Abstract
Neovascularization is frequently observed in Achilles tendinopathy. It remains unclear whether neovascularization has a positive or negative impact on the prognosis of Achilles tendinopathy, and whether treatment should include the eradication or positive influence of neovessels. The purpose of this scoping review was to investigate the effect of ultrasound-guided interventions in the treatment of neovascularization in Achilles tendinopathy. Five different ultrasound-guided interventions were identified, which are characterized by an opposite effect. Whereas platelet-rich plasma (PRP) is used to positively influence neovascularization, sclerosing agents, high-volume image-guided injections, electrocoagulation, and hyaluronic acid are used to eradicate neovascularization. Therapies eradicating neovessels, through sclerosis or high-volume image-guided injections, have a long-term effect on the reduction of neovascularization. Moreover, eradication seems to improve pain and function in the short and long term compared to therapy that positively influences neovascularization, such as PRP. PRP induces neovascularization in the short term, but this effect fades out after this period. This review focusses on the role of neovascularization in Achilles tendinopathy and provides evidence supporting the theory that neovascularization is a pathological process rather than a positive impact on healing and remodeling of the tendon. Therapy that positively influences neovascularization in the form of PRP show contradictory results in the treatment of Achilles tendinopathy, while interventions eradicating neovessels demonstrate positive effects in the short and long term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Achilles tendinopathy is one of the most common tendinopathies in the general and sporting population with an incidence of 9% in recreational runners and prevalence of up to 54% in professional runners and can end the careers of 5% of professional athletes [1]. Achilles tendinopathy is commonly defined as a combination of pain, impaired function, and swelling [2]. Moreover, the burden of Achilles tendinopathy is high, resulting in reduced work productivity and higher absence at work due to sick leave [3].
Tendons are generally poorly vascularized, while certain regions most prone to injury are almost avascular resulting in a virtually non-existent tissue turnover [4]. However, tendinopathic tendons are found to have an increased tissue turnover [5]. The combination of pre-existing hypoxia in combination with a required increase in tissue turnover results in the activation of hypoxia-inducible factor 1α, a transcription factor leading to the expression of a large range of genes encoding angiogenic growth factors [4]. As tissue regeneration requires a sufficient supply of oxygen and nutrients, neovascularization could be interpreted as a necessary mechanism for healing.
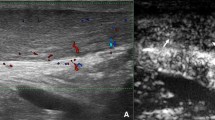
One issue with neovascularization is that these hypoxia-induced neovessels contain many branches and little vessel lumenization, resulting in leakage of mostly blood plasma (Fig. 1) [6]. Therefore, these neovessels can be regarded as non-functional by nature [4]. The hyperpermeable neovessels are non-functioning blood vessels that fail to deliver oxygen and nutrients required for tissue maintenance and possible regeneration [7]. It is believed that the absence of the R-Ras enzyme might be responsible for the abnormal structure of the neovessels [6]. R-Ras is responsible for the stabilization of microtubules and lumen structure and promotes endothelial lumenogenesis [6]. Li et al. demonstrated that the re-introduction of R-Ras leads to the stabilization of blood vessels, resulting in well-functioning blood vessels [6]. A simultaneous enhancement of vascular endothelial growth factor (VEGF) and R-Ras could be used as a possible strategy to generate an increased number of patent blood vessels to help reestablish capillary or microcirculation in the ischemic tissues [6]. Knobloch et al. [8] studied the oxygenation of tendinopathies using color Doppler ultrasound and concluded that no difference in oxygenation could be found if tendinopathic tendons are compared to non-tendinopathic tendons. This can possibly be explained by the presence of arterio-venous anastomoses, which are common in tendinopathic tendons [4, 9]. These anastomoses provide a bypass route for circulation and might lead to a false negative outcome in detecting reduced oxygen saturation. Overall, it would seem that neovessels are part of a pathological process and not a response to physiological healing after tendon insult.
Neovascularization in Achilles tendinopathy. The tendon responds to hypoxia by secreting angiogenic growth factors that induce the growth of neovessels and neurovascular ingrowth in tendinopathy. These hypoxia-induced neovessels contain many branches and little vessel lumenization, resulting in leakage of mostly blood plasma. This leakage results in failure to deliver oxygen and nutrients required for tissue regeneration
Neovessels may be involved in pain-generating mechanisms in pathological tendons. Using color or power Doppler or microflow imaging, neovascularization is mostly seen in the degenerative stage of tendinopathy [10]. Studies using microdialysis have shown that several sensory neurotransmitters are found within and around tendon neovessels in tendinopathies, but not in normal tendons [8]. These findings have created the hypothesis that neovascularization and accompanying newly formed nerves, known as neurovascular ingrowth, are a potential source of pain in chronic midportion Achilles tendinopathy [11]. In a study investigating painful tendons using color Doppler ultrasound, increased blood flow and neovascularization were found in painful tendons compared to tendons of an asymptomatic control group [11, 12].
