Abstract
Juvenile localised scleroderma (JLS) is a condition that results in inflammation and fibrosis of the skin in children and young people. Systemic treatment with immunomodulation is most commonly with Methotrexate (MTX) or Mycophenolate Mofetil (MMF). Other treatments include DMARDs, biologic therapies, topical treatments and phototherapy. This scoping review considers the available information on the relative safety and efficacy of MTX and MMF. A scoping review was conducted in accordance with PRISMA-ScR guidelines. A search was conducted in three bibliographic databases (Cochrane Library, Medline (OVID) and Embase (OVID)) to identify relevant studies for inclusion . A single reviewer identified published articles eligible for the review based on the inclusion and exclusion criteria. The relevant key findings were summarised in a word document by the first reviewer and then checked by a second reviewer. From 1233 unique references, 109 were identified as meeting the inclusion criteria. MTX is the most commonly used first-line systemic treatment for JLS with the greatest evidence for its use in JLS. The evidence for the efficacy of MMF is restricted to a small number of retrospective studies. Both MTX and MMF are described to be relatively safe medications with a low rate of adverse events. Information regarding the tolerability of these medications is limited. The rarity of JLS and the paucity of validated measures of disease activity makes comparison between these two treatments challenging and should be reflected in the design of future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile localised scleroderma (JLS), also described as morphoea, is a paediatric rheumatological condition primarily affecting the skin. It is characterised by inflammation, fibrosis and atrophy which may extend to damage the underlying muscle and bone [1]. JLS is rare with an estimated incidence of 3.4 cases per million per year [2] and prevalence of 3.2 to 3.6 per 10,000 children per year [3]. It can present in various forms including linear scleroderma, circumscribed morphoea, en coup de sabre and Parry-Romberg syndrome (hemifacial atrophy) [4]. Systemic disease complications include: arthritis, eye inflammation, eyelid or dental abnormalities, headaches, seizures or other neurological effects [5]. Progressive disease can lead to disability, need for corrective surgery, biomechanical dysfunction (e.g. from leg length discrepancies), pain and negative psychological impact [6].
Drug therapy for JLS aims to halt active inflammation, prevent tissue damage and to gain disease control without the need for ongoing corticosteroid therapy. Traditionally systemic corticosteroid therapy has been the mainstay of treatment for induction, with methotrexate (MTX) used first line as a steroid-sparing agent. The second most commonly used immunomodulator is mycophenolate mofetil (MMF). The rare nature of this disease had led to a paucity of evidence on the efficacy of these medications in clinical management [7]. Both MMF and MTX are generally accepted as safe, although there is limited information on both safety and tolerability.
Treatment is currently based on a randomised controlled trial (RCT), observational cohorts, expert opinion and consensus guidelines. The limited robust quality data available from the small patient cohorts make any results from meta-analysis and systematic reviews uncertain in this area. A pragmatic scoping review is therefore more appropriate to map out current knowledge, inform clinical practice and to highlight targets for future research. Therefore, this scoping review aims to identify and describe existing studies reporting the efficacy and safety of MTX compared to MMF for children and young people in the treatment of JLS. Outcome measures of interest are the reported efficacy, adverse events and tolerability of remission induction and treatment regimes.
Methods
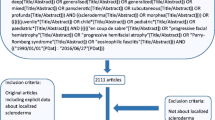
The scoping review was conducted in accordance with PRISMA-ScR guidelines (See Appendix 1 for PRISMA-ScR checklist) [8]. A sensitive search of the Cochrane Library, Medline (OVID) and Embase (OVID) was performed in May 2022 from the start of each database [8]. Search strategies were designed separately for each database with terms for MTX, MMF, or corticosteroids combined with JLS terms (See Appendix 2 for full Medline search strategy). After the searches were imported into EndNote reference management software and duplicates removed, results were initially screened by a single reviewer to identify published articles eligible for the review based on the inclusion and exclusion criteria. The relevant key findings were summarised in a word document by the first reviewer and then checked by a second reviewer.
Studies were selected if they met the following inclusion criteria: Study population of children up to age of 18 years with JLS or morphoea, recruited from any location or setting, with exposure to MTX or MMF regimes, whose outcomes included measures of pharmaceutical efficacy, adverse events and/or tolerability. Studies were excluded if they were non-English language guidelines, recommendations, systematic reviews, overviews or clinical opinions as these could not be translated within the timeframe of the review. Non-English language primary research articles with English abstracts were included if the abstract met the inclusion criteria.
Results
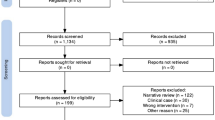
As summarised in Fig. 1, 1233 unique references were identified by database searches, 720 of which were excluded during title and abstract screening as they were not related to the subject of this review. Of the remaining full-text 513 articles, 109 were identified as meeting the inclusion criteria: 3 guidelines and treatment recommendations [1, 9, 10] (Table 1); 4 systematic reviews and meta-analyses [11,12,13,14] (Table 2); 42 primary studies including 9 prospective studies [15,16,17,18,19,20,21,22,23] (Table 3) and 33 retrospective observational studies [4, 24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] (Table 4); 35 case reports of MTX [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90] (Table 5), 8 of MMF [91,92,93,94,95,96,97,98] and 1 of MMF and MTX [99]; and 16 overviews and expert opinions [5, 7, 100,101,102,103,104,105,106,107,108,109,110,111,112,113] (Table 6).
Efficacy of MTX
MTX is the most commonly used first line systemic medication for JLS, either as a monotherapy or (more commonly) in combination with corticosteroids and has the greatest evidence for use in JLS. A 2016 UK national audit of 149 patients with JLS by Lythgoe et al. (2016) found that 95.5% of patients were treated with MTX as first-line therapy [37].
