non-ORN groups in OPC patients at a single institution. Significant differences were observed in mandibular V60 and V70 values, and it was confirmed that mandibular V60≧25% and V70≧15% are risk factors for ORN. There is no previous paper that examine and clarify these points, and our results are very useful clinically.
In recent years, the increase in HPV-positive OPC and the combined use of immune checkpoint inhibitors (ICI) have improved the prognosis. ORN is refractory, and even if the disease stage is completely cured, patients will suffer from pain, ulceration of the soft tissue, exposure of necrotic bone, and trismus caused by ORN throughout their lifetime. This leads to dietary restrictions, reduced food intake, weight loss, and sarcopenia. When prognosis improvement is anticipated, avoiding late adverse events becomes a very important point. Based on the present findings, we aim to develop treatment plans that avoid hotspots with mandibular V60≧25% and V70≧15% without compromising target coverage.
Conclusion
In this study, we found that IMRT is more useful for reducing the risk of xerostomia and ORN than 3D-CRT. Our study indicated that IMRT irradiation to reduce mandibular V60 and V70 can decrease the risk of ORN. Furthermore, it can be said that reducing mandibular V60 < 25% and V70 < 15% contributes to a decrease in the incidence of ORN. IMRT irradiation can significantly contribute to maintaining the QOL for patients with OPC.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Code availability
Not applicable.
Abbreviations
- IMRT:
-
Intensity-modulated radiation therapy
- OPC:
-
Oropharyngeal cancer
- 3D-CRT:
-
Three-dimensional conformal radiation therapy
- ORN:
-
Osteoradionecrosis
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- LRC:
-
Locoregional control
- HPV:
-
Human papillomavirus
- RT:
-
Radiation therapy
- QOL:
-
Quality of life
- ECOG-PS:
-
Eastern Cooperative Oncology Group performance status
- UICC:
-
Union for International Cancer Control
- CT:
-
Computed tomography
- PET:
-
Positron emission tomography
- MRI:
-
Magnetic resonance imaging
- CDDP:
-
Cisplatin
- CBDCA:
-
Carboplatin
- CDGP:
-
Nedaplatin
- 5-FU:
-
5-Fluorouracil
- ICT:
-
Induction chemotherapy
- SQB:
-
Sequential-boost
- GTV:
-
Gross tumor volume
- CTV:
-
Clinical target volume
- PTV:
-
Planning target volume
- RTOG:
-
Radiation Therapy Oncology Group
- VMAT:
-
Volumetric Modulated Arc Therapy
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- MVA:
-
Multivariate analysis
- CCCH:
-
Concurrent chemotherapy
- DVHs:
-
Dose-volume histograms
- PEG:
-
Percutaneous endoscopic gastrostomy
- DM:
-
Diabetes mellitus
- HBO:
-
Hyperbaric oxygen therapy
- ICI:
-
Immune checkpoint inhibitors
References
Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (National Cancer Registry, Ministry of Health, Labour and Welfare) https://ganjoho.jp/reg_stat/statistics/data/dl/en.html. Accessed 10 Aug 2023.
Hama T, Tokumaru Y, Fujii M, Yane K, Okami K, Kato K, et al. Prevalence of Human papillomavirus in oropharyngeal cancer: a multicenter study in Japan. Oncology. 2014;87:173–82.
Fein DA, Lee WR, Amos WR, Hinerman RW, Parsons JT, Mendenhall WM, et al. Oropharyngeal carcinoma treated with radiotherapy: a 30-year experience. Int J Radiat Oncol Biol Phys. 1996;34:289–96.
Foote RL, Schild SE, Thompson WM, Buskirk SJ, Olsen KD, Stanley RJ, et al. Tonsil cancer. Patterns of failure after surgery alone and surgery combined with postoperative radiation therapy. Cancer. 1994;73:2638–2647.
Monitoring of Cancer Incidence in Japan - Survival 2009–2011 Report (Center for Cancer Control and Information Services, National Cancer Center, 2020)
Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jap J Clin Oncol 2011; 41: 40–51
Garden AS, Kies MS, Morrison WH, Weber RS, Frank SJ, Glisson BS, et al. Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat Oncol. 2013;8:21.
Numasaki H, Teshima T, Nishimura T, Akuta K, Ando Y, Ikeda H, et al. Japanese Structure Survey of Radiation Oncology in 2011. J Radiat Res. 2019;22;60(6):786–802.
Tsai CJ, Hofstede TM, Sturgis EM, Garden AS, Lindberg ME, Wei Q, et al. Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2013;85:415–20.
De Felice F, Musio D, Tombolini V. Osteoradionecrosis and intensity modulated radiation therapy: An overview. Crit Rev Oncol. 2016;107:39–43.
Gregoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, et al. Delineation of the neck node levels for head and neck tumors: a 2013 up date. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110:172–81.
