Abstract
Microcystins (MCs) are found in aquatic settings and have detrimental effects on both human and animal health. Their occurrence is influenced by the deposition of nutrients in water and further increased by high temperatures which favor the proliferation of harmful algal blooms (HABs). Climatic conditions in Africa favor the growth of HABs. Therefore, determination of MC contamination of African water sources is of paramount importance. The use of molecularly imprinted polymers (MIPs) for the adsorption of MCs has recently gained increasing interest in the selective and rapid determination of MCs in water. MIPs are ideal adsorbents for this purpose because of their versatility and environmental friendliness and thus have potential to replace conventional adsorbents such as activated carbon and silica composites. This review summarizes the occurrence of MCs in Africa as well as the detection and determination methods used for analysis. Secondly, common methods for the synthesis of MIPs are compared and their application and efficacy towards determination of MCs in water is investigated, with focus on the African continent. An assessment of current challenges and proposed solutions is also presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
HABs are most frequently seen in lakes and oceans, where they can flourish due to high levels of phosphorus, nitrogen, and organic chemicals. Factors such as temperature, light intensity, nutrition, and pH greatly influence the ability of the blooms to produce poisonous substances known as cyanotoxins. It has been assumed that cyanobacteria produce cyanotoxins as a form of chemical defense against other microbes and higher trophic levels [1] and as a form of chemical signaling [2]. These secondary metabolites pose a serious threat to marine life and the availability of safe drinking water. The two major ways that people and animals get cyanotoxin poisoning are either ingesting contaminated water or bacteria cells that are generally discovered on the scum that covers water bodies in lakes and ponds [3]. As such, exposure to cyanobacteria can result in gastro-intestinal and hay fever symptoms or pruritic skin rashes [4]. Furthermore, ingestion of β-Methylamino-l-alanine (BMAA), a cyanobacteria neurotoxin, could lead to progressive nervous system disorders such as Amyotrophic Lateral Sclerosis (ALS), Parkinson’s disease, and Alzheimer’s disease [5].
Reports of cyanotoxin poisoning are usually associated with fresh water sources, and throughout the years, more fatal incidents have been documented [6]. The first death of marine mammals was reported [7] after sea otters died from ingestion of cyanotoxins. Recently, 330 elephants died after drinking water infested with cyanobacteria in Botswana [8]. Despite government authorities’ claims that they tested waterholes for algal blooms, no official report on the collection and analysis of water and carcass samples was ever published. The head veterinarian of the department of wildlife in Botswana only mentioned that cyanobacterial neurotoxins were shown to be the cause of mortality in their studies [9]. While most focus is on the detrimental impacts cyanotoxin poisoning has on human and animal (mostly livestock) health, it should also be noted that it may greatly jeopardize the tourism industry.
1.1 Microcystins
Amongst the most toxic cyanotoxins, are microcystins which are produced by several cyanobacterial genera, such as Anabaena, Anabaenopsis, Aphanocapsa, Aphanizomenon, Cylin-drospermopsis, Fischerella, Hapalosiphon, Lyngbya, Microcystis, Nostoc, Oscillatoria (Planktothrix), Phormidum, Rivularia, and Synechococcus. The most frequent occurrence is reported in the strain of Anabaena and Microcystis. MCs were named after the cyanobacterial species first discovered to produce them, Microcystis aeruginosa [10]. The general structure of MCs is made up of proteinogenic (l) and non-proteinogenic (d) amino acids, and they have a generalized sequence of cyclo-[d-Ala]-[X]-[d-Masp]-[Z]-[Adda]-[d-Glu]-[Mdha] (Fig. 1).
MCs vary according to the amino acids in the X and Z locations of the structure. A list of common MC variants with their respective amino acids is presented in Table 1. More than 300 different types of MCs have been recorded, and many of them can be simultaneously produced during a bloom [11]. This high number of variants makes analysis challenging due to their different physicochemical parameters [12]. Microcystin-Leucine-Arginine (MC–LR) is the most prevalent and dangerous variant and its structure is illustrated in Fig. 2 [13]. It is primarily linked to mammalian immune system dysfunction, which often results in numerous illnesses, including cancer [14]. Many reports link MC–LR with liver disease. Freshwater HABs producing MCs were associated with primary liver cancer incidence and death in central Serbia [15]. An earlier study in China also linked primary liver cancer to freshwater MCs in drinking water sources [16]. Even though no MC-related deaths in humans have been documented in Africa, serious effects from direct contact with MCs have been observed. These include hepatoxicity, gastrointestinal diseases, dermal irritation, and nausea [17].
MCs have also been determined to be the primary reason for random fish deaths. Cyanobacteria and MCs have been detected in various water sources across Africa, including artificial dams, lakes, fishponds, and rivers. Great lakes and rivers in Africa, such as Lake Victoria and the Limpopo River, cut across several countries and have been the subject of many cyanobacteria and MC studies. The abundance of these toxins in Africa has influenced several scientists to focus on the development of greener, simpler, and more sensitive MC determination strategies, and molecular imprinting techniques prove to be the most effective and economically friendly approach for this purpose.
1.2 Molecularly Imprinted Polymers
The term “molecular recognition” refers to a particular interaction between two or more molecules through noncovalent bonding. Molecular recognition controls connections between enzyme and substrate, antigen and antibody, or ligand and receptor systems [18]. This idea sparked the creation of MIPs, which bind with target molecules (templates) with high specificity and selectivity to capture target analytes for analysis or removal [19, 20]. Due to their inexpensive production costs, resilience to extreme environmental factors (such as pH, temperature, and chemical solvents), and recognition capabilities that are comparable to those of natural receptors, MIPs have become increasingly popular [21, 22]. To facilitate interactions with a targeted molecule, inert macromolecular polymers with multi-functional receptor groups have been created utilizing the imprinting approach [23].