The most often-cited evidence for the use of MTX comes from a RCT of 70 children with active localised scleroderma [22]. Zulian et al. (2011) found oral MTX to be more effective than placebo, when used alongside 3 months of oral prednisolone. Both arms showed a response in the first 6 months but at month 12, the MTX group showed a significant reduction in disease activity measured by a computerised skin score (no expansion of lesions) and improved thermographic findings. The MTX group were also less likely to have new lesions and to experience a flare. Both this study and the follow-up cohort study [23] concluded that MTX is efficacious in the management of JLS. This remains the only RCT conducted within JLS. The rarity of the condition makes it difficult to conduct further RCTs with meaningful comparators, i.e. the second most-commonly used medication MMF [7].
The other non-controlled prospective studies in Table 3 represent a diversity of approaches to management in JLS but also report the efficacy of MTX in JLS. Other retrospective observational studies shown in Table 4 have further reported clinical improvement with MTX use.
The data recommending MTX use is summarised in systematic reviews of JLS. A Cochrane review of 14 RCTs with 429 patients (aged 3-76 years) by Albuquerque et al. (2019) concluded that compared to placebo plus oral prednisone, oral MTX plus oral prednisone may improve disease activity or damage in JLS, although there may be a slightly increased chance of experiencing at least one adverse event [11]. Equally, Marrani et al. (2018) compared the use of ultraviolet light A phototherapy (UVA) with MTX in combination with corticosteroids for the treatment of JLS across 8 studies [13]. The authors concluded that MTX treatment was superior to UVA and the overall efficacy of MTX with or without corticosteroids was as high as 87.5% with only 24 patients showing disease worsening under treatment.
However other systematic reviews were unable to draw a conclusion, such as De Peufheiloux et al. (2018) who reviewed 28 studies featuring 463 children with JLS [12]. This was due to the design of the reviewed studies which showed variation in dosing, administration routes, duration of treatment and outcome measures. They found MTX (10-25mg/ m2/week) in combination with oral corticosteroids (1-2mg/kg/day for 1 to 3 months then tapering over 1 to 4 months) was the most studied treatment, included in 11 studies and used for the treatment of 210 patients. This body of evidence also forms the basis of recommendations for the use of MTX in consensus-based treatment plans. This review highlights three commonly cited consensus treatment plans (CTPs) as summarised in Table 1.
The North American Childhood Arthritis and Rheumatology Research Alliance (CARRA) JLS subgroup devised three consensus treatment plans, described by Li et al. (2012) [10]. These are MTX monotherapy, MTX with oral prednisolone and MTX with intravenous (IV) methylprednisolone. The subgroup composed a core group of paediatric rheumatologists, dermatologists, and a lay advisor. Do et al. 2020 [30] found in a retrospective cohort study that publication of the CARRA CTPs significantly changed prescribing practice in JLS at a single North American centre. A pilot study comparing efficacy between the CTPs was not adequately powered to detect a difference [16].
European recommendations come firstly from the Hamburg Scleroderma Consensus Group composed of paediatric rheumatologists from the Paediatric Rheumatology European Society (PRES) Scleroderma working group and paediatric dermatologists with a research interest in JLS, described by Constantin et al. (2018) [9]. More recently, they also come from the Single Hub and Access point for paediatric Rheumatology in Europe (SHARE) guidelines, described by Zulian et al (2020) [1]. The consensus group for this composed of 15 experienced European rheumatologists and 2 young fellows.
All the identified consensus based-guidelines within this review recommend MTX as the first-line treatment for JLS (Table 1). In certain cases of limited JLS lesions, topical treatment may be used initially. If there is no disease response MTX should then be prescribed. Although most commonly used with systemic corticosteroids, there is some evidence for MTX use as a monotherapy [15, 16, 32, 115, 116].
The heterogeneity in MTX dosage regimes used was highlighted in the formation of the CARRA CTPs. Li et al. (2012) [10] described 82 discrete MTX and 86 discrete corticosteroid regimens from 114 responders. There is a disparity is dosage regimes between the predominantly European PRES & SHARE guidelines (which favour dosing by body surface area at 15mg/m2/week) and North American CARRA guidelines (which dose by body weight at 1mg/kg/week). They do however agree on a maximum dosage of 25mg/week. This is similar to the doses reported in the primary studies in this review. Some primary studies use a lower dose of 0.3-0.6mg/kg/week, such as Hardy et al. (2019) [33], who identify this lower dosage as potential cause for resistance to MTX. Torok et al. (2012) also report increasing the maximum dose to 30mg/week in a patient with extensive disease [19].
There is also a disparity between the optimal route for MTX usage. The best evidence for MTX from the aforementioned RCT [22] used an oral regime. Despite this, CARRA CTPs recommend subcutaneous use to optimise bioavailability, as 78% of respondents to the JLS CARRA survey preferred subcutaneous use. PRES and SHARE guidelines advise either oral or subcutaneous use. There equally appears to be no consensus between the optimal route for concurrent corticosteroid use, which may be given orally or intravenously.
There is also a lack of definition of optimal duration of treatment. The guidelines all agree MTX treatment should be continued for a minimum of 12 months either as a monotherapy or with a tapering corticosteroid regime. The CARRA guidelines found there was insufficient information to specify duration of treatment beyond 12 months. The PRES & SHARE guidelines advise that systemic treatment should not be stopped before at least 12 months of inactivity. In the follow up cohort study to the RCT, Zulian et al (2012) found that use of MTX for at least 24 months was associated with prolonged disease remission [23].
Estimates of resistance to MTX treatment remain small at 6-10% [33]. In a retrospective multicentre French study, Hardy et al. (2019) were unable to identify a clinical profile for those resistant to MTX [33]. Weibel et al. (2006) found that ongoing disease on MTX was associated with younger age of onset [53].