Jacobson AS, Buchbinder D, Hu K, Urken ML. Paradigm shifts in the management of osteoradionecrosis of the mandible. Oral Oncol. 2010;46:795–801.
NIH. National Cancer Institute. DCTD Division of Cancer Treatment & Diagnosis. CTEP Cancer Therapy Evaluation Program. 2020. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc. htm#ctc_50.
Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Kanda Y. Investigation of the freely available easy-to use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Kuppersmith RB, Greco SC, Teh BS, Donovan DT, Grant W, Chiu JK, et al. Intensity-modulated radio-therapy: First results with this new technology on neoplasms of the head and neck. Ear Nose Throat J. 1999;78:238–51.
Alterio D, Gugliandolo SG, Augugliaro M, Marvaso G, Gandini S, Bellerba F, et al. IMRT versus 2D/3D conformal RT in oropharyngeal cancer: A review of the literature and meta-analysis. Oral Dis. 2021;27(7):1644–53.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging(ICON-S):a multicentre cohort study. Lancet Oncol. 2016;17:440–51.
Horne ZD, Glaser SM, Vargo JA, Ferris RL, Balasubramani GK, Clump DA, et al. Confirmation of proposed human papillomavirus risk-adapted staging according to AJCC / UICC TNM criteria for positive oropharyngeal carcinomas. Cancer. 2016;122:2021–30.
Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomized controlled trial. Lancet Oncol. 2011;12:127–36.
Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10-19.
Reuther T, Schuster T, Mende U, Kübler A. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients: a report of a thirty year retrospective review. Int J Oral Maxillofac Surg. 2003;32:289–95.
Wahl MJ. Osteoradionecrosis prevention myths. Int J Radiat Oncol Biol Phys. 2006;64:661–9.
Chang DT, Sandow PR, Morris CG, Hollander R, Scarborough L, Amdur RJ, et al. Do pre-irradiation dental extractions reduce the risk of osteoradionecrosis of the mandible? Head Neck. 2007;29:528–36.
MD Anderson Head and Neck Cancer Symptom Working Group. Dose volume correlates of mandibular osteoradionecrosis in Oropharynx cancer patients receiving intensity-modulated radiotherapy: results from a case-matched comparison. Radiother Oncol. 2017;124:232–9.
Kubota H, Miyawaki D, Mukumoto N, Ishihara T, Matsumura M, Hasegawa T, et al. Risk factors for osteoradionecrosis of the jaw in patients with head and neck squamous cell carcinoma. Radiat Oncol. 2021;16(1):1.
Murray CG, Daly TE, Zimmerman SO. The relationship between dental disease and radiation necrosis of the mandible. Oral Surg Oral Med Oral Pathol. 1980;49:99–104.
Murray CG, Herson J, Daly TE, Zimmerman S. Radiation necrosis of the mandible: a 10 year study. Part I. Factors influencing the onset of necrosis. Int J Radiat Oncol Biol Phys. 1980;6:543–548.
Murray CG, Herson J, Daly TE, Zimmerman S. Radiation necrosis of the mandible: a 10 year study. Part II. Dental factors; onset, duration and management of necrosis. Int J Radiat Oncol Biol Phys. 1980;6:549–553.
Alterio D, Gugliandolo SG, Augugliaro M, Marvaso G, Gandini S, Bellerba F, et al. IMRT versus 2D/3D conformal RT in oropharyngeal cancer: A review of the literature and meta-analysis. Oral Dis. 2021;27(7):1644–53.
Sathasivam HP, Davies GR, Boyd NM. Predictive factors for osteoradionecrosis of the jaws: A retrospective study. Head Neck. 2018;40:46–54.
Owosho AA, Tsai CJ, Lee RS, Freymiller H, Kadempour A, Varthis S, et al. The prevalence and risk factors associated with osteoradionecrosis of the jaw in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT): The Memorial Sloan Kettering Cancer Center experience. Oral Oncol. 2017;64:44–51.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design, interpretation of the findings, revision of the manuscript, and approved the final version of the manuscript. KH acquired, analysed, and verified the data and performed the statistical analysis and drafted the initial manuscript. YS supervised the study.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
Not applicable.
Ethics approval
Ethical approval was granted by the institutional review board of the National Kyushu Cancer Center (Reference number 2018–30).
Consent to participate
Informed consent was obtained in the form of opt-out on the website.
Informed consent
Informed consent was obtained in the form of opt-out on the website.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hiramine, K., Kunitake, N., Shirakawa, Y. et al. Clinical Efficacy and Complications of Intensity-modulated Radiation Therapy in Patients With Oropharyngeal Carcinoma: Especially Focus On Osteoradionecrosis. SN Compr. Clin. Med. 5, 236 (2023). https://doi.org/10.1007/s42399-023-01562-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-023-01562-5