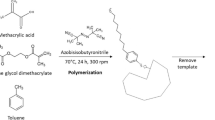
The general route for synthesizing MIPs is illustrated in Fig. 3. Functional monomers, crosslinkers, and initiators are the fundamental building blocks in the synthesis of MIPs. The effectiveness of the imprinting process depends on the development of a robust complex that must be maintained throughout the whole polymerization step. Functional monomers engage with templates through covalent, noncovalent, or semi-covalent interactions to create complexes [24]. The active substituents or functional hydrogen bonds offered by the functional monomer molecules not only influence the affinity of the template to the functional monomer molecules but also control the mechanical stability and porosity of the polymer [25, 26]. As a result, MIPs are particular to the template and have excellent selectivity. Electrostatic interactions, hydrogen bonds, hydrophobic contacts, or weak van der Waals forces are frequently used to recognize those target molecules.
The schematic diagram of MIPs synthesis [27]
Due to the exothermic nature of the polymerization reaction, the complex is susceptible to conformational changes of the monomer and template, which results in the irreversible loss of a portion of the complex. By creating robust contacts between the functional monomer and the template, these alterations and losses can be reduced. Therefore, choosing the right monomers is crucial. Thermodynamic simulations and combinational screening have been utilized to effectively identify suitable monomers for imprinting [28, 29].
Traditionally, basic organic compounds, including methanol (MeOH), acetonitrile (MeCN), methacrylic acid (MAA), and ethylene dimethacrylate (EDMA), have been used to prepare MIPs for the selective identification or adsorption of MCs. Because of the enormous library of monomers that contains thousands of polymerizable chemicals, this field of research was initially challenging. Chianella et al. [24] solved this problem via computational chemistry by using molecular modeling software and narrowing down the functional monomers that can be used for the preparation of MIPs suitable for the recognition of MCs. Some methods also employed the actual toxins as templates [30, 31] an approach that is potentially dangerous because of the exposure of analyst to the toxins. Further this approach leads to leakage of the template molecules, slow mass transfer, and difficulties in removing the templates. Because of these shortcomings, surface molecular imprinting was adopted in modern approaches where graphene oxide (GO) is used as the supporting material [29]. GO is used as a supporting material for the manufacture of imprinted polymers because of its unique properties, including a large surface area, high mechanical strength, and an abundance of functional groups containing oxygen. The huge delocalized p-electron system of graphene is also preserved in GO, giving it a strong attraction for carbon-based ring formations [30, 31]. Instead of using the actual toxins as templates, some scientists used dummy templates [32] and fragments of the MCs such as arginine (R) [33] to create the desired cavities in the MIPs.
In this review, the occurrence of MCs in Africa and analytical methods for their determination are summarized, with a focus on the use of LC–MS/MS. Progress in the synthesis of MIPs and their application to the analysis and removal of MIPs over the past 10 years is critically assessed. Challenges and future perspectives in this field are also outlined. This paper gives an overview of MC abundance in Africa and a necessary foundation for promoting the further development of MIPs not only for MC determination but also for mitigation purposes.
2 Analysis and Occurrence of MCs in Africa
MCs are found everywhere in the world, especially in fresh stagnant waters such as lakes, ponds, and dams [2, 13, 34] and have also been reported in desert environments [35]. Their occurrence threatens the availability of safe drinking water [3]. Hence, the World Health Organization (WHO) has set the maximum permitted level of MC–LR in drinking water at 1.0 µg/L, and the tolerable daily intake at 0.04 µg/Kg [36].
In recent years, cyanobacterial blooms and the production of MCs have been reported in several African countries. Recent reviews provide the context of cyanobacterial blooms in Africa and critically assess existing information regarding cyanobacteria’s morphological, genetic, and chemical diversity in water bodies in Africa. These reviews highlight that the abundance of cyanobacteria may indicate MC exposure. However, no review summarizes work done on the analysis of MCs in African water sources, particularly with a focus on analytical methods that determine MC concentrations even at trace levels. Table 2 is a summary of the existing literature (2013–2023) that reports detection and quantification of MCs in Africa. The information in this table has been compiled through a thorough analysis of online publications and reports. MCs are indeed present in various water sources in Africa, and their concentrations are mostly above the maximum permitted level of MC-LR in drinking water by the WHO (1.0 µg/L).
Analysis and quantification of MCs in Africa (Fig. 4) have been carried out using immunochromatographic and chromatographic techniques such as enzyme-linked immunosorbent assay (ELISA), protein phosphatase type 2A (PP2A) inhibition assay, polymerase chain reactions (PCR), high-performance liquid chromatography coupled with a diode array detector (HPLC–DAD), and liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS).
It is well established that LC–MS/MS is an excellent technique for analysis of many contaminants due to its numerous advantages, such as a more extensive linear dynamic range, lower limits of detection (LODs), higher precision, and accuracy [58]. In a majority of studies (42% of reports from the literature), MCs have been analyzed by LC–MS/MS (Table 3 and Fig. 4). He et al. [59] stated that due to its great sensitivity and selectivity, LC–MS/MS is the standard approach for detecting, identifying, and confirming cyanotoxins in environmental samples. In every analytical procedure, important processes like extraction, purification, and chromatographic separation need to be clearly described to significantly reduce matrix effects. The most popular sample pre-treatment method prior to analysis of MCs appears to be solid phase extraction (SPE).
3 Strategies for Adsorption of MCs
3.1 Carbon-Based Adsorbents
The harmful effects MCs have on marine ecosystems, animals, and humans can be minimized by adding conventional toxin adsorbents to water. The removal of MCs from water has been reported using a variety of adsorbing materials [60]. Since its discovery as an effective and strong adsorbent, activated carbon (AC) has been widely employed in the adsorption and removal of MCs. AC surpasses other adsorbents due to desirable properties such as good performance, affordability, non-toxicity, and a large surface area that can easily absorb biological contaminants. Zhu et al. [61] investigated the adsorption of MC–LR and MC–RR onto different forms of powdered AC: wood, shell, and coal. Their study provides important information on the selection of a suitable AC based on the temperature, pH, and anion content of the water. Zhu et al. found that wood AC had better adsorption capacity compared to shell and coal ACs. They also showed that decreasing temperatures increased the adsorption rates, while increasing pH decreased the adsorption capacity of wood AC for MCs. Additionally, anions were found to hinder adsorption and reduce the removal rate by 14–24%. Similar findings were reported on a study carried out to investigate the effect of natural organic matter (NOM) on the adsorption capacity of wood, coal blend, bituminous coal, and coconut shell ACs [62]. The authors also indicated that wood AC eliminated MC–LR the fastest and most effectively, perhaps because it has the most mesopores. However, the authors indicated that the adsorption of MC–LR by AC was significantly reduced in the presence of NOM. Thus, suggesting competition for binding sites in AC between MC–LR and NOM.