MTX appears to be effective in many subtypes of localised scleroderma. In a systematic review, Marrani et al. (2018) were unable to extrapolate data regarding the efficacy of MTX in the different subtypes of JLS [13]. Hardy et al. (2019) did not find that subtype was a predictor of MTX resistance [33]. In another systematic review of 34 studies featuring 59 children and adults with en coup de sabre lesions, Ulc et al. (2021) reported good clinical outcomes with MTX [14]. Evidence from case reports also suggests that MTX is effective in treating extracutaneous manifestations of disease, such as en coup-de-sabre related epilepsy [117].
Relapses are common after treatment discontinuation of MTX. This was reported to be as high as 44% after treatment discontinuation in one retrospective study of 34 patients [53]. Many patients who relapse respond to further treatment with MTX, which may include increase in dose or longer course of concurrent corticosteroid use [110].
In conclusion, although MTX remains the most commonly used first-line treatment for JLS and has demonstrated efficacy for its use, there remain a number of questions about its use – including optimal dose, route, duration, use with/without corticosteroids. Superiority to other medication such as MMF also remains to be determined.
Safety of MTX
MTX is frequently used in JLS, with any adverse events reported to be transient and mild. In the RCT of 70 patients detailed above, 56.5% of patients treated with MTX for 12 months/until treatment failure developed side effects, compared with 45.8% in the placebo arm group [22]. The most common side effect observed over the 12-month follow-up period was nausea, seen in 17.4% of patients. Other side effects included alopecia, headache, fatigue and hepatotoxicity. There were no serious adverse events related to treatment in this group and no patients were withdrawn from the trial due to side effects. The results from the follow-up cohort study (mean follow up of 40.3 months) found similar rates of adverse events [23].
Gastric intolerance, including nausea, appears to be the most frequent side effect. In a single centre study of 36 patients who were treated with 36 months of MTX (24 months subcutaneous followed by 12 months oral), Torok et al. (2012) 19% of patients developed anticipatory nausea and vomiting (median follow-up = 36.4 months) [19]. A national UK audit found that of 79 children with JLS observed over a 12-month period who stopped MTX, 37 (46.8%) did so due to intolerance (median duration of treatment =16 months) [37]. Although oral and subcutaneous forms of MTX are recommended by European guidelines as having equivalent efficacy, it is notable that in one prospective study of 34 patients, 26% were switched from oral to subcutaneous administration due to gastric intolerance (mean duration of maintenance treatment with MTX = 32 months, mean follow up = 2.9 years) [53].
Patients are infrequently discontinued on MTX due to adverse events. In a prospective cohort study of 10 patients described by Uziel et al. (2000) only one patient was discontinued on treatment after a year due to leukopenia [20]. Other effects noted were elevation of hepatic enzymes and nausea. When reported, adverse events also frequently include the effects of concurrent corticosteroid use, such as hypertension, cushingoid facies and cutaneous striae.
Little comment is made on the safety of MTX in the guidelines identified. The SHARE guidelines acknowledge that low dose MTX is safe and well-tolerated and the CARRA guidelines recommend supplementation with folic acid or folinic acid. Mertens et al. (2016) also recommend folic acid supplementation, and minimising delay to initiation to minimise treatment failure of MTX in clinical practice [118]. In the case reports reviewed, many of the above noted adverse effects were mentioned. MTX was also reported to cause mood disturbances in one patient with JLS which resolved with cessation of use [59].
Efficacy of MMF
MMF is recommended as second-line treatment in cases of MTX resistance or intolerance by the SHARE & CARRA guidelines. The PRES guidelines advise that there is limited evidence for MTX non-responders and that MMF can be considered alongside abatacept and infliximab. Lythgoe et al. (2018) reported that second line treatment was MMF for 89.5% of patients [37]. Other surveys of clinician use in the UK and North America have reported similar results on the popularity of MMF as a second line-option [5, 106].
The evidence for MMF use in JLS comes from a series of retrospective studies. These mostly feature patients who had not responded to MTX or were intolerant of MTX. For the main part, review of these studies highlighted the lack of robust evidence for treatment of JLS and the absence of a standardised approach.
The most commonly cited evidence comes from Martini et al. (2009), a retrospective case series of 10 patients with methotrexate-resistant JLS treated with MMF [38]. All patients showed an improvement through clinical examination and thermography findings.
Mertens et al. (2016) report another retrospective case series of 7 patients with localised scleroderma (aged 7-73 years) treated with MMF [42]. 3 were treated with MMF due to MTX ineffectiveness and 4 due to MTX intolerance. 6 out of 7 patients showed a favourable response to MMF treatment based on clinical examination.
Martini et al. (2021) describe a recent retrospective longitudinal study comparing the outcomes of 22 patients with JLS treated with MMF compared to 47 treated with MTX [40]. This study identified no significant difference in relapse-free survival with MMF compared to MTX. MMF did however seem more likely to provide persistent remission than MTX. They found that combination of MMF and MTX did not increase its efficacy.
As with MTX, there is variation in the dosing regime used for MMF (e.g. 600 to 1200 mg/m2/day twice daily [38] and 700 to 1000 mg/m2/day [40]). A number of case reports also describe successful treatment with MMF in the case of MTX resistance, either as a monotherapy or in combination with other agents [92,93,94, 96]. None of the systematic reviews were able to draw a conclusion on the use of MMF in JLS, due to the lack of prospective studies.
When considering extracutaneous disease, a RCT comparing MMF and MTX in 216 adult patients with uveitis did not find MMF to be superior [119].
