Abstract
Acitretin is a systemic retinoid that is used in dermatology for treatment of various inflammatory and especially hyperkeratotic diseases. Elevation of liver enzymes may occur occasionally but normally resolves spontaneously, at the latest after termination of acitretin. However, it can very rarely develop into a life-threatening adverse event including drug-induced liver injury (DILI). A 45-year-old man with classical pityriasis rubra pilaris, a frequently severe, inflammatory skin disease, was started on acitretin. After a seemingly harmless elevation of transaminases, a few weeks after initiation of acitretin, the patient experienced a dramatic course of liver injury with hepatic jaundice though acitretin was stopped immediately. Eventually, laboratory values recovered upon high-dose oral prednisolone therapy. Prescribing physicians should keep in mind that acitretin might induce severe liver injury. Even after termination of acitretin laboratory values should be monitored for a while in order to recognize symptomless but harmful drug-induced liver injury in time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acitretin, a systemic retinoid receptor agonist, is administered for a broad variety of inflammatory and hyperkeratotic diseases. Its application has to be considered carefully with special attention to its harmful teratogenicity. Common side effects include dry skin and mucous membranes, dyslipoproteinemia and hair loss. Another frequent, but mostly harmless and reversible adverse effect is the elevation of liver enzymes [1].
Here, we report the case of a 45-year-old man with pityriasis rubra pilaris (PRP), who developed severe drug-induced liver injury (DILI) upon administration of acitretin leading to temporary liver dysfunction although acitretin was stopped. PRP is a rare papulosquamous, inflammatory skin disease that is characterized by a broad spectrum of presentations ranging from mild manifestations confined to extremities to severe disease developing into erythroderma. Its typical feature are follicular keratotic papules with reddish-orange hue and in more generalized subtypes islands of sparing (so called “nappes claires”). The pathogenesis of PRP is poorly understood, and treatment options are limited. Retinoids are considered first-choice systemic treatment [2].
Case Presentation
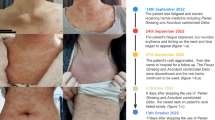
A 45-year-old man with an otherwise unremarkable medical history was referred to our department presenting with the typical clinical picture of classical adult PRP with confluent follicular, hyperkeratotic, reddish-orange papules and plaques that were intervened by nappes claires on his extremities and his trunk and with keratoderma on his palms and soles (Fig. 1). Two weeks before the rash had begun with a rough reddish plaque on the forehead. Despite of topical treatment with corticosteroids and the initiation of oral prednisolone 50 mg once daily skin manifestations continued to spread in a cephalocaudal direction ending up in suberythroderma. Histopathology substantiated the diagnosis of PRP. After admission, acitretin was initiated in a dose of 50 mg (0.66 mg/kg body weight) once daily, and prednisolone was terminated. Topical treatment with potent corticosteroids was applied twice daily. Nine days later, the patient was discharged in good general condition and with inconspicuous routine laboratory values. At his first appointment in our outpatient clinic 2 weeks later, the skin had improved significantly showing less infiltration and desquamation. Except for dry lips and a certain degree of hair loss acitretin was well tolerated in the first months of treatment. However, 3 months after initiation of acitretin, a substantial elevation of liver enzymes (alanine aminotransferase (ALT) > 15 × upper limit of normal (ULN), aspartate aminotransaminase (AST) > 7 × ULN) and gamma-glutamyltransferase (GGT, > 2.5 × ULN) was noted accompanied by generalized itch (Fig. 2). Despite immediate termination of acitretin therapy, the transaminases (ALT > 28 × ULN, AST > 13 × ULN) and GGT (> 3 × ULN) continued to increase. Simultaneously, hyperbilirubinemia and a scleral icterus developed indicating a cholestatic disorder. The patient was referred to the department of internal medicine where the presumptive diagnosis of acute drug-induced liver injury (DILI) was histopathologically confirmed by a liver biopsy that showed centrilobular (zone 3) necroses and resorption but no signs of autoimmunological, viral, or hereditary causes. Complementary serological tests ruled out viral infections (hepatitis A, B, C, and E, cytomegalovirus) and syphilis. Interestingly, elevated antinuclear antibodies (ANA) (titer 1:640) and a human leucocyte antigen (HLA) constellation indicating susceptibility for autoimmune hepatitis (HLAB08 and HLADRB1*03) were detected [3]. Even four weeks after treatment termination hepatocellular and cholestatic laboratory values (ALT > 35 × ULN U/I, AST > 20 × ULN, GGT > 9 × ULN, bilirubin 1,2 x ULN) kept on rising (Fig. 2). A marked liver dysfunction was noticed by an increased international normalized ratio (INR) of 1.33 and a decrease of cholinesterase. All led to the initiation of oral prednisolone in a daily dose of 70 mg. The systemic therapy resulted in rapid improvement of laboratory parameters. Two weeks later, the patient was dismissed with stabilizing liver and cholestatic values (ALT > 3 ULN, AST > 15 × ULN, GGT > 7 × ULN, bilirubin 3.6 mg/dL, normalized INR) (Fig. 2). However, due to an undulating course of liver enzymes, prednisolone had to be continued at low levels for a period of 9 months in total. In the meantime, the patient was offered the induction of secukinumab, an IL-17-antagonist, to treat his skin manifestations. Biologicals, especially IL-17-antagonists, have been described to be a promising and safe off-label treatment option for PRP [4]. As the patient had experienced severe hepatotoxic side effect, he expressly dispensed with a new therapy. Eventually, skin lesions almost resolved without further systemic therapy. The patient had no further discomfort regarding his DILI and PRP until today.