Other researchers modified AC by adding biological components to enhance the adsorption of MCs. For instance, Ren et al. [63] modified AC fibers with sodium alginate (SA) to act as an adhesive and Sphingopyxis sp. for the removal of MC–RR. AC fibers and Sphingopyxis sp. were suspended in SA to create the adsorbent. Like the study by Zhu et al., the effectiveness of the adsorbent was examined in relation to the effects of rising temperature and pH. The elimination of MCs by the adsorbent increased because of raising the two, according to the results. However, the biologically modified AC fibers adsorption effectiveness was reduced by temperatures and pH levels over 32.8 °C and 6.8. Furthermore, high reusability of the adsorbent was observed, as it removed over 70% of MCs after the 7th cycle [64].
Different forms of modified non-traditional carbon, such as, biochar, mesoporous carbon, and GO, have been investigated for their efficiency in MC removal. Abbas et al. reviewed these adsorbents [60] and state that carbon-based adsorbents, including AC, are inefficient in removing large MC-LR due to the presence of micropores. They further state that to achieve greater removal of MCs from water, higher doses of these adsorbents need to be used. This is uneconomical and environmentally unfriendly since the production of the adsorbents relies on the use of natural resources. Additionally, higher doses of carbon-based adsorbents translate to large volumes of saturated or exhausted carbon, which, depending on the kind of carbon, is frequently disposed of with or without additional treatment. Consequently, the adsorbate could be leached into the environment.
Various forms of oxides have also been studied for their ability to absorb MCs. In most cases, these oxides are combined with carbon-based materials for improved adsorption. Even though some of the reported adsorbents are applied as sorbents in SPE for MC analysis, they can be further developed for mitigation purposes. For instance, Mashile et al. [65] synthesized an activated carbon@iron oxide@ manganese oxide composite (AC@Fe2O3@MnO2) which they used as solid phase material for the preconcentration and determination of MC-LR in environmental matrices. The adsorbent showed excellent extraction, separation, preconcentration, and determination of MC-LR, with an adsorption capacity of 389 µg/g. Mashile et al. concluded that their AC@Fe2O3@MnO2 adsorbent was excellent during their developed dispersive solid phase microextraction (DSPME) and UV–vis spectrophotometry methods due to the presence of AC and magnetic nanoparticles.
Photocatalytic composites made of titanium oxide (TiO2) nanotubes coated with carbon nanotubes were designed by Chae et al. [66]. The composites were employed for the adsorption and degradation of cyanotoxins in water and showed an excellent removal efficiency of MC–LR (> 95).
While the removal of MCs from water using conventional and modified carbon-based adsorbents is effective, it has notable limitations. The challenges of regeneration and the disposal of used AC are reasons to advance toward the use of environmentally friendly adsorbents. Molecular imprinting technology could be a better alternative for the development of robust, inexpensive, and greener MC adsorbents.
3.2 Synthesis of MIPs for MCs Adsorption
3.2.1 Bulk Polymerization
One of the first techniques for synthesis of MIPs, bulk polymerization is a quick and easy procedure where the template, functional monomer, crosslinker, and initiator are mixed in a porogen solvent [67]. The polymerization process is started by thermal or light irradiation. After the polymer is created, the template is removed using grinding and sieving, leaving behind particles of inconsistent shapes and sizes. Additionally, due to mechanical disintegration during this process, the binding group and heterogeneous absorption sites in the polymer are partially destroyed [68]. As a result, the polymer exhibits low selectivity and reproducibility. Perhaps this is the reason why bulk polymerization has not recently been used in the creation of MC–MIPs. Krupadam et al. used bulk polymerization to synthesize MC–LR MIPs using MC–LR as a template, itaconic acid (IA) as a functional monomer, and EGDMA as a cross-linking monomer [69]. Their investigation was first guided using computational simulations to come up with the best recipe for highly specific MIPs. Figure 5 shows the chemical molecules employed in this investigation. The polymer showed binding ability for MC–LR, effectively removing 90% of the toxin from water. However, beyond the chemical oxygen demand (COD) of 380 mg/L, adsorption of MC–LR was reduced due to the presence of hydrophilic organics, which form the majority of NOM.
The use of MC–LR to prepare MIPs is risky because it exposes researchers to the dangerous toxin and leads to leakage of template molecules. Further, it is often difficult to remove the template after the polymer has been formed, especially when it’s a bulk polymer. So Mbukwa, Msagati, and Mamba [33] employed a fragment imprinting approach to synthesize bulk polymers using l-arginine (instead of MC–LR) as a template (Fig. 6). This is so that carbonyl functionalized MIPs can be created using the guanidinium characteristics of l-arginine (R, an amino acid present in practically all MCs. They employed 2, 2′-azobis (2, 4-diethylvaleronitrile (AIVN as an initiator, acidified acetonitrile (MeCN as a porogen, ethylene dimethacrylate (EDMA as a cross-linker, and methacrylic acid (MAA as a functional monomer. The polymers demonstrated a high selective recognition capacity towards the guanidinium terminal in [arginine]-MCs.