Safety of MMF
Like MTX, MMF is a relatively well-tolerated medication with a favourable safety profile. Many of the adverse effects are transient and infrequently result in treatment discontinuation. In the studies described above, Martini et al. (2009) noted in a case series of 10 patients that one patient had mild abdominal discomfort, with no haematological or biochemical abnormalities found (mean duration of treatment = 20 months) [38]. Mertens et al. (2016) reported in a case series of seven patients that one patient had to discontinue MMF after three months due to elevated liver enzymes [42]. Another patient experienced diarrhoea at doses greater than 1000mg daily. In a case report, Arkin et al. [91] also describe mood changes five weeks after starting treatment with MMF that resolved with cessation.
In larger retrospective case series, Martini et al. (2021) [40] report the following adverse events in a cohort of 22 JLS patients followed up over a mean of 9.4 years: headache (22.7%), mild increase in transaminases (18.2%), nausea/vomiting (9.1%) and fatigue (9.1%). There was no treatment discontinuation due to side effects.
Discussion
To date, there is an absence of robust evidence regarding the management of JLS. There is only one small RCT and a number of observational studies reporting on the use of MTX in JLS with some success. There is even less evidence available for the use of MMF. This includes no RCTs or prospective observational studies. As such recommendations for the use of MMF in JLS are based on retrospective observational studies, case reports and consensus clinical opinion. Further evidence comes from in vitro studies show that MMF inhibit lymphocyte proliferation [120] and has a direct antifibrotic role [121]. However, it is possible there is publication bias in the published articles included in this scoping review, with studies reporting success in treatment and unusual adverse events more likely to be published.
Reassuringly the findings reported by the systematic reviews and consensus statements are consistent in that they all recommend MTX as a first line treatment for JLS. It is also widely reported as well tolerated. A concomitant course of corticosteroids should also be considered, options for which include a tapering course of oral prednisolone or periodic IV methylprednisolone. Although intolerance can be high, the reporting of adverse events leading to discontinuation in the literature remains low.
MMF is mainly advised in the context of unsuccessful treatment with MTX or intolerance to MTX. MMF is suggested as an alternative treatment choice in severe disease. However there is no available consensus as to whether MMF should be used as a monotherapy or in combination, nor whether MMF should be considered primary treatment. Like MTX, MMF has good evidence for safety, with limited evidence for tolerability.
Other therapies, such as biologic therapies, have been employed in JLS with varying evidence for their use. Tocilizumab was found to be effective in JLS in a retrospective study of 11 patients with JLS who had not responded to previous therapy [122]. A case report of two patients with pansclerotic morphoea found that treatment with tocilizumab reduced disease activity and stopped disease progression [123]. Another case series of 5 patients also reports the successful treatment of 5 patients with tocilizumab [124]. There is also evidence for abatacept which is commissioned for use in JLS in the UK. A retrospective study of 18 patients with JLS by Li at al. (2021) found abatacept to be a safe and effective treatment in patients refractory to MTX/MMF/corticosteroid treatment [125]. This was also found in a case series of 6 patients with JLS reported by Kalampokis et al. (2020) [126]. In an accompanying systematic review, the authors were unable to draw a conclusion on abatacept use in JLS due to high risk of bias in the identified studies. Case studies that are limited to single patients report on the successful use of other biologic treatment, such as infliximab [127] and of the tyrosine kinase inhibitor imatinib [63].
PRES guidelines also recommend the use UV phototherapy for the treatment of children above the age of 12 in small superficial lesions [9]. The safety of these treatments remains to be reported.
A key difficulty when comparing the efficacy and safety of MMF and MTX comes from a lack of adequate measures of these properties. The studies included here feature a range of outcomes for efficacy, from clinician-reported measures (which may or may not be validated) to imaging-based measures such as ultrasound and thermography. The landmark RCT conducted by Zulian et al. (2011) used a combination of clinical scores: physician’s global assessment of disease severity visual analogue scale (VAS), parent’s global assessment VAS and childhood health assessment questionnaire (CHAQ), thermographic findings and a computerised skin scoring system) [22]. It is notable that the physician and patient/parent VAS and CHAQ score did not reach statistical significance in this trial, which was thought to be due to low sensitivity in JLS. In a follow-up study, the authors also note that the lack of availability of objective measures, e.g. infrared thermography and computerised skin scoring, makes it difficult to compare this to previous studies [23]. Newer studies based on expert consensus have recommended validated clinician reported outcome measures such as the LoSCAT, which combines activity (mLoSSi) and damage (LoSDI) [7, 9]. The diversity of outcome measures and lack of adequate sensitivity makes effective comparison between existing studies challenging.
Another challenge in comparing the efficacy of MMF and MTX is that the rarity of JLS makes a traditional RCT between the two impractical. Desai et al. (2021) argue it would take 15 years to complete a clinical trial if 50% of patients from every available specialty centre in the UK consented to enrolment [7]. The authors argue a Bayesian framework for a multicentre RCT of MMF and corticosteroids versus MTX and corticosteroids would offer an achievable alternative. This prior elicitation sought to describe experts’ current beliefs on the efficacy and tolerability of MTX and MMF in JLS. The prevailing outcome of this was that there is uncertainty amongst experts as to the most appropriate use of MTX and MMF in JLS, particularly on the efficacy of MMF.
It is equally challenging to measure the relative safety for these two medications. Both drugs appear to be relatively safe with a low rate of serious adverse events as outlined above. However adverse event reporting in published literature is low and may underrepresent the burden of intolerability of drugs. Patient reported outcome measures (PROMs) are uniquely placed to report the effect of medication side-effects on patients’ health-related quality of life and thus their tolerability. The methotrexate intolerance severity score (MISS) PROM has been used to measure tolerability of MTX in juvenile idiopathic arthritis [128], but no such patient reported outcome exists for MMF [7]. The Localised Scleroderma Quality of Life Instrument (LoSQI) PROM features a medication subscale that quantifies the tolerability of medication used in JLS in aggregate [129]. In developing the LoSQI, Zigler et al. (2020) highlighted the burden of medication side effects on the lives of patients with JLS, particularly those from corticosteroid medications [130].