Contradictory findings complicate the understanding of the pain pathophysiology in tendinopathy. Pain can occur at any stage in the process of tendinopathy, and painful tendons can be observed without any pathological changes [13]. Moreover, two-thirds of degenerative tendons reach a state of possible rupture without accompanying pain sensation [14]. These findings support the dissociation between pain and pathological findings in tendinopathy.
Treatment of Achilles tendinopathy is considered challenging. Exercise therapy is recommended as the initial strategy, as studies suggest reduced pain, improved healing of the tendon and stiffening and lengthening of the myotendinous units, and reduced neovascularization [15,16,17,18]. In recent years, researchers have become more interested in the role of neovascularization and neurovascular ingrowth in the pathophysiology of pain in tendinopathy as this might be an effective target in treatment [19, 20]. Targeting these structures with ultrasound-guided interventions is increasingly seen as the next step in the treatment of Achilles tendinopathy. The advantage of ultrasound-guided injections over blind injections (i.e., non-image guided) is that they are precision injections where there is no need to doubt whether the injection has been placed in the right place. In addition, it gives real-time feedback whether the neovessels were eradicated if that was the target. For example, ultrasound-guided sclerotherapy, known to inhibit neovascularization, shows an immediate effect on neovessels and has shown promising results regarding pain reduction and improved tendon structure [21]. However, controversy exists as to whether neovascularization is necessarily pathological, with some practitioners believing that neovascularization can exert a positive effect on healing and remodeling of the tendon in tendinopathy [22]. No reviews yet exist focusing on the role of neovascularization in the process of Achilles tendinopathy.
In this context of these contradictory theories on the role of neovascularization in tendinopathy, it is pertinent to perform a scoping review with the aim of identifying [1] which ultrasound-guided interventions are used in the treatment of neovascularization in Achilles tendinopathy and [2] the effectiveness of those interventions on neovascularisation.
Methods
This scoping review is conducted and described in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping reviews [23].
Scoping Review Research Question
What types of ultrasound-guided interventions are effective in the treatment of neovascularization in Achilles tendinopathy?
Search Strategy
A systematic literature search was conducted in PubMed, Embase, and Cochrane Library up to the 12th of April 2021. The following keywords and their synonyms were used: ultrasound, sonography, Achilles tendon, and tendinopathy. The search procedures can be found in Appendix 1. References of relevant articles were screened as well. The search was restricted to human studies and articles written in English.
Eligibility criteria
The following inclusion criteria were used: (a) assessment of neovascularization in the Achilles tendon with ultrasound at baseline and during follow-up; (b) neovascularization reported on a (semi)quantitative scale; (c) the relevance of neovascularization reported in the context of pain (e.g., a VAS-scale) or function (e.g., VISA-A-scale). Studies focusing on post-surgical Achilles tendinopathy or investigating Achilles full-tendon rupture were excluded from the review. Regarding the study design, case reports were excluded.
Definition Outcome Measures
Neovascularization had to be evaluated with color or power Doppler ultrasound using a (semi-)quantitative grading system and scored on a scale from 0 to 5, 0 to 4, or 0 to 3, where 0 was equal to no vascularization. Secondary outcome measurements were pain, pain combined with function and activity, or maximal tendon thickness. Pain had to be assessed with a pain score using a visual analog scale (VAS) from 0 to 100 [24], a Likert pain scale from 0 to 11 [25], an 11-point numeric pain rating scale (NRS-11) from 0 to 11 [26], or the Victorian Institute of Sports Assessment-Achilles questionnaire (VISA-A) that measures the severity of Achilles tendinopathy [27]. VISA-A covers the domain of pain, functionality, and activity, and scores are summed to give a maximum score of 100. A low score pertains to higher pain and reduced function and activity. Maximal thickness of the Achilles tendon had to measured, e.g., in millimeters, using gray-scale ultrasound.
Study Selection
Two independent reviewers (PVZ and RO) evaluated all papers based on title and abstract. Disagreement between the two authors was resolved by discussion. The full-text paper was reviewed when title and abstract were insufficient for correct assessment. Relevant data was extracted from eligible studies by one reviewer (PVZ) including first author, publication year, study design, sample size, duration of Achilles tendinopathy symptoms, intervention type, and outcome measures.
Results
Literature Search
The PRISMA flow diagram demonstrates the identified and selected studies in Fig. 2. Among the 1178 articles identified for screening, 113 studies were eligible for further assessment. Full-text articles were examined and excluded for reasons delineated in Fig. 2. The most common reasons for exclusion were not having neovascularization measurements, not having human participants, not having (semi-)quantitative data for neovascularization, not performing an ultrasound-guided intervention, case reports, and patients having previous surgery. Ultimately, 17 studies were included in this review. Of these studies, one study was selected through reference screening of included articles.