Although MIPs prepared by bulk polymerization have several advantages, such as being easy to prepare and inexpensive, they still have several shortcomings. These include low mass transfer, poor selectivity due to poor site accessibility, and a high diffusion barrier. Additionally, reproducing polymers that have been synthesized via the bulk polymerization approach is challenging because the technique involves the use of mechanical force to break down polymer particles. Moreover, the bulk polymerization process itself is laborious and time-consuming. Therefore, to improve the efficiency of MIPs for MC adsorption, regular-shaped (spherical) MIPs with controlled size have been developed using a variety of polymerization methods such as suspension, emulsion, and precipitation polymerization.
3.2.2 Surface Imprinting Polymerization
Surface polymerization involves the grafting of thin MIP layers on carrier surfaces such as carbon materials, silica gel, magnetic spheres, and quantum dots [33]. These types of polymers are mostly used for separation, catalysis, and sensing applications. Compared with the above-mentioned approaches, surface imprinting produces highly selective polymers with more binding sites, better specific recognition, an increased mass transfer rate, and improved adsorption capacity. Additionally, the process prevents permanent entrapment of template molecules, which facilitates removal and recombination of the template molecules. Due to these advantages, surface imprinting has been the most followed approach for preparing MC-MIPs using either GO, silica gel, salinized glass beads, or quantum dots (QDs) as the carrier surface.
Graphene and GO are characterized by high surface areas, strong mechanical strength, and exceptional electrical and thermal conductivity. Also, due to the presence of hydrophilic hydroxyl, epoxide, and carboxylic groups, GO disperses easily in water [71]. These features make it suitable for various applications in technological fields such as SPE, water treatment [72], drug delivery, sensors [73], and proteomics [74]. In a study [30, 31], a GO-based ternary magnetic MIP hybrid (T-MMIP hybrid) was developed. GO was first modified with iron oxide (Fe3O4) to prepare magnetic GO–Fe3O4, which was then used as a substrate for additional in situ co-polymerization in the presence of a functional monomer, acrylamide (AM), a cross-linking agent, divinylbenzene (DVB), and a template molecule (MC–LR). The molar ratios of template molecule to functional monomer to cross-linker (T:F:C), polymerization time, and temperature were optimized to obtain high adsorption capacity and selectivity of the T-MMIP hybrid. From this, the authors found that the optimum molar ratio of T:F:C is 1:4:10, and higher adsorption capacity is obtained after 8 h of polymerization at 70 °C. In aqueous solution under ideal circumstances, the resulting T-MMIP hybrid showed an exceptional affinity for eight structurally related microcystins (MC–RR, MC–YR, MC–LR, MC–LW, MC–LY, MC–LA, MC–LF, and MC–WR) ranging from 85.5 to 100%.
Similarly, based on magnetic GO–Fe3O4, Tian et al. [75] synthesized a MIP for the extraction of MC–LR. Tian et al. state that functionalized GO surpasses ordinary GO because, once functionalized, the GO is endowed with more functional groups, making it more selective, less aggregating, and easily dispersive in aqueous systems. Dopamine was used to functionalize GO, act as a functional monomer, as a crosslinker, and MC–LR as a template in the imprinting process. The developed MIP showed a higher imprinting factor and specific adsorption capacity toward MC–LR than the structurally similar analytes MC–RR and MC–YR.
The benefits of silica gel include high stability, superior biocompatibility, non-swelling properties, and low cost making it an excellent support when used as carrier surface for MIPs [76]. A few studies have utilized this type of support to synthesize MIPs suitable for immunoassay detection of MC–LR. Wu et al. [77] used 3-(trimethoxysilyl)propyl methacrylate functionalized silica, EGDMA as the crosslinker, MAA as the functional monomer, and MC–LR as the template for the preparation of their imprinted polymer microspheres. A much more intricate process was adopted by Garcia et al. [78], who mixed silinized glass beads with MC–LR and a pre-polymerization mixture consisting of eight monomers to form the surface-imprinted nanoMIPs (Fig. 7). The polymers exhibited excellent selectivity for MC–LR and low cross-reactivity towards another toxin (MC–YR).
Steps involves in solid phase synthesis of nanoMIPs for MC-LR detection. a Covalent immobilisation of MC–LR on the support, b Formation of the nanoMIPs around of the template, c removal of low-affinity nanoMIPs and unreacted monomers, d Elution of high-affinity nanoMIPs suited for pseudo-ELISA [78]
As a new class of fluorescent materials, QDs have gained a lot of attention in recent years thanks to their impressive size tunability, narrow emission spectra, broad excitation spectra, excellent signal intensity, outstanding photostability, and biocompatibility. MIPs are highly selective; therefore, combining them with QDs creates an advantageous new tool for analyte recognition. Li et al. [32] prepared a MIP on the surface of Mn-doped ZnS QDs using two fragment-dummy templates to reduce the toxicity and cost of MCs as templates in the synthesis process. The two dummy templates used were L-arginine (R), which is a fragment present in almost all MCs, and phenylpentane, which is structurally like the ADDA group that is present in all MC structures. Tetraethoxysilane (TEOS) was used as a cross-linker. The developed FMIP-Mn-doped ZnS QDs showed better selectivity for MC–LR, MC–YR, and MC–RR compared to MCs without the R group, MC–LF, MC–LY and MC–LA. Additionally, the polymer displayed good selectivity for probing MC–LR in the presence of relevant metal ions, amino acids, and biomolecules. The use of two molecules, like the parts of the MCs, as molecular templates to form two specific recognition site cavities proved to be a cost-effective technique for preparing highly selective adsorbents.
A novel type of MIP-coated carbon QDs (MIP@CQDs@SiO2) was developed for the selective and sensitive determination of MC-LR [79]. The material was synthesized via sol–gel polymerization using l-leucine as the segment template molecule, 3-aminopropyltriethoxysilane as the monomer, and tetraethylorthosilicate as the crosslinker. CQDs were used as carrier surfaces and as signal materials.