Discussion
PRP is a rare inflammatory skin disease. While medical guidelines are yet not available, oral retinoids used in higher doses are widely considered a first-line treatment for PRP. Acitretin, the preferred retinoid, is given in doses of 0.5–0.75 mg/kg body weight daily with the principal meal [2, 5,6,7]. Even in the era of biologicals and small molecules, acitretin is regarded as a promising and effective medication not only for PRP.
By activating nuclear retinoid receptors, it is involved in the regulation of cell proliferation and differentiation (keratinisation). Furthermore, it has anti-inflammatory effects. Acitretin is the major metabolite of the formerly used etretinate, which it has replaced completely in clinical practice because of better pharmacokinetics. Its main advantages are a shorter half-life and minor accumulation in adipose tissue resulting in a more favourable side effect profile. Of note, acitretin can undergo reverse metabolism to etretinate, in particular in combination with consumption of alcohol. After its metabolisation, acitretin is eliminated in the bile or through the kidneys [8].
However, mucocutaneous dryness, lipid dysfunction, or hair loss often leads to premature termination, and its use may further be limited by potentially severe side effects. Adverse effects to the liver are the reason for regular laboratory tests especially in the beginning of therapy. Reported prevalences of transaminase elevation during acitretin treatment range from less than 1 to up to 16% [9]. Marked elevations (> 3 × ULN) are estimated to occur in 1–5% of acitretin-treated patients. In most of the cases, abnormalities resolve spontaneously even with sustained use or elsewise after discontinuation of acitretin and are not accompanied by other symptoms. DILI developing upon systemic retinoid administration is a rare event and estimated to occur in 0.1–0.5% [10]. Several studies conducted with patients suffering from different psoriasis subtypes confirmed that severe liver injury is a very rare side effect of acitretin. In 1990, a prospective, open-label trial demonstrated that even long-term therapy with acitretin did not cause clinically significant biopsy-proven hepatotoxicity [11]. Liver biopsies performed on 128 patients before and 2 years after systemic treatment with acitretin (25–75 mg/day) did neither indicate a correlation between elevation of transaminases nor the cumulative dose with the degree of liver injury. A retrospective study published in 2004 confirmed that significant elevation of transaminases is very unlikely to appear under long-term low-dose acitretin [12]. In 2019, another observational study attested that severe hepatic side effects under low-dose acitretin are very uncommon. None of 104 patients who were treated with acitretin in a mean dose of 20 mg daily over a mean time of 3.2 years developed severe liver side effects [13].
However, few cases of DILI under acitretin have been described [14,15,16,17,18]. In our case, unlike other observations, discontinuation of acitretin did not lead to an improvement of complications. Instead, liver enzymes continued to rise to a very high extent and were accompanied by scleral icterus, nausea, itching, weakness, and poor concentration and eventually ending up in a liver dysfunction. Especially, the detection of elevated ANAs and a typical HLA-constellation opened the discussion of an autoimmunological component having aggravated the progression and long duration of the acitretin-induced liver injury. Cofactors (e.g., alcohol consumption) might have contributed to acitretin having been sequestrated in fatty storage sites leading to systemic distribution and liver damage despite discontinuation. However, our patient denied alcohol consumption. Otherwise, due to its half-life of 50 to 60 hs, acitretin must have been eliminated a few days after termination.
The actual reason for the long duration of liver injury with transaminases staying at a high level remains unclear and the limitation of the reported case.