Study Characteristics
Baseline characteristics of the included studies are summarized in chronological order in Table 1. The selected studies were published between 2002 and 2020, consisting of a total sample size of 686 patients. Of the included studies, five different intervention types were applied targeting neovascularization in the Achilles tendon. Four studies assessed the effects of a sclerosing agent using polidocanol injections, one study assessed the effects of electrocoagulation, seven studies used platelet-rich plasma (PRP), four studies assessed high-volume image-guided injections (HVIGI), and one study assessed the effects of hyaluronic acid (HA) as treatment. The majority of the studies used a randomized controlled trial design (n = 7), prospective cohort study design (n = 5), or prospective pilot study design (n = 3). Other study designs used were a retrospective cohort study design (n = 1) and a case series (n = 1).
Ultrasound-Guided Interventions
Of the five different identified interventions, two studies administered injectate as the neovessels entered the Achilles tendon from the ventral side (sclerosing agents and electrocoagulation), one study administered intratendinous (PRP), one study administered an injectate between the Achilles tendon and Kager’s fat pad (HVIGI), and one study administered an injectate between the paratendon and endotendon (HA). The anatomical location is shown per intervention in Fig. 3. The results are summarized per intervention in Table 1.
Intervention types. a Sclerosing agent: Under Doppler guidance, Polidocanol is injected at the neovessels entering the Achilles tendon at the ventral side until the vessels are no longer visible at all. b Electrocoagulation: Using a coagulation needle under Doppler guidance the needle is placed against the neovessels entering the Achilles tendon at the ventral side. Coagulation is carried out until the Doppler signal disappears. c Platelet-rich plasma (PRP): under ultrasound guidance, multiple depots of PRP are left at several sites in the degenerative areas of the tendon. d High-volume image-guided injection: A 21G needle is inserted under ultrasound guidance between the ventral aspect of the Achilles tendon and Kager’s fat pad. The injection targets the area of maximal neovascularization. e Hyaluronic acid (HA): Under ultrasound guidance, HA is administered between the paratenon and epitenon
Sclerosing Agents ( Fig. 3a )
Four studies assessed the effect of Polidocanol. Polidocanol is a sclerosing agent used to sclerose neovascularization in the diseased tendon. Polidocanol has a selective effect on the vascular intimae, causing thrombosis of the vessel. The agent also has an effect if the injection is performed extravascularly [28]. The sclerosing effect of polidocanol on the vessels also affects the nerves adjacent to the neovessels, either directly by destruction or indirectly by ischemia, leading to reduced pain in the patient [29]. Polidocanol is injected, guided with ultrasound, adjacent to the neovessels entering the Achilles tendon from the ventral side of the tendon until the neovessels are no longer visible with color Doppler. Commonly, about 2–4 ml is injected during each intervention. Polidocanol is injected every 6–8 weeks for 2–3 times. The tendon is iced after injection and the patient is instructed to rest for a few days after intervention. At 2 weeks post-injection, exercise treatment is instigated.
In a prospective pilot study (n = 10), Öhberg et al. [30] observed that four out of five patients showed a significant reduction of neovascularization after intervention and a significant decrease in pain during activity on the VAS scale from 74.0 before intervention to 8.0 after intervention. One-fifth of the patients exhibited no pain relief after intervention and these patients also showed no reduction in neovascularization. The authors concluded that sclerosing neovessels appears to be an effective treatment for painful chronic Achilles tendinopathy.
Subsequently, Öhberg et al. [31] performed a similar study (n = 11) with a comparable outcome. In the following years, Alfredson et al. [21] and Lind et al. [32] performed a randomized controlled trial and retrospective cohort study, with a larger sample size, n = 20 and n = 42 respectively. These authors concluded that Polidocanol is an effective therapy to reduce neovascularization and that reduction in neovascularization results in lower pain, reduced maximal Achilles tendon thickness, and improvement in tendon structure. The follow-up period of 24 months by Lind et al. showed that these results remain for the long term as well.
Electrocoagulation ( Fig. 3b )
One study accessed the effect of electrocoagulation. This is an intervention that uses a radiofrequency probe to thermally sclerose neovessels entering the tendon. This procedure destroys neovessels together with the sensory nerves that accompany them. Under ultrasound guidance, the tip of the electrocoagulation probe targets neovessels entering the Achilles tendon from the ventral side of the tendon (the site is identical to the site used during sclerosing therapy).
Boesen et al. [33] performed a pilot study (n = 11) of ultrasound-guided coagulation, with a unipolar 16G coagulation needle. In this study, neovascularization at baseline and at 6 months of follow-up was compared through a quantitative scoring system (color pixels/total pixels on color Doppler in the region of interest) and a semiquantitative scoring system (neovascularization was scored on a scale of 0 to 4). In summary, no difference was found in the neovascularization score at follow-up compared to baseline. Using the quantitative scoring system, mean vascularization was 23.7% at baseline and 26.6% at follow-up. Using the semi-quantitative scoring system, mean neovascularization was 2.3 at baseline and 2.0 at follow-up. Reduction of pain was demonstrated, as measured on a Likert pain scale, with a median score of 7.0 at baseline compared to 0.0 at follow-up. The authors concluded that electrocoagulation appears to be an effective treatment for painful chronic mid-tendinous Achilles tendinopathy. However, there was no effect on the intratendinous Doppler activity suggesting that improvement in symptoms is independent of change in blood flow.