3.2.3 Polymerization on an Electrode Surface
Over the years, a lot of effort has been put into synthesizing molecularly imprinted sensors, particularly with an emphasis on improving their sensitivity. Many methods, such as drop-coating a solution on a prepared polymer, in-situ chemical polymerization [30, 31], and electro-polymerization [80] have been applied to produce MIP films on the surfaces of transducers. By regulating the polymerization factors (such as the applied voltage and cyclic scanning), electro-polymerization offers greater control over the final MIP film's thickness and density than other methods [81]. Hence, it is the most preferred technique.
The photocatalyst copper(I) oxide (Cu2O) absorbs visible light, is non-toxic, and is easy to manufacture. This means Cu2O can easily harvest solar energy and photo-catalytically oxidize MC–LR, then measure target molecule concentration as a function of photocurrent [82]. Chen et al. [83] developed an MC–LR adsorbent by modifying the surface of a Cu2O/indium-doped tin oxide-coated (ITO) glass electrode with molecularly imprinted polypyrrole (PPy). MC–LR was used as the template molecule and pyrrole as the functional monomer to form the active sites of the sensor. With a scan rate of 50 mV/s, the polymerization potential ranged from − 0.2 V to + 1.0 V. The template molecules were then removed from the PPy after 15 cycles by electrochemical treatment at + 1.3 V for 15 min in a 0.2 M K2HPO4 solution.
4 Characterization of MIPs Synthesized for Adsorption of MCs in Water
MIPs are capable of adsorbing target molecules due to the tridimensional cavities that are formed during the polymerization process. Affinity for the target compounds is also increased by the polymer’s surface area, pore volume, and pore size. The ideal characteristics of the polymers are determined using various instruments, and the observations influence the synthesis procedure. De Middeleer, Dubruel, and De Saegaer [84] reviewed general characterization techniques of MIPs using an ergot alkaloids MIP to demonstrate other techniques such as scanning electron microscopy (SEM). Here in, a comprehensive overview of techniques that have been applied to MC–MIPs is given.
4.1 Chemical Characterization
Studying the chemical structure of the generated polymer is crucial since the synthesis of MIPs relies on the use of functional groups to create holes that are comparable in structure and shape to the target molecule.
4.1.1 Fourier Transform Infrared Spectroscopy (FT–IR)
IR spectroscopy gives information about functional groups present in the polymer via adsorption bands. Upon exposure to infrared radiation, molecules adsorb specific wavelengths, which lead to a change in their dipole moments. This change results in the transfer of vibrational energy levels from the ground state to the excited state, and the energy gap is used to determine the frequency of the adsorption peak [85]. One can readily obtain chemical and structural information about a polymer via FT–IR because the number of absorption peaks is related to the number of vibrational degrees of freedom of the molecule. Furthermore, the intensity of absorption peaks corresponds to the change in dipole moment and the probability of energy level transitions. As a result, various functional groups have various adsorption bands, and various bond types have various adsorption characteristics. FT–IR spectroscopy was used to characterize different functional groups in their MC–MIP and non-imprinted polymer (NIP), which were prepared using arginine and MAA [33]. Carbonyl functional groups were expected to be found on the polymer cavities because of the MAA, and they hold the template through hydrogen bonding. FT–IR confirmed their presence in the MIP and NIP at 1728 and 1727 cm−1 respectively. Complete removal of the template from the crude MIP after washing was also confirmed via the absence of the guanidium terminal functional groups (1671 cm−1 (NH2 band), 1612 cm−1 (NH2 vibration band), 1574 cm−1 (C = O stretch), and broad band around 3000–3300 cm−1 (NH stretch)).
Similarly, FT–IR was used to confirm complete template removal from the developed polymer [68]. This technique has also been applied to study the successful grafting of polymers onto graphene surfaces to form GO–Fe3O4–MIPs [28,29,30,31, 75]. Moreover, FT–IR can be used to demonstrate modification of silica microspheres prior to immobilizing the template onto the solid support [78].
4.1.2 Raman Spectroscopy
Raman spectroscopy [86] (different font), relies on the use of visible or near-infrared monochromatic laser light to study and provide chemical structural information about materials nondestructively. Raman scattering occurs when a portion of photons loses or acquires energy due to molecular vibrations. These vibrations’ energy depends on the molecules' composition and structure, hence why Raman is called a chemical fingerprint technique. Light gathered by sensitive detectors and spectrographs produces comprehensive and information-rich spectra that have excellent and unique sharp peaks that can be utilized to determine sample identity, concentration, phase, morphology, and other features. Raman spectroscopy has been used to analyze the interactions and carbon structural changes in the prepared GO–Fe3O4–MIP (T-MMIP) hybrid by Pan et al. (Fig. 8) [30, 31]. Chen et al. [83] and Song et al. [87] also utilized Raman spectroscopy to verify the chemical structures of their prepared sensors.
4.2 Morphological Characterization
Knowing the size and form of the polymer particles is crucial to account for back pressure issues during washing and elution because most MIPs created for MC adsorption are intended for SPE applications. The geometry and size of polymers can be determined using electron microscopy techniques, which is important information because particle size affects the polymer’s capacity to rebind to the target chemical. Additionally, studies of particle size are crucial for validating polymerization methods because different methods produce varying particle sizes [84].
4.2.1 Scanning Electron Microscopy (SEM)
Visualizing polymer particles requires the use of SEM. The entire surface is scanned using an electron beam that is concentrated on the particles. The detection of secondary electrons that are emitted from the surface is then used to create a picture of the entire surface [88]. Several research articles report the use of SEM to visualize MIPs synthesized for MC adsorption [12, 28,29,30, 75]. These studies show that SEM can be used to differentiate MIPs from NIPs. Li et al. [89] confirmed the imprinting of the MC-LR dummy template on the polymer using SEM images, which showed that the surface of the MIP was more coarse and bulgy than that of the NIP because of the template imprinting on it. Tian et al. [75] also employed SEM to confirm and visualize the surface imprinting of the MIPs on the GO surface (Fig. 9). Figure 9a shows a crumpled and rippled structure made of GO, while Fig. 9b shows a structure made of Fe3O4 with a lot of sphere-shaped particles. Figure 9c then showed that the combination of the GO layer and Fe3O4 created irregularly scattered Fe3O4 spheres, whereas on the synthetic GO-Fe3O4-MIP, considerably more porous and homogeneous particles were found. This implied the development of holes that the template molecule could access. Similar findings were also reported [30, 31]. SEM was used to study the morphology of their PPy/Cu2O molecularly imprinted composite and determine its particle sizes [83].
