Abstract
The high prevalence of asymptomatic patients infected with SARS-CoV-2 during the pandemic peaks and the common occurrence of in-hospital transmission urges the need for SARS-CoV-2 testing before admission of all patients with non-COVID-related symptoms. RT-PCR testing however is costly, time-consuming, and increases the length of stay in the emergency department. For the aforementioned reasons, we propose that the admission of non-suspected COVID-19 patients to the appropriate department should be based on the sole use of the rapid test result. In order to assess the safety of this suggestion, we assessed the negative predictive value of our rapid antigen tests that was calculated at 96.38%. This value was considered acceptable and the proposed strategy was applied in our hospital improving the overall turnaround times. However, since various rapid tests may perform differently, we propose that hospitals assess their own methodologies before implementing our proposal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
During the first months of the COVID-19 pandemic, there was a considerable worldwide decline in emergency department (ED) visits for non-COVID-related symptoms, resulting in a decrease in the usual overcrowding of the ED [1]. Lockdowns, government restrictions, social distancing, telephone or online medical consultations, reduction of non-emergency hospital admissions and procedures, the fear of the unknown, and the untreatable prevented the ED overcrowding [1, 2].
As the new normal settles and the world resumes “business as usual,” we observe an expected rise in ED visits for non-COVID-related symptoms. However, the bifurcation of the pathway to emergency health services and resources for suspected and non-suspected COVID-19 patients is still applied for the safety of all involved, resulting in further overcrowding and increased length of stay in the ED.
Due to the common occurrence of in-hospital transmission of severe acute respiratory coronavirus 2 (SARS-CoV-2), especially during the pandemic peaks and the high prevalence of asymptomatic patients infected with SARS-CoV-2, our hospital initially implemented an obligatory testing for SARS-CoV-2 with real-time polymerase chain reaction (RT-PCR) before admission of all patients with non-COVID-related symptoms. The aim was to ensure the safety of the hospital environment for both the patients and the staff, to reduce the hospital-acquired COVID-19 as well as the need for in-hospital transfer of patients and quarantining of hospital departments.
RT-PCR testing however is costly and time-consuming. Its use in the emergency setting for the admission of non-suspected for COVID-19 patients increases the risk and duration of potential exposure in the finite and overcrowded emergency department and the length of stay in the ED (which is linked to increased morbidity, mortality, and longer duration of hospital stays [3,4,5]) and influences the quality and timeliness of the emergency health services provided.
Rapid antigen lateral flow assays on the other hand are less expensive, easy to perform, and provide results in a few minutes. It is well known however that they are less reliable than RT-PCR. Their sensitivity and specificity have been extensively studied and are commonly within the range of 85–92% and 95–99%, respectively [6]. Their limit of detection is lower than that of molecular methods [7] and their results depend also on the timing of testing regarding the onset of symptoms, with positive results being more probable to occur in already symptomatic individuals [8].
In an effort to improve the quality and timelines of our ED services in the context of an ongoing pandemic, we suggest that the admission of non-suspected COVID-19 patients to the appropriate department should be based on the sole use of the rapid test result. In order to assess the safety of this suggestion, we calculated the negative predictive value of our rapid antigen tests.