Platelet-Rich Plasma Injection ( Fig. 3c )
Seven studies assessed the effect of PRP injections. PRP is injected at several sites in the degenerative areas of the tendon. PRP is rich in platelets and derived from centrifuged autologous whole blood. When platelets are activated, cytokines and granules are produced releasing growth factors such as transforming growth factor beta 1, insulin-like growth factor, epidermal growth factor, and platelet-derived growth factor [34, 35]. In addition, PRP accelerates the repair of wound sites by stimulating vascular endothelial cell division, vascular proliferation, capillary growth, and collagen synthesis in the transplanted area [36].
After a PRP injection for mid-portion Achilles tendinopathy, De Jonge et al. [37] found a slight increase in neovascularization score at 1 -weeks (2.3 at baseline to 3.0 at 12 weeks of follow-up), but a decrease in neovascularization score to 1.4 at 12 months of follow-up. The function on the VISA-A scale improved steadily from 46.7 at baseline to 78.2 at 12 months of follow-up. However, increased neovascularization was not associated with decreased function as measured by the VISA-A scale. There was no difference in neovascularization and VISA-A in patients treated with PRP compared with the placebo. De Vos et al. [38] performed a similar study with a similar result where an increase in neovascularization was found at first but returned to pretreatment levels after a long-term follow-up. Ferrero et al. [39], Boesen et al. [40] and Ooi et al. [41] each performed a study showing a gradual decrease in neovascularization after PRP injection. In the study of Ferrero et al., a neovascularization score of 2.0 at baseline was found compared to a score of 1.0 at 6 months of follow-up. Regarding the VISA-A score in this study, a score of 58.0 was found at baseline, compared to a score of 77.0 at 6 months of follow-up. The author concluded that PRP injection results in a significant and lasting improvement of clinical symptoms. In the study of Boesen et al., a neovascularization score reduced from 3.2 at baseline to 1.8 at 24 weeks of follow-up. In the same study, a decrease was found in VAS score, namely a score of 53.0 at baseline compared to a score of 15.9 at 24 weeks of follow-up. The VISA-A score was found to increase from 58.1 at baseline to 77.7 at 24 weeks of follow-up. The authors concluded that PRP in combination with eccentric training seems to be more effective than eccentric training alone. In the study of Ooi et al., neovascularization decreased from 2.4 at baseline to 0.6 at 12 months of follow-up with an increase in the VISA-A score of 38.4 at baseline to 81.2 at 12 months of follow-up. Moreover, Abate et al. [42] demonstrated an increase in VISA-A score in Achilles tendons with and without neovascularization. However, the group without neovascularization scored substantially better on the VISA-A scale. Finally, Krogh et al. [43] found no clinical difference between PRP injection and placebo at 3 months of follow-up. They even found a slightly better outcome of pain and neovascularization in the placebo group compared to the PRP group. The authors concluded that PRP injections did not result in an improvement in VISA-A score over a 3-month period in patients with chronic Achilles tendinopathy compared with placebo.
High-Volume Image-Guided Injections ( Fig. 3d )
Four studies assessed the effect of HVIGI. This intervention consists of ultrasound-guided injection of saline and local anesthetic with or without corticosteroids in the interface between the ventral aspect of the Achilles tendon and Krager’s fat pad in the region with the highest grade of neovascularization. The mechanism of action is not yet fully understood but it is thought that HVIGI disrupts neovessels and neonerves by mechanical pressure of the fluid injected. This disruption may diminish neurogenic inflammation, decrease tendon pain, promote tendon healing, and improve functioning [44, 45].
Humphrey et al. [46] demonstrated HVIGI treatment resulted in a reduction in mean neovascularization grade from grade 3.0 at baseline to grade 1.1 at 3 weeks of follow-up. VISA-A score increased from 46.3 at baseline to 84.1 after 3 weeks of follow-up. They concluded that HVIGI is an effective treatment to improve the symptoms of resistant Achilles tendinopathy. Boesen et al. [47] found a significantly improved outcome on the VISA-A scale and VAS scale in HVIGI combined with corticosteroids in comparison to HVIGI without corticosteroids. Regarding neovascularization, a reduction was found for both groups. However, in the group receiving both HVIGI and corticosteroids, a greater decrease in neovascularization was found at mid-term follow-up, but the neovascularization scores were the same at 12 months. The authors concluded that HVIGI seems to be an effective treatment for Achilles tendinopathy, but the effect might be due to a cortisone effect rather than the high volume per se. In contrast, van der Vlist et al. [48] performed a study with HVIGI without corticosteroids, finding no difference in neovascularization, VISA-A score, and VAS score in the intervention group compared to the placebo group. They concluded that HVIGI without corticosteroids is not an effective treatment for Achilles tendinopathy. Finally, Maffuli et al. [49] did a cross-over study, with patients first receiving HVIGI without corticosteroids and a second HVIGI with corticosteroids if the first intervention showed insufficient improvements after two weeks. Approximately 50% of the patients received a second injection. At 12 months of follow-up, the mean neovascularization grade decreased from 3.0 to 2.0 and the VISA-A score increased from 41.7 to 74.6. The authors concluded that HVIGI significantly reduces pain and improves function in patients with Achilles tendinopathy.