In conclusion, there is a lack of robust trials of MTX and MMF in the treatment of JLS and little in the way of reliable research into their safety and tolerability profiles in this context. Future trials in this area are vital for the effective and safe management of JLS. Due to the small population of JLS patients, traditional frequentist clinical trials are unfeasible and therefore future studies should consider more novel trial design.
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- CARRA :
-
Childhood arthritis and rheumatology research alliance
- CHAQ :
-
Childhood health assessment questionnaire
- CTP :
-
Consensus treatment plan
- DMARD :
-
Disease Modifying Anti-Rheumatic Drug
- JLS :
-
Juvenile Localised Scleroderma
- LoSQI :
-
Localised Scleroderma Quality of Life Instrument
- RCT :
-
Randomised Controlled Trial
- SHARE :
-
Single Hub and Access point for paediatric Rheumatology in Europe
- MISS :
-
Methotrexate Intolerance Severity Score
- MMF :
-
Mycophenolate Mofetil
- MTX :
-
Methotrexate
- PRES :
-
Paediatric Rheumatology European Society
- PROM :
-
Patient Reported Outcome Measure
- UVA :
-
Ultraviolet Light A Phototherapy
- VAS :
-
Visual Analogue Scale
References
Zulian F, et al. Consensus-based recommendations for the management of juvenile localised scleroderma. Ann Rheum Dis. 2019;78(8):1019–24.
Herrick AL, et al. Incidence of childhood linear scleroderma and systemic sclerosis in the UK and Ireland. Arthritis Care & Research: Official J Am College Rheumatol. 2010;62(2):213–8.
Beukelman T, Xie F, Foeldvari I. The prevalence of localised scleroderma in childhood assessed in the administrative claims data from the United States. J Scleroderma Relat Disord. 2019;4(1):77–8.
Zulian F, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford). 2006;45(5):614–20.
Li SC, et al. Treatment of pediatric localized scleroderma: results of a survey of North American pediatric rheumatologists. J Rheumatol. 2010;37(1):175–81.
Khanna D, et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2020;8(10):963–74.
Desai Y, et al. Prior elicitation of the efficacy and tolerability of Methotrexate and Mycophenolate Mofetil in Juvenile Localised Scleroderma. AMRC Open Research. 2021;3(20):20.
PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7): 467-473.
Constantin T, et al. Development of minimum standards of care for juvenile localized scleroderma. Eur J Pediatr. 2018;177(7):961–77.
Li SC, et al. Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care Res. 2012;64(8):1175–85.
Albuquerque JV, et al. Interventions for morphea. Cochrane Database Syst Rev. 2019;7:CD005027.
de Peufheiloux LLS, et al. Treatments and outcomes in juvenile linear scleroderma: a narrative systematic review. Eur J Dermatol. 2018;28(5):718–20.
Marrani E, et al. Comparing ultraviolet light A photo(chemo) therapy with Methotrexate protocol in childhood localized scleroderma: Evidence from systematic review and meta-analysis approach. Semin Arthritis Rheum. 2018;48(3):495–503.
Ulc E, et al. Therapeutic and Reconstructive Management Options in Scleroderma (Morphea) en Coup de Sabre in children and adults. A systematic literature review. J Clin Med. 2021;10(19):29.
Herrick AL, et al. Clinical features of childhood localized scleroderma in an incidence cohort. Rheumatology. 2011;50(10):1865–8.
Li SC, et al. Initial results from a pilot comparative effectiveness study of 3 methotrexate-based consensus treatment plans for juvenile localized scleroderma. J Rheumatol. 2020;47(8):1242–52.
Li SC, et al. Extracutaneous involvement is common and associated with prolonged disease activity and greater impact in juvenile localized scleroderma. Rheumatology. 2021;60(12):5724–33.
Porta F, et al. High frequency ultrasound can detect improvement of lesions in juvenile localized scleroderma. Mod Rheumatol. 2014;24(5):869–73.
Torok KS, Arkachaisri T. Methotrexate and corticosteroids in the treatment of localized scleroderma: a standardized prospective longitudinal single-center study. J Rheumatol. 2012;39(2):286–94.
Uziel Y, et al. Methotrexate and corticosteroid therapy for pediatric localized scleroderma. J Pediatr. 2000;136(1):91–5.
Weibel L, et al. Prospective evaluation of treatment response and disease reversibility of paediatric localized scleroderma (morphoea) to steroids and methotrexate using multi-modal imaging. J Eur Acad Dermatol Venereol. 2020;34(7):1609–16.
Zulian F, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2011;63(7):1998–2006.
Zulian F, et al. A long-term follow-up study of methotrexate in juvenile localized scleroderma (morphea). J Am Acad Dermatol. 2012;67(6):1151–6.
Adrovic A, et al. Juvenile scleroderma: a referral center experience. Arch Rheumatol. 2018;33(3):344–51.
Beltramelli M, et al. Localized severe scleroderma: a retrospective study of 26 pediatric patients. Pediatr Dermatol. 2010;27(5):476–80.
Christen-Zaech S, et al. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59(3):385–96.
Condie D, Grabell D, Jacobe H. Comparison of outcomes in adults with pediatric-onset morphea and those with adult-onset morphea: a cross-sectional study from the morphea in adults and children cohort. Arthritis Rheum. 2014;66(12):3496–504.
Cox D, et al. Juvenile localised scleroderma: a retrospective review of response to systemic treatment. Ir J Med Sci. 2008;177(4):343–6.
De Somer L, et al. Overlap between linear scleroderma, progressive facial hemiatrophy and immune-inflammatory encephalitis in a paediatric cohort. Eur J Pediatr. 2015;174(9):1247–54.
Do N, et al. A retrospective study: Impact of consensus treatment plans on systemic therapy of pediatric morphea. Pediatr Dermatol. 2020;37(2):278–83.