SEM images of GO (a), Fe3O4 (b), GO–Fe3O4 (c) and GO–Fe3O4-MIPs (d) [75]
4.2.2 Transmission Electron Microscopy (TEM)
Like SEM, TEM is also used for visualization purposes due to an electron beam interacting with the particles. However, instead of detecting electrons being ejected from the surface, TEM measures transmitted electrons to study and elucidate the geometry and size of the polymer particles [90]. TEM is also used to investigate differences between MIPs and NIPs. In the studies to synthesize MIPs for binding MCs, TEM has been applied to confirm the structures observed by the SEM technique [28,29,30,31, 75].
4.2.3 Brunauer, Emmett and Teller (BET) method
The BET theory explains how gas molecules adsorb on solid surfaces, and this translates into measures of a material’s specific surface area and pore size distribution [91]. The most frequently utilized gas is nitrogen gas (N2), which does not chemically react with most substances being analyzed. The N2 adsorption isotherm method can be used to determine the total pore volume, surface area, and pore size distribution of particles because the pressures used during the adsorption of the gas onto the surface and the size and volume characteristics of the different pores are inversely proportional. The specific surface area is then computed using the BET technique, which assumes uniform pore wall coverage. Since MCs have masses ranging from 800 to 1400 g/mol, their successful extraction with MIPs as SPE sorbents requires polymers with large pore-sized particles and surface areas (> 100 m2/g) [28,29,30,31, 75]. Therefore, during polymer synthesis, the template: functional monomer ratio needs to be optimized as it affects the final pore sizes. It was concluded that using arginine (template) and MAA (functional monomer) in the ratio 1:10 yields polymers with desirable surface area (> 100 m2/g), pore volume (> 0.6 cm3/g), and pore sizes (≈ 88 Å) are suitable for MC binding [33]. Pan et al. [30, 31] also determined that a MIP with a high surface area (1015 m2/g) and a large total pore volume (0.75 m3/g) is ideal for MC adsorption. However, the BET surface area and average pore size of GO–Fe3O4–MIPs prepared by [75] were 81.92 m2/g and 0.31 cm3/g, respectively.
4.2.4 X-ray Diffraction Analysis (XRD)
XRD is used for the determination of the crystallographic structure, chemical composition, and physical properties of a material [92]. XRD uses Bragg's equation to reflect collimated X-ray beam incidence on a sample's crystal plane. Hence, the technique is suitable for ordered material, particularly long-range ordered crystalline material. X-rays flow through the specimen and are diffracted by atoms. Bragg’s law and a properly positioned detector observe X-ray scattering interference and establish the material’s crystalline structure. Several researchers have utilized XRD to elucidate the crystal structures of the developed MC-MIPs [87, 89]. For example, Pan et al. elucidated the crystal structures of GO, magnetic GO–Fe3O4, and the surface imprinted polymer T-MMIP (Fig. 10) [30, 31].
4.3 Thermal Characterization
4.3.1 Thermogravimetric Analysis (TGA)
Thermal characterization techniques examine the dynamic interactions between various system parameters, such as mass, volume, and reaction rate, and how a controlled temperature program impacts these properties. TGA is the most popular method [93]. The temperature range in which a polymer can be employed is revealed by TGA. To obtain a degradation profile, the weight loss of the polymer is measured as a function of temperature [75, 84]. TGA also allows for the detection of solvent residues, unreacted monomers, and cross-linkers, which may highlight the need for procedure improvement. Previously [94] used TGA analysis to study the stability of GO before and after surface imprinting and to further confirm the grafting of the MIP onto the GO–Fe3O4 surface.
4.4 Characterization of MIP Structures on Surfaces
MIP structures are often used as recognition elements in a wide range of sensor applications, such as electrochemical sensors, conductometric sensors, light scattering sensors, and many more [84]. Because MIPs are employed in such a wide variety of sensor applications, methods for characterizing immobilized particles and imprinted surfaces are now needed. Specific techniques for this purpose have been engineered, and X-ray photoelectron spectroscopy (XPS) analysis has been applied in MC–MIPs.
4.4.1 X-ray Photoelectron Spectroscopy
In XPS, a surface is exposed to X-rays, which cause photoelectrons to be emitted. The orbital and atomic origins of these electrons are related to their energy. Therefore, the chemical composition of a surface can be determined using XPS spectra. Depending on their surroundings, elements can exhibit a peak shift indicative of the presence of distinct groupings of elements (i.e., functional groups). The characterization of MC-MIPs on surfaces using XPS has been published for studying template molecule imprinting, verifying their removal, and evaluating target molecule rebinding. For example, Pan et al. used XPS spectra to verify MIP grafting onto the GO–Fe3O4 surface and to further confirm components of the prepared T-MMIP [30, 31]. XPS was also used to elucidate the compositions and chemical states of the obtained Cu2O and Cu2O/PPy materials during the synthesis of an electrochemical sensor for MC–LR (MIP/Cu2O/ITO) [83]. Likewise, Li et al. performed XPS measurements to confirm the element composition of the Mn-doped ZnS QDs and the functionalized MIPs [32].
4.4.2 Energy-dispersive X-ray Spectroscopy (EDS)
EDS, also known as EDX, is a technique that enables the chemical or elemental characterization of materials. An electron beam is used to excite a sample, which then releases some of the absorbed energy by ejecting a core–shell electron [91]. A higher-energy outer-shell electron replaces the core–shell electron, releasing the energy difference as an X-ray with an atom-specific spectrum. This makes it possible to do a compositional analysis on a volumetric sample that has been excited by the energy source. The spectrum peaks identify the element, while the signal intensity indicates its concentration. Chen et al. used EDS to study the element composition on the surface of the MIP/Cu2O/ITO electrode. The spectrum displayed the chemical composition of Cu, O, C, and N on the electrode surface, verifying the structure of the developed material [83].