Hyaluronic Acid ( Fig. 3e )
One study accessed the effect of HA. The mechanism of action of HA is not fully understood and the role of HA in tendinopathies is still controversial. HA potentially exerts disease-modifying effects on chronic tendinopathy through several mechanisms, leading to increased tenocyte viability and proliferation in combination with a decreased expression of proinflammatory cytokines, cyclooxygenase-2, and prostagladine E2 and a reduction of apoptosis in a dose-dependent manner [50, 51].
Frizziero [50] injected 2 ml of HA between the paratendon and tendon. One weekly injections were given for 3 consecutive weeks. The authors found that HA injections resulted in improvements in pain, function, and neovascularization in patients with mid-portion Achilles tendinopathies. At 90 days of follow-up, 43% of the patients showed no visible blood vessels. The VISA-A score improved from 44.8 to 67.2 and the pain on the NRS-11 score reduced to 4.52.
Discussion
We conducted this scoping review to identify currently performed ultrasound-guided interventions in the treatment of neovascularization in Achilles tendinopathy and the effectiveness of these interventions. We only included studies reporting on neovascularization as a study outcome, as we were interested in the role of neovascularization in Achilles tendinopathy. Overall, this review identified 17 studies assessing five different ultrasound-guided interventions: sclerotherapy, electrocoagulation, PRP, HVIGI, and HA. The studies were predominantly randomized controlled trials and prospective cohort studies, with rather low sample sizes (range 10–94). There were no systematic reviews included.
The five different ultrasound-guided interventions are characterized by an opposite intended effect on neovascularization. Whereas PRP is used to positively influence neovascularization, the other four interventions are used to eradicate neovascularization. Sclerotherapy, electrocoagulation, and HVIGI are known to destroy neovessels. This is in contrast with PRP, which is believed to promote repair by stimulating vascular endothelial cell division, vascular proliferation, capillary growth, and collagen synthesis in the transplant area. [34] The mechanism of HA is not fully understood, but it could possibly lead to increased tenocyte viability and proliferation in combination with a decreased expression of proinflammatory cytokines. [50, 51] Also, the target location differs between the interventions. Sclerotherapy and electrocoagulation target the neovessels entering the tendon, while PRP targets the intratendinous area of maximal neovascularization. Moreover, HVIGI is injected in the area of maximal neovascularization between the ventral aspect of the tendon and Krager’s fat pad, while HA is injected between the paratenon and tendon.
Sclerotherapy resulted in significant reduction in neovascularization at 3 months and at 24 months [21], with simultaneous improvement in pain and function. It is hypothesized that the destruction of neovascularization may be long lasting and accompanied by a significant reduction in pain. These findings of reduced pain and increased function are in line with a recent systematic review investigating the effects of sclerotherapy in Achilles tendinopathy [52, 53].
Electrocoagulation was also found to have beneficial effects on pain at the 6 months of follow-up. However, there were no accompanying changes in neovascularization at follow-up. Since only a single study investigated the effects of electrocoagulation in Achilles tendinopathy, these results should be interpreted with caution.
HVIGI therapy is often used in combination with corticosteroids, since this leads to better overall results [47]. Interestingly, significant reductions are found in neovascularization in the initial stages after HVIGI administration, but neovascularization gradually increases at longer term follow-up. The reason for the increase is unknown, but could be explained by persisting hypoxia in chronic tendinopathy stimulating further neovascularization. However, neovascularization levels do not return to pre-treatment levels. Moreover, in combination with the decrease in neovascularization, reductions in pain and improvement in function are found. The reduction in pain is most profound shortly after intervention. This is in line with the most recent literature review investigating the effect of HVIGI that concluded that HVIGI improves pain and function in Achilles tendinopathy [45].