Fadanelli G, et al. Methotrexate in linear scleroderma: long-term efficacy in fifty children from a single pediatric rheumatology center. Arthritis Care Res. 2021;73(9):1259–63.
Fitch PG, et al. Treatment of pediatric localized scleroderma with methotrexate. J Rheumatol. 2006;33(3):609–14.
Hardy J, et al. Clinical profile of methotrexate-resistant juvenile localised scleroderma. Acta Derm Venereol. 2019;99(6):539–43.
Kashem SW, et al. Inflammatory arthritis in pediatric patients with morphea. J Am Acad Dermatol. 2018;79(1):47–51 e2.
Koch SB, et al. Linear morphea: a case series with long-term follow-up of young, methotrexate-treated patients. J Dermatol Treat. 2013;24(6):435–8.
Lo CY, et al. Juvenile scleroderma: experience in one institution. Asian Pac J Allergy Immunol. 2010;28(4):279–86.
Lythgoe H, et al. Multi-centre national audit of juvenile localised scleroderma: describing current UK practice in disease assessment and management. Pediatr Rheumatol Online J. 2018;16(1):80.
Martini G, et al. Successful treatment of severe or methotrexate-resistant juvenile localized scleroderma with mycophenolate mofetil. Rheumatology. 2009;48(11):1410–3.
Martini G, et al. Disease course and long-term outcome of juvenile localized scleroderma: Experience from a single pediatric rheumatology Centre and literature review. Autoimmun Rev. 2018;17(7):727–34.
Martini G, et al. Mycophenolate mofetil for methotrexate-resistant juvenile localized scleroderma. Rheumatology. 2021;60(3):1387–91.
Mertens JS, et al. Disease recurrence in localized scleroderma: a retrospective analysis of 344 patients with paediatric- or adult-onset disease. Br J Dermatol. 2015;172(3):722–8.
Mertens JS, et al. Use of mycophenolate mofetil in patients with severe localized scleroderma resistant or intolerant to methotrexate. Acta Derm Venereol. 2016;96(4):510–3.
Milovanova K, et al. Predictors of family impact of juvenile localized scleroderma. Pediatr Dermatol. 2021;38(5):1137–42.
Noh JW, Kim J, Kim JW. Localized scleroderma: a clinical study at a single center in Korea. Int J Rheum Dis. 2013;16(4):437–41.
Piram M, et al. Short- and long-term outcome of linear morphoea in children. Br J Dermatol. 2013;169(6):1265–71.
Rattanakaemakorn P, Jorizzo JL. The efficacy of methotrexate in the treatment of en coup de sabre (linear morphea subtype). J Dermatol Treat. 2018;29(2):197–9.
Reiff D et al. Characteristics of coexisting localized scleroderma and inflammatory arthritis. Eur J Rheumatol. 2019; 1-5.
Saxton-Daniels S, Jacobe HT. An evaluation of long-term outcomes in adults with pediatric-onset morphea. Arch Dermatol. 2010;146(9):1044–5.
Schoch JJ, et al. Orthopedic complications of linear morphea: Implications for early interdisciplinary care. Pediatr Dermatol. 2018;35(1):43–6.
Tollefson MM, Witman PM. En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol. 2007;56(2):257–63.
Valões CCM, et al. Esophageal abnormalities in juvenile localized scleroderma: is it associated with other extracutaneous manifestations? Rev Bras Reumatol Engl Ed. 2017;57(6):521–5.
Virdi A, et al. A retrospective study on clinical subtypes and management of morphea in 10 Italian Dermatological Units. Italian J Dermatol Venereol. 2021;156(4):446–54.
Weibel L, et al. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155(5):1013–20.
Wu EY, et al. Baseline description of the juvenile localized scleroderma subgroup from the childhood arthritis and rheumatology research alliance legacy registry. ACR Open Rheumatol. 2019;1(2):119–24.
Yee J, Orchard D. Monitoring recommendations for oral azathioprine, methotrexate and cyclosporin in a paediatric dermatology clinic and literature review. Australas J Dermatol. 2018;59(1):31–40.
Agata K, et al. Clinical usefulness of magnetic resonance imaging in four children with scleroderma. Arch Rheumatol. 2018;33(2):230–5.
Appelhans C, et al. Unilateral generalized morphea is a rare variant of localized scleroderma. Eur J Med Res. 2006;11(4):152–6.
Asghar A, Riaz S, Ahmad TJ. Generalized morphea-a case report. J Pak Assoc Dermatol. 2017;27(2):180–2.
Bhat T, Coughlin CC. Mood changes with methotrexate therapy for dermatologic disease. Pediatr Dermatol. 2018;35(2):253–4.
Perez Crespo M, et al. Rapid response to cyclosporine and maintenance with methotrexate in linear scleroderma in a young girl. Pediatr Dermatol. 2009;26(1):118–20.
Forsea AM, et al. Disabling pansclerotic morphea of childhood--unusual case and management challenges. J Med Life. 2008;1(3):348–54.
Hirt P, et al. Morphea with oral mucosa involvement and unilateral nevoid telangiectasia as an early presentation of morphea: a case report and review of the literature. J Clin Aesth Dermatol. 2020;13(1):38–40.
Inamo Y, Ochiai T. Successful combination treatment of a patient with progressive juvenile localized scleroderma (morphea) using imatinib, corticosteroids, and methotrexate. Pediatr Dermatol. 2013;30(6):e191–3.
Jin K, et al. Successful treatment of low-dose methotrexate in combination with systemic steroids for juvenile multiple and symmetrical circumscribed morphea. J Dermatol. 2017;44(10):e256–7.
Jindal AK, et al. Thrombocytopenia associated with localized scleroderma: report of four pediatric cases and review of the literature. Pediatr Dermatol. 2017;34(4):e174–8.