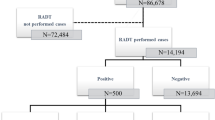
We retrospectively reviewed 636 negative rapid test results (Rapid Test Ag 2019-nCoV, ProGnosis Biotech S.A., Larissa, Greece, manufactured to detect the SARS-CoV-2 nucleocapsid protein) of nasopharyngeal specimens collected in our emergency department between November 1, 2021, and January 21, 2022, that were tested also by RT-PCR on the same day using the NeuMoDx SARS-CoV-2 or the Abbott RealTime SARS-CoV-2 assay. The Rapid Test Ag 2019-nCoV has a sensitivity of 85.5% and a specificity of 99.8% [9]. From November 1 to mid-December 2021, Delta was the only variant present in our hospital. On December 20, 2021, the Omicron variant emerged and prevailed within the range of 2 weeks. Delta however continued to be detected in lower rates. The national prevalence of COVID-19 was 0.67% on November 1, 2021, and 3.15% on January 21, 2022. During this period, the maximum prevalence was 4.43% on January 9, 2022 (https://www.worldometers.info/coronavirus/country/greece/). Among the tested specimens, 23 had a positive PCR result and were considered false negatives (Table 1). The negative predictive value of the rapid antigen test was calculated at 96.38%. Even though the cycle threshold values (Cts) of the false negative specimens varied and were not all higher than the positivity limits applied in our laboratory (≤ 30 for NeuMoDx SARS-CoV-2 and ≤ 27 for Abbott RealTime SARS-CoV-2), the negative predictive value of our rapid antigen test was considered acceptable and the proposed strategy was applied in our hospital with satisfactory results. More precisely, we propose rapid testing for all patients at a pre-triage office. Patients with negative test results proceed to a “clean” non-COVID ED. This way, the COVID ED is not burdened with additional patients that would delay the already complicated procedures that have to be followed for COVID patients. This decision improved the overall turnaround times because rapid testing is performed on site and lasts a few minutes whereas RT-PCR results may need from 2 up to 8 h depending on the workload and the laboratory workflow.
These results however cannot be generalized, since various lateral flow assays may perform differently. We therefore propose that hospitals assess their own methodologies before implementing our strategy.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Lucero A, Sokol K, Hyun J, et al. Worsening of emergency department length of stay during the COVID-19 pandemic. JACEP Open. 2021;2(3): e12489.
Ugglas B, Skyttberg N, Wladis A, et al. Emergency department crowding and hospital transformation during COVID-19, a retrospective, descriptive study of a university hospital in Stockholm, Sweden. Scand J Trauma Resusc Emerg Med. 2020;28(1):107.
Hoot NR, Aronsky D. Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med. 2008;52(2):126–36.
McCusker J, Vadeboncoeur A, Levesque JF, et al. Increases in emergency department occupancy are associated with adverse 30-day outcomes. Acad Emerg Med. 2014;21(10):1092–100.
Morley C, Unwin M, Peterson GM, et al. Emergency department crowding: a systematic review of causes, consequences and solutions. PLoS ONE. 2018;13(8): e0203316.
Zhou Y, Wu Y, Ding L, et al. Point-of-care COVID-19 diagnostics powered by lateral flow assay. Trends Analyt Chem. 2021;145: 116452.
Mak GC, Cheng PK, Lau SS, et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol. 2020;129: 104500.
Linares M, Pérez-Tanoira R, Carrero A, et al. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J Clin Virol. 2020;133: 104659.
Kyritsi M, Vontas A, Voulgaridi I, et al. Rapid Test Ag 2019-nCoV (PROGNOSIS, BIOTECH, Larissa, Greece); Performance evaluation in hospital setting with Real Time RT-PCR. Int J Environ Res Public Health. 2021;18:9151.
Funding
Open access funding provided by HEAL-Link Greece
Author information
Authors and Affiliations
Contributions
B.F.: conceptualization, methodology, data collection, writing, and review.
G.M.: methodology, data analysis, writing, and review.
S.G.: investigation, data collection, and review.
I.G.: investigation, data collection, and review.
A.T.: investigation, data collection, and review.
L.S.: conceptualization, supervision, review, and editing.
Corresponding author
Ethics declarations
Ethics Approval
The publication of the laboratory results was approved by the AHEPA University Hospital bioethics committee (protocol number: 29694–3/6/22).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Covid-19
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fyntanidou, B., Meletis, G., Gkarmiri, S. et al. Negative Predictive Value of the Rapid Test Ag 2019-nCoV During the Predominance of Omicron over the Delta Variant and Implications in the Emergency Department. SN Compr. Clin. Med. 4, 213 (2022). https://doi.org/10.1007/s42399-022-01294-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01294-y