Overall, the included studies on PRP showed improvements in pain and function, apart from Krogh et al. who demonstrated no clinical difference compared with a placebo group. Regarding neovascularization and PRP injections, three studies showed no change in neovascularization [43], or an increase in neovascularization at short-term follow-up [37, 38]. However, the study of Boesen et al. showed an opposite effect of a decrease in neovascularization in the short term [40]. All studies comparing baseline and follow-up results after 3 months showed a decrease in neovascularization [37,38,39,40,41]. The initial increase in neovascularization in some of these studies could be explained by the mechanism of action of PRP, namely positively influencing neovessels. It should be noted that the results from most of the included studies of a positive effect of PRP on improvement in pain and function compared to placebo are not consistent with a recent systematic review and meta-analysis investigating the effect of PRP in Achilles tendinopathy [54]. The selection process of our review with neovascularization as the primary outcome, in addition to the other advantages of a systematic review with meta-analysis, might be an explanation for this inconsistency.
Finally, HA therapy showed reductions in neovascularization in half of the patients studied, accompanied by improvement in pain and function [50]. However, only one study was performed with HA so the results need to be interpreted with caution.
Pain pathophysiology in Achilles tendinopathy is complex and multivariable and is not fully understood [55]. In this review, we addressed neovascularization, generally viewed as an important component of tendinopathy-related pain. We chose not to focus on all possible involved pain-related variables. There is evidence to suggest that the relationship between neovascularization and pain is biologically plausible. Neovascularization is an endogenic response to Achilles tendinopathy as a potential mechanism for healing and remodeling [4]. The neovessels contain many branches and little vessel lumenization, making these hyperpermeable neovessels non-functional blood vessels that fail to deliver oxygen and nutrients [4]. This hypothesis is supported by the observation that no improvements in pain or function are seen in combination with an increase in neovascularization in PRP treatment [43]. The effect of these non-functional neovessels on the healing and remodeling of the Achilles tendinopathy seems thereby minimal. The neovessels are accompanied by neonerves, possibly resulting in increased pain experience since one study showed an increase in pain in patients with high neovascularization scores compared to patients with low neovascularization scores [42]. The eradication of these non-functional neovessels results in a simultaneous reduction in neurovascular ingrowth and thus in reduced pain sensation [21].
This scoping review has some limitations. Our intention was to study the role of neovascularization in Achilles tendinopathy by assessing the effect of ultrasound-guided interventions. To do this, we restricted inclusion to studies reporting on neovascularization at baseline and as a study outcome. Of the identified studies, we had to exclude 71 studies that did not measure neovascularization at follow-up. In the included studies that did quantify neovascularization after baseline, significant heterogeneity existed in the neovascularization scales used. This limits comparability between studies, which was also hindered by the addition of eccentric training in some studies. As such, we are unable to infer the most effective intervention on neovascularization, although it was not our intention to investigate which intervention is most effective in improving pain and function. Other limitations of this review include limiting our studies to English written studies only, and we did not include gray literature. Finally, we didn’t assess the risk of bias, but that is inherent in a scoping review [56], which is therefore different from a systematic review.
Perspectives
This review focused on the role of neovascularization in Achilles tendinopathy and provides evidence supporting the theory that neovascularization is a pathological process rather than a positive impact on healing and remodeling of the tendon. Therapy positively influencing neovascularization in the form of PRP failed to show benefits in the treatment of Achilles tendinopathy, while interventions eradicating neovessels demonstrated positive effects in the short and long term.
While this review does provide some direction on the role of neovascularization, the existing controversy surrounding neovascularization has not been resolved. Perhaps the promotion of well-functioning neovessels with stable microtubules might give better results. So far, it is unknown if this also positively affects the accompanying neural ingrowth, which contributes to the experienced pain. Currently, the most effective intervention for chronic Achilles tendinopathy seems to be the eradication of neovascularization and neural ingrowth simultaneously.
Future research should focus on which eradication intervention is most effective on the one hand and on exploring the possibilities of interventions that stabilize neovascularization in Achilles tendinopathy on the other hand. To gain insight into the role of neovascularization in Achilles tendinopathy, these studies should also focus on neovascularization as an outcome measure in addition to pain and function. Moreover, it is important that quantification of the neovascularization is done in a uniform manner.
Data Availability
All data generated and analyzed for the elaboration of this manuscript are included in this published article.
Code Availability
Not applicable.
References
Janssen I, van der Worp H, Hensing S, Zwerver J. Investigating Achilles and patellar tendinopathy prevalence in elite athletics. Res Sports Med. 2018;26(1):1–12.
Aicale R, Tarantino D, Maffulli N. Overuse injuries in sport: a comprehensive overview. J Orthop Surg Res. 2018;13(1):309.
Bonde JP, Mikkelsen S, Andersen JH, Fallentin N, Baelum J, Svendsen SW, et al. Prognosis of shoulder tendonitis in repetitive work: a follow up study in a cohort of Danish industrial and service workers. Occup Environ Med. 2003;60(9):E8.
Järvinen TA. Neovascularisation in tendinopathy: from eradication to stabilisation? Br J Sports Med. 2020;54(1):1–2.