Joshi A, Al-Mutairi N, Nour-Eldin O. Congenital skin lesions presenting as morphea in a 4-year-old. Pediatr Dermatol. 2006;23(1):94–5.
Kanoh H, et al. Localized scleroderma presenting as port-wine stains: report of two cases and a literature review. Acta Derm Venereol. 2015;95(8):1003–4.
Kashiwagi Y, et al. Thermography for evaluation of localized scleroderma treated with methotrexate and corticosteroid. Indian J Pediatr. 2013;80(11):980–1.
Kawashima H, et al. Therapy of childhood generalized morphea: case reports and reviews of the literature of Japanese cases. Pediatr Int. 2006;48(3):342–5.
Khaled A, et al. Postvaccination morphea profunda in a child. Pediatr Dermatol. 2012;29(4):525–7.
Khan MA, et al. Radiologic improvement after early medical intervention in localised facial morphea. Pediatr Dermatol. 2016;33(2):e95–8.
Lehman N, Moorthy LN. Case 3: Purplish-brown, shiny upper extremity lesion and stiff hand in a 9-year-old. Pediatr Rev. 2011;32(10):447.
Laverde-Saad A, et al. Dermatologic ultrasound in the management of childhood linear morphea. Dermatol Online J. 2021;27(7):15.
Lu M-C, et al. Unilateral generalized morphea: First case report in Taiwan. Asian Pac J Allergy Immunol. 2020;38(2):120–3.
McCarthy S, et al. Subtle erythema of the forehead. Clin Experiment Dermatol. 2020;45(4):470–1.
Merlin E, Breton S, Fraitag S, Stéphan JL, Wouters C, Bodemer C, Bader-Meunier B. Fibrous Arthropathy Associated With Morphea: A New Cause of Diffuse Acquired Joint Contractures. Pediatrics. 2017;140(4)4):e20161899. https://doi.org/10.1542/peds.2016-1899.
Mirsky L, et al. Relapse after systemic treatment in paediatric morphoea. Br J Dermatol. 2012;166(2):443–5.
Nagai Y, Hattori T, Ishikawa O. Unilateral generalized morphea in childhood. J Dermatol. 2002;29(7):435–8.
Najeeb N, Thomas J, Manoharan D. Linear morphea in a child: a case report. Biomed Pharmacol J. 2015;8(SpecialOct):583.
Niklander S, et al. Morphea “en coup de sabre”: An unusual oral presentation. J Clin Exp Dent. 2017;9(2):e315.
Plachouri KM, et al. A pediatrician's alert: misdiagnosis of mixed localized scleroderma in a child. Pediatr Int. 2020;62(6):759–61.
Roulez FM, et al. Orbital myositis in a child with linear scleroderma en coup de sabre. J Pediatr Ophthalmol Strabismus. 2007;44(5):264–6.
Santos G et al. Linear morphea-A case treated with calcipotriol and betamethasone dipropionate. Eur J Pediatr Dermatol. 2012;22(4):284-284.
Siddiqui F, Kumar M. A 13-year-old girl with a linear dark patch on her forehead: A case of scleroderma en coup de sabre in a child with skin of color presenting with a bruise-like appearance. JAAD Case Reports. 2018;4(5):418–20.
Sugiura K, Muro Y, Tomita Y. A case of a childhood linear scleroderma with limb asymmetry. Mod Rheumatol. 2004;14(3):254–6.
Uchiyama M, et al. Case of localized scleroderma successfully treated with bath psoralen and ultraviolet A therapy. J Dermatol. 2010;37(1):75–80.
Ventejou S, et al. Case report: pansclerotic morphea-clinical features, differential diagnoses and modern treatment concepts. Front Immunol. 2021;12:656407.
Vazquez Sanchez M, et al. Linear morphea in saber coup: about a case. Arch Argent Pediatr. 2022;120(2):e75–9.
Vieira Martins M, et al. Linear scleroderma en coup de sabre - a different clinical presentation. Acta Reumatol Port. 2021;46(1):72–6.
Weinberg J, et al. Morphoea of the breast in a young girl. Clin Exp Dermatol. 2001;26(6):497–8.
Arkin L, Talasila S, Paller AS. Mycophenolate Mofetil and mood changes in children with skin disorders. Pediatr Dermatol. 2016;33(3):e216–7.
Cuellar-Barboza A, et al. A case of bullous morphea resistant to methotrexate and phototherapy successfully treated with mycophenolate mofetil. J Drugs Dermatol JDD. 2018;17(10):1123–5.
Küçükoğlu R, Yılmaz Z, Kutlay A. Treatment of recalcitrant generalized morphea with mycophenolate mofetil and intravenous immunoglobulin. Dermatol Ther. 2018;31(5):e12674.
Kurtzman DJ, et al. Segmental stiff skin syndrome (SSS): Two additional cases with a positive response to mycophenolate mofetil and physical therapy. J Am Acad Dermatol. 2016;75(6):e237–9.
Rose R, Goodfield M. Combining PUVA therapy with systemic immunosuppression to treat progressive diffuse morphoea. Clinic Experiment Dermatol Clinic Dermatol. 2005;30(3):226–8.
Schlaak M, et al. Successful therapy of a patient with therapy recalcitrant generalized bullous scleroderma by extracorporeal photopheresis and mycophenolate mofetil. J Eur Acad Dermatol Venereol. 2008;22(5):631–3.
Soh HJ, et al. Challenges in the diagnosis and treatment of disabling pansclerotic morphea of childhood: case-based review. Rheumatol Int. 2019;39(5):933–41.
Song P, et al. Resolution of pansclerotic morphea after treatment with antithymocyte globulin. Nat Rev Rheumatol. 2009;5(9):513–6.