Conclusions
The presented case emphasizes the harmful potential of a well-known, but underestimated side effect of a frequently used drug. Prescribing physicians should not fear to initiate acitretin but always bear in mind its potential hepatotoxic adverse effects even after termination.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Berbis P. Acitretine. Ann Dermatol Venereol. 2001;128(6–7):737–45.
Ross NA, Chung HJ, Li Q, Andrews JP, Keller MS, Uitto J. Epidemiologic, clinicopathologic, diagnostic, and management challenges of pityriasis rubra pilaris: a case series of 100 patients. JAMA Dermatol. 2016;152(6):670–5. https://doi.org/10.1001/jamadermatol.2016.0091.
de Boer YS, van Gerven NM, Zwiers A, Verwer BJ, van Hoek B, van Erpecum KJ et al. Genome-wide association study identifies variants associated with autoimmune hepatitis type 1. Gastroenterology. 2014;147(2):443–52 e5. https://doi.org/10.1053/j.gastro.2014.04.022.
Napolitano M, Abeni D, Didona B. Biologics for pityriasis rubra pilaris treatment: a review of the literature. J Am Acad Dermatol. 2018;79(2):353–9 e11. https://doi.org/10.1016/j.jaad.2018.03.036.
Dicken CH. Treatment of classic pityriasis rubra pilaris. J Am Acad Dermatol. 1994;31(6):997–9. https://doi.org/10.1016/s0190-9622(94)70271-3.
Allison DS, El-Azhary RA, Calobrisi SD, Dicken CH. Pityriasis rubra pilaris in children. J Am Acad Dermatol. 2002;47(3):386–9. https://doi.org/10.1067/mjd.2002.124619.
Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451–7. https://doi.org/10.2147/ccid.S124351.
Zito PM, Mazzoni T. Acitretin. Treasure Island: StatPearls; 2022.
Sauder MB, Cheung L, Beecker J. Acitretin-induced hepatitis: when to monitor cholestatic enzymes. J Cutan Med Surg. 2015;19(2):115–20. https://doi.org/10.2310/7750.2014.14051.
LiverTox: Clinical and research information on drug-induced liver injury. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
Roenigk HH Jr, Callen JP, Guzzo CA, Katz HI, Lowe N, Madison K, et al. Effects of acitretin on the liver. J Am Acad Dermatol. 1999;41(4):584–8.
Lee E, Koo J. Single-center retrospective study of long-term use of low-dose acitretin (Soriatane) for psoriasis. J Dermatolog Treat. 2004;15(1):8–13. https://doi.org/10.1080/095466303100184473.
Chularojanamontri L, Silpa-Archa N, Wongpraparut C, Limphoka P. Long-term safety and drug survival of acitretin in psoriasis: a retrospective observational study. Int J Dermatol. 2019;58(5):593–9. https://doi.org/10.1111/ijd.14349.
Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN Prospective Study. Gastroenterology. 2015;148(7):1340–52 e7. https://doi.org/10.1053/j.gastro.2015.03.006.
van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, van de Kerkhof PC, Yap SH. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11(2):185–8. https://doi.org/10.1016/0168-8278(90)90111-4.
Leithead JA, Simpson KJ, MacGilchrist AJ. Fulminant hepatic failure following overdose of the vitamin A metabolite acitretin. Eur J Gastroenterol Hepatol. 2009;21(2):230–2. https://doi.org/10.1097/MEG.0b013e32830dffd0.
Kreiss C, Amin S, Nalesnik MA, Chopra K, Shakil AO. Severe cholestatic hepatitis in a patient taking acitretin. Am J Gastroenterol. 2002;97(3):775–7. https://doi.org/10.1111/j.1572-0241.2002.05581.x.
Ramak G, Marjan M, Ali Zare M, Alireza Aziz A, Amirhosein F, Alireza H. Severe hepatotoxic injury and cirrhosis due to acitretin: a case review. J Liver Res Disord Ther. 2017;3(7):183–5.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Interdisziplinäres Zentrum für Klinische Forschung (IZKF), Medical Faculty, University of Würzburg (grant to A.K., AdvCSP-2).
Author information
Authors and Affiliations
Contributions
All authors were involved in medical care of the patient and critically revised the manuscript to bring it into its final version. All authors gave final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Written consent for publication was given by the patient.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rak, K., Hamm, H., Kerstan, A. et al. Severe and Prolonged Liver Damage in Pityriasis Rubra Pilaris Treated with acitretin: a Case Report. SN Compr. Clin. Med. 4, 230 (2022). https://doi.org/10.1007/s42399-022-01309-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01309-8