5 Applications of MC-MIPs
5.1 Adsorptive Removal of MCs from Water
Synthesized MIPs were tested for the adsorption of MC-LR in lake water collected from Lake Ambazari, Nagpur, India [30, 31, 89]. The effectiveness of the synthesized MIPs was evaluated in comparison to that of commercial powdered AC, and the interference of environmental variables, including total organic carbon (TOC) also expressed as chemical oxygen demand (COD), and total dissolved solids (TDS), was also studied. Additionally, utilizing adsorption isotherm studies, the competitive binding ability of MC Issaabadi LR to MIPs (MIP–IA and MIP–MAA) was assessed. The study’s findings showed that, despite MI–IA's higher adsorption capacity than MIP–MAA, both MIPs were successful at adsorbing MC–LR. Additionally, environmental factors like TOC and TDS (concentrations less than 300 mg/L) had no impact on the MC–LR adsorption capacity of MIP, and this capacity was 40% higher than the powdered AC that is typically employed in the water purification industry. The MIPs created demonstrated good selectivity in addition to water remediation.
5.2 Enrichment of Water Samples Prior to MCs Determination
The majority of the MIPs that have been synthesized were applied as SPE sorbents for the extraction of MCs prior to analytical determination using HPLC or LC–MS/MS. Mbukwa and co-authors used MIPs for the selective recognition and extraction of [arginine]-MCs in water samples collected from Hartbeespoort Dam (South Africa) [33]. MC–LR, MC–YR, and MC–RR were successfully extracted and analyzed by LC–ESI–MS. Similarly, LC–MS/MS analysis was used to study the adsorptive efficiency of synthesized T-MMIP [30, 31, 70]. The T-MIP was used for enrichment of river water samples, and it showed excellent performance, proving to be a better alternative SPE. Moreover, GO–Fe3O4–MIP was used in sample preparation for the rapid and sensitive determination of MC-LR in water samples [28].
Seeing that the chromatographic methods, such as HPLC and LC–MS/MS, rely on the use of sophisticated and expensive equipment that cannot meet the high throughput detection required by food safety authorities, efforts are now concentrated on pairing MIPs with immunochromatographic techniques. Wu et al. [77] combined an MIP suitable for trapping MC–LR with an enzyme-assisted calorimetric method to enhance the sensitivity of the lateral flow immunochromatographic assay (LFICA) towards MC–LR (Fig. 11). The polymer was used as an SPE sorbent to separate MC–LR from the complex matrix, and then the obtained MC–LR was detected by LFICA. This approach resulted in a wider linear range and a more sensitive detection of MC–LR. NanoMIP synthesized was used to replace antibodies in Enzyme-linked immunosorbent assay (ELISA) for determination of MC–LR in water [78]. Cross-reactivity in the presence of another analogous toxin (MC–YR) indicated that the assay had a high selectivity for MC–LR. Impressive results (Table 4) were obtained in real samples as well, confirming that these “plastic antibodies” have a high potential for diagnostic and remedial applications.
Schematic illustration of the principles of the proposed LFICA method [77]
5.3 Sensors
Analysis methods such as SPE–HPLC and SPE–LC–MS/MS for MCs are expensive as they require expensive instruments and complicated sample pretreatment techniques. Sensors such as biosensors and electrochemical sensors have a promising potential for rapid, simple, and efficient analysis of MCs. MIPs’ exceptional quality is their selectivity for individual analytes. A physical or chemical signal is produced when the MIPs interact with the template molecules. This signal is then converted into a quantitative output signal by the converter, and by continuously monitoring this signal, the studied molecule can be determined in real time. MIPs are highly desirable materials to produce a wide range of sensor-sensitive membranes because of their high levels of selectivity and environmental tolerance [68]. Currently, the applications of MC–MIPs for use as sensors include electrochemical sensors and fluorescence sensors.
Chen et al. developed a visible light-responsive photoelectrochemical (PEC) sensor based on PPy/Cu2O molecular imprinting composite film for MC–LR [83]. The sensor demonstrated a linear relationship between the change in photocurrent density and the logarithm of the MC–LR concentration from 1.0 ng/L to 100 ng/L and 100 ng/L to 10.0 g/L. Furthermore, when subjected to a predetermined concentration of interference solutions, the sensors displayed a selectivity toward MC–LR.
Song et al. recently developed a novel label-free nonenzymatic PEC sensor for detection of MC–LR [95]. The sensor was fabricated by functionalizing MIPs onto reduced GO, which was grown on Ti–Fe–O nanotubes (NTs). The designed PEC sensor showed high sensitivity toward MC–LR with an LOD as low as 10 pM. Furthermore, high selectivity toward MC–LR was observed for the sensor, with recoveries in real water samples ranging from 91.78 to 97.40%.
CQDs have excellent optical properties, unique photoluminescence (PL) properties, strong photostability, are easy to prepare, and are chemically inert. Therefore, they exhibit absolute superiority as fluorescent materials in the development of fluorescence sensors. Recently, Qi et al. prepared a novel type of fluorescence sensor (MIP@CQDs@SiO2), which was used for rapid and sensitive detection of MC–LR in real water samples [79].
6 Present Challenges and Future Perspectives
Even though Africa is slow to conduct research and share findings, many countries have recently documented algal blooms and their hazardous effects. More efforts need to be made to advance the monitoring of contamination of African water sources with MCs. Particularly with regards to use of advanced, rapid and highly sensitive techniques such as UPHPLC–LC–MS/MS. Africans are at danger due to climate change and the current increase in temperature because cyanobacterial blooms will proliferate, further raising the quantities of MCs in drinking water. Therefore, researchers and governments should give top attention to finding effective and economical ways to remove MCs from African water sources.