Heinemeier KM, Schjerling P, Øhlenschlæger TF, Eismark C, Olsen J, Kjær M. Carbon-14 bomb pulse dating shows that tendinopathy is preceded by years of abnormally high collagen turnover. Faseb j. 2018;32(9):4763–75.
Li F, Sawada J, Komatsu M. R-Ras-Akt axis induces endothelial lumenogenesis and regulates the patency of regenerating vasculature. Nat Commun. 2017;8(1):1720.
McIntyre A, Harris AL. Metabolic and hypoxic adaptation to anti-angiogenic therapy: a target for induced essentiality. EMBO Mol Med. 2015;7(4):368–79.
Knobloch K. The role of tendon microcirculation in Achilles and patellar tendinopathy. J Orthop Surg Res. 2008;3:18.
Järvinen M, Józsa L, Kannus P, Järvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports. 1997;7(2):86–95.
Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43(6):409–16.
Tol JL, Spiezia F, Maffulli N. Neovascularization in Achilles tendinopathy: have we been chasing a red herring? Knee Surg Sports Traumatol Arthrosc. 2012;20(10):1891–4.
Alfredson H, Ohberg L, Forsgren S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):334–8.
Malliaras P, Cook J. Patellar tendons with normal imaging and pain: change in imaging and pain status over a volleyball season. Clin J Sport Med. 2006;16(5):388–91.
Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507–25.
Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19(1):54–64.
Meyer A, Tumilty S, Baxter GD. Eccentric exercise protocols for chronic non-insertional Achilles tendinopathy: how much is enough? Scand J Med Sci Sports. 2009;19(5):609–15.
Rowe V, Hemmings S, Barton C, Malliaras P, Maffulli N, Morrissey D. Conservative management of midportion Achilles tendinopathy: a mixed methods study, integrating systematic review and clinical reasoning. Sports Med. 2012;42(11):941–67.
Li HY, Hua YH. Achilles tendinopathy: current concepts about the basic science and clinical treatments. Biomed Res Int. 2016;2016:6492597.
Maffulli N, Longo UG, Kadakia A, Spiezia F. Achilles tendinopathy. Foot Ankle Surg. 2020;26(3):240–9.
Alfredson H. The chronic painful Achilles and patellar tendon: research on basic biology and treatment. Scand J Med Sci Sports. 2005;15(4):252–9.
Alfredson H, Ohberg L. Sclerosing injections to areas of neo-vascularisation reduce pain in chronic Achilles tendinopathy: a double-blind randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. 2005;13(4):338–44.
Maffulli N, Oliva F, Testa V, Capasso G, Del Buono A. Multiple percutaneous longitudinal tenotomies for chronic Achilles tendinopathy in runners: a long-term study. Am J Sports Med. 2013;41(9):2151–7.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56.
Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;22(140):55.
Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19.
Robinson JM, Cook JL, Purdam C, Visentini PJ, Ross J, Maffulli N, et al. The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med. 2001;35(5):335–41.
Goldman MP, Bennett RG. Treatment of telangiectasia: a review. J Am Acad Dermatol. 1987;17(2 Pt 1):167–82.
Lake JE, Ishikawa SN. Conservative treatment of Achilles tendinopathy: emerging techniques. Foot Ankle Clin. 2009;14(4):663–74.
Ohberg L, Alfredson H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: pilot study of a new treatment. Br J Sports Med. 2002;36(3):173–5 (discussion 6-7).
Ohberg L, Alfredson H. Sclerosing therapy in chronic Achilles tendon insertional pain-results of a pilot study. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):339–43.
Lind B, Ohberg L, Alfredson H. Sclerosing polidocanol injections in mid-portion Achilles tendinosis: remaining good clinical results and decreased tendon thickness at 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2006;14(12):1327–32.
Boesen MI, Torp-Pedersen S, Koenig MJ, Christensen R, Langberg H, Hölmich P, et al. Ultrasound guided electrocoagulation in patients with chronic non-insertional Achilles tendinopathy: a pilot study. Br J Sports Med. 2006;40(9):761–6.
Lopez RG, Jung HG. Achilles tendinosis: treatment options. Clin Orthop Surg. 2015;7(1):1–7.
Liu CJ, Yu KL, Bai JB, Tian DH, Liu GL. Platelet-rich plasma injection for the treatment of chronic Achilles tendinopathy: a meta-analysis. Medicine (Baltimore). 2019;98(16):e15278.
Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. 2018;4(1):18–24.
de Jonge S, de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39(8):1623–9.
de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387–92.
Ferrero G, Fabbro E, Orlandi D, Martini C, Lacelli F, Serafini G, et al. Ultrasound-guided injection of platelet-rich plasma in chronic Achilles and patellar tendinopathy. J Ultrasound. 2012;15(4):260–6.
Boesen AP, Hansen R, Boesen MI, Malliaras P, Langberg H. Effect of high-volume injection, platelet-rich plasma, and sham treatment in chronic midportion Achilles tendinopathy: a randomized double-blinded prospective study. Am J Sports Med. 2017;45(9):2034–43.