Sotgiu S, et al. Anti-GAD epileptic encephalopathy in a toddler with Parry-Romberg syndrome. Neurol Sci. 2020;41(3):705–8.
Asano Y, et al. Diagnostic criteria, severity classification and guidelines of localized scleroderma. J Dermatol. 2018;45(7):755–80.
Adrovic A, et al. Juvenile scleroderma-what has changed in the meantime? Curr Rheumatol Rev. 2018;14(3):219–25.
Fett NM. Morphea: evidence-based recommendations for treatment. Indian J Dermatol Venereol Leprol. 2012;78(2):135–41.
Foeldvari I. New developments in juvenile systemic and localized scleroderma. Rheum Dis Clin N Am. 2013;39(4):905–20.
Foeldvari I. Update on the systemic treatment of pediatric localized scleroderma. Paediatr Drugs. 2019;21(6):461–7.
George R, George A, Kumar TS. Update on management of morphea (localized scleroderma) in children. Indian Dermatol Online J. 2020;11(2):135–45.
Hawley DP, et al. United Kingdom survey of current management of juvenile localized scleroderma. Rheumatology. 2014;53(10):1849–54.
Kaushik A, et al. Paediatric morphoea: a holistic review. Part 2: diagnosis, measures of disease activity, management and natural history. Clin Experiment Dermatol. 2020;45(6):679–84.
Knobler R, et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: localized scleroderma, systemic sclerosis and overlap syndromes. J Eur Acad Dermatol Venereol. 2017;31(9):1401–24.
Kreuter A, et al. German guidelines for the diagnosis and therapy of localized scleroderma. J Dtsch Dermatol Ges. 2016;14(2):199–216.
Li SC, Zheng RJ. Overview of Juvenile localized scleroderma and its management. World J Pediatr. 2020;16(1):5–18.
Pena-Romero AG, Garcia-Romero MT. Diagnosis and management of linear scleroderma in children. Curr Opin Pediatr. 2019;31(4):482–90.
Weibel L. Diagnosis and management of morphoea in children: an overview. Clin Experiment Dermatol. 2021;46(3):487–94.
Zulian F, Tirelli F. Treatment in Juvenile Scleroderma. Curr Rheumatol Rep. 2020;22(8):45.
Zhang W, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312–24.
Kroft EB, et al. Effectiveness, side-effects and period of remission after treatment with methotrexate in localized scleroderma and related sclerotic skin diseases: an inception cohort study. Br J Dermatol. 2009;160(5):1075–82.
Platsidaki E, et al. Methotrexate: an effective monotherapy for refractory generalized morphea. Clin Cosmet Investig Dermatol. 2017;10:165–9.
Nguyen K, Atty C, Ree A. Linear scleroderma en coup de sabre presenting with seizures. Radiol Case Rep. 2020;15(11):2164–70.
Mertens JS, et al. Drug survival and predictors of drug survival for methotrexate treatment in a retrospective cohort of adult patients with localized scleroderma. Acta Derm Venereol. 2016;96(7):943–7.
Rathinam SR, et al. Effect of corticosteroid-sparing treatment with mycophenolate mofetil vs methotrexate on inflammation in patients with uveitis: a randomized clinical trial. JAMA. 2019;322(10):936–45.
Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 2005;14(Suppl 1):s2–8.
Roos N, et al. In vitro evidence for a direct antifibrotic role of the immunosuppressive drug mycophenolate mofetil. J Pharmacol Exp Ther. 2007;321(2):583–9.
Foeldvari I, et al. Tocilizumab is a promising treatment option for therapy resistant juvenile localized scleroderma patients. J Scleroderma Related Disorders. 2017;2(3):203–7.
Martini G et al. Tocilizumab in two children with pansclerotic morphoea: a hopeful therapy for refractory cases? Clin Exp Rheumatol, 2017. 35 Suppl 106(4); 211-213.
Lythgoe H, et al. Tocilizumab as a potential therapeutic option for children with severe, refractory juvenile localized scleroderma. Rheumatology. 2018;57(2):398–401.
Li SC, et al. Preliminary evidence on abatacept safety and efficacy in refractory juvenile localized scleroderma. Rheumatology (Oxford). 2021;60(8):3817–25.
Kalampokis I, Yi BY, Smidt AC. Abatacept in the treatment of localized scleroderma: A pediatric case series and systematic literature review. Semin Arthritis Rheum. 2020;50(4):645–56.
Ferguson ID, Weiser P, Torok KS. A case report of successful treatment of recalcitrant childhood localized scleroderma with infliximab and leflunomide. Open Rheumatol J. 2015;9:30–5.
Fatimah N, et al. Frequency of methotrexate intolerance in rheumatoid arthritis patients using methotrexate intolerance severity score (MISS questionnaire). Clin Rheumatol. 2016;35(5):1341–5.
Zigler CK, et al. A novel patient-reported outcome for paediatric localized scleroderma: a qualitative assessment of content validity. Br J Dermatol. 2020;182(3):625–35.
Zigler CK, et al. Exploring the impact of paediatric localized scleroderma on health-related quality of life: focus groups with youth and caregivers. Br J Dermatol. 2020;183(4):692–701.
Funding
SS is supported by a National Institute of Health Research (NIHR) Academic Clinical Fellowship.
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by EH, NC, JJ and CP. The scoping review process was undertaken by SS and EH who undertook the acquisition, analysis and interpretation of the data. SS and EH drafted the manuscript with substantial revisions from JJ, NC and CP. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Written Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singhal, S., Heaf, E., Jordan, J.L. et al. A Scoping Review of the Efficacy and Safety of Methotrexate Compared to Mycophenolate Mofetil in the Treatment of Juvenile Localized Scleroderma in Children and Young Adults. SN Compr. Clin. Med. 5, 212 (2023). https://doi.org/10.1007/s42399-023-01546-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-023-01546-5