Ooi CC, Schneider M, Malliaras P, Png MA, Chadwick M, Jones D, et al. Real-time sonoelastography evaluation of the Achilles tendon following ultrasound-guided platelet-rich plasma injection and eccentric exercise for the treatment of refractory Achilles tendinopathy. Ultrasound. 2019;27(3):138–47.
Abate M, Di Carlo L, Belluati A, Salini V. Factors associated with positive outcomes of platelet-rich plasma therapy in Achilles tendinopathy. Eur J Orthop Surg Traumatol. 2020;30(5):859–67.
Krogh TP, Ellingsen T, Christensen R, Jensen P, Fredberg U. Ultrasound-guided injection therapy of Achilles tendinopathy with platelet-rich plasma or saline: a randomized, blinded, placebo-controlled trial. Am J Sports Med. 2016;44(8):1990–7.
Peck E, Jelsing E, Onishi K. Advanced ultrasound-guided interventions for tendinopathy. Phys Med Rehabil Clin N Am. 2016;27(3):733–48.
Chaudhry FA. Effectiveness of dry needling and high-volume image-guided injection in the management of chronic mid-portion Achilles tendinopathy in adult population: a literature review. Eur J Orthop Surg Traumatol. 2017;27(4):441–8.
Humphrey J, Chan O, Crisp T, Padhiar N, Morrissey D, Twycross-Lewis R, et al. The short-term effects of high volume image guided injections in resistant non-insertional Achilles tendinopathy. J Sci Med Sport. 2010;13(3):295–8.
Boesen AP, Langberg H, Hansen R, Malliaras P, Boesen MI. High volume injection with and without corticosteroid in chronic midportion achilles tendinopathy. Scand J Med Sci Sports. 2019;29(8):1223–31.
van der Vlist AC, van Oosterom RF, van Veldhoven PLJ, Bierma-Zeinstra SMA, Waarsing JH, Verhaar JAN, et al. Effectiveness of a high volume injection as treatment for chronic Achilles tendinopathy: randomised controlled trial. BMJ. 2020;370: m3027.
Maffulli N, Spiezia F, Longo UG, Denaro V, Maffulli GD. High volume image guided injections for the management of chronic tendinopathy of the main body of the Achilles tendon. Phys Ther Sport. 2013;14(3):163–7.
Frizziero A, Oliva F, Vittadini F, Vetrano M, Bernetti A, Giordan N, et al. Efficacy of ultrasound-guided hyaluronic acid injections in Achilles and patellar tendinopathies: a prospective multicentric clinical trial. Muscle Ligaments Tendons J. 2019;09:305.
Mitsui Y, Gotoh M, Nakama K, Yamada T, Higuchi F, Nagata K. Hyaluronic acid inhibits mRNA expression of proinflammatory cytokines and cyclooxygenase-2/prostaglandin E(2) production via CD44 in interleukin-1-stimulated subacromial synovial fibroblasts from patients with rotator cuff disease. J Orthop Res. 2008;26(7):1032–7.
Maffulli N, Papalia R, D’Adamio S, Diaz Balzani L, Denaro V. Pharmacological interventions for the treatment of Achilles tendinopathy: a systematic review of randomized controlled trials. Br Med Bull. 2015;113(1):101–15.
Morath O, Kubosch EJ, Taeymans J, Zwingmann J, Konstantinidis L, Südkamp NP, et al. The effect of sclerotherapy and prolotherapy on chronic painful Achilles tendinopathy-a systematic review including meta-analysis. Scand J Med Sci Sports. 2018;28(1):4–15.
Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27(5):486–95.
Rio E, Moseley L, Purdam C, Samiric T, Kidgell D, Pearce AJ, et al. The pain of tendinopathy: physiological or pathophysiological? Sports Med. 2014;44(1):9–23.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143.
Author information
Authors and Affiliations
Contributions
All authors were involved in study conception, design, interpretation of data, drafting and revising the manuscript, and approving the final manuscript for publication. PvZ and RO performed data collection.
Corresponding author
Ethics declarations
Ethics Approval
Ethics approval was not required. This article does not contain any studies with human participants performed by any of the authors.
Consent to Participate
Not applicable.
Consent for Publication
The authors give full permission to the journal to publish the work presented here, including all the research findings, figures, and tables.
Conflict of Interest
PvZ declares he has no conflict of interest. MS is the owner of Sonoskills, a company developing and teaching courses in musculoskeletal ultrasound. MS, RO, and LM are teachers for Sonoskills in addition to their clinical and/or academic work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Zundert, P.G.E., Masci, L., Schmitz, M.J.H. et al. Effectiveness of Ultrasound-Guided Interventions on Neovascularization in Achilles Tendinopathy: a Scoping Review. SN Compr. Clin. Med. 4, 231 (2022). https://doi.org/10.1007/s42399-022-01308-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01308-9