The advancement of MIPs has allowed for their use as SPE materials in the separation and enrichment of MCs in sample pretreatment processes prior to analysis with centralized methods such as HPLC–DAD and LC–MS/MS. However, there are several obstacles that must be overcome before these methods of analysis can be widely implemented. Since methods such as LC–MS/MS offer a greater level of analytical reliability, the adoption of MIP-based methods should be taken into consideration and assessed in terms of field adaptability. Procedures for the preparation of MC–MIPs have been reported. However, not much has been reported on the optimization of factors and conditions during preparation, such as the types and amounts of template, crosslinker, solvents, initiators, and functional monomers. Pan et al. optimized the molar ratios of template molecule to functional monomer to cross-linker, polymerization time, and temperature to improve the selectivity of their polymer [79]. Additionally, not much has been done in Africa concerning the synthesis of MIPs for the MC adsorption. Most of the research reported is done by researchers from other continents. In the future it will be crucial to incorporate mathematical and statistical tools such as design of experiment (DOE) in the optimization of MC–MIPs. Computational methods could also be used as a guide in the process, particularly in selecting the best recipe for their synthesis [96].
One of the most frequently mentioned causes of errors is inadequate processes that often lead to template bleeding or leakage, which often results in MIP with irregular cavities, different particle sizes, and low binding capacities. To prevent false-positives and false-negatives due to remaining template molecules, care must be taken to ensure the complete removal of the template. Nanoscale MIPs have remarkable binding properties and selectivity compared to conventional MIPs. These types of MIPs are ideally suited as sensitive materials for sensors for the analytical detection of real samples because of their resistance to high temperature and pressure, acid and alkali, and compact storage compared to conventional MIPs. However, there is still a challenge to the stability and reusability of nanoscale sensors. The analytical sensitivity of MIP sensors often seems to deteriorate after just one use and after exposure to very low pH. For example, Song and co-workers observed a drop in the photoelectric density of their PEC sensor by 16.4% under pH = 4, which led to a decrease in the response signal [87]. Therefore, if optimizing the re-extraction conditions does not result in a consistent sensor, MIP-based sensors may be more suited for single-use. With respect to on-site monitoring of MCs in water sources, it is ideal for MC sensors to detect total MC concentrations rather than just one toxin, MC–LR. The development of sensors for this purpose can be achieved by using a segment that is present on all MCs as a template. ADDA is suitable for this, and the sensors developed would be able to detect all MCs.
The application of MIPs for MC remediation purposes is understudied, perhaps because procedures for the removal of toxins in water are complex and require large amounts of adsorbents and organic solvents. Thus, further exploration of effective MIPs that can remove MCs without the need for cumbersome post-treatment procedures is required. This can be achieved by combining MIPs or MIT with novel technological advances such as nanotechnology.
7 Conclusions
Due to their significant prevalence in many African water sources, MCs pose a serious health risk to Africans and animals. They will continue to have an impact if nothing is done to develop materials that can quickly remove them from the water that locals use for drinking, irrigation, and fishing. Several adsorbents, including AC, have been proposed for MC adsorption, but they have disadvantages that greatly limit their use for practical detection or removal of MCs. While MC–MIPs can be made using a variety of techniques, surface imprinting is the most effective and results in polymers with high adsorption capabilities. More research needs to be conducted in this area, especially with a focus on fabricating these polymers for MC removal in water. It should also be noted that developing such adsorbents in large quantities may be dangerous, expensive, and challenging if the actual toxins are used as templates. Although several dummy or MC segments have been adopted for use as templates, they are not easy to prepare and are expensive. The resulting MIPs are therefore not selective enough for practical use. Extracting template molecules from MIPs is a time-consuming and labor-intensive process that currently requires a lot of organic solvents. It is therefore necessary to do further research into efficient post-treatment methods for MIPs. Notably, the detection of MCs using a mix of MIPs, nanotechnology, and cutting-edge spectrum technology is a promising area of study.
Data Availability
No specific data is available for this review paper.
References
Drobac Backović D, Tokodi N, Marinović Z, Lujić J, Dulić T, Simić SB, Svirčev Z (2021) Cyanobacteria, cyanotoxins, and their histopathological effects on fish tissues in Fehérvárcsurgó reservoir Hungary. Environ Monit Assess 193(9):1–14. https://doi.org/10.1007/S10661-021-09324-3/FIGURES/5
Rastogi RP, Madamwar D, Incharoensakdi A (2015) Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front Microbiol 6(1254):1–22. https://doi.org/10.3389/fmicb.2015.01254
Rastogi RP, Sinha RP, Incharoensakdi A (2014) The cyanotoxin-microcystins: current overview. Rev Environ Sci Biotechnol 13:215–249
Kubickova B, Babica P, Hilscherová K, Šindlerová L (2019) Effects of cyanobacterial toxins on the human gastrointestinal tract and the mucosal innate immune system. Environ Sci Eur 31(1):1–27. https://doi.org/10.1186/S12302-019-0212-2
Holtcamp W (2012) The emerging science of BMAA: do cyanobacteria contribute to neurodegenerative disease? Environ Health Perspect 120(3):a110–a116. https://doi.org/10.1289/ehp.120-a110
Bouaïcha N, Miles CO, Beach DG, Labidi Z, Djabri A, Benayache NY, Nguyen-quang T (2019) Structural diversity, characterization and toxicology of. Toxins 11:714–714
Miller MA, Kudela RM, Mekebri A, Crane D, Oates SC, Tinker MT, Jessup DA (2010) Evidence for a novel marine harmful algal bloom: cyanotoxin (microcystin) transfer from land to sea otters. PLoS ONE 5(9):1–11. https://doi.org/10.1371/journal.pone.0012576
News BBC (2020) [Botswana: Mystery elephant deaths caused by cyanobacteria - BBC News]
Weston P (2020) Botswana says it has solved mystery of mass elephant die-off. The Guardian. https://www.theguardian.com/environment/2020/sep/21/botswana-says-it-has-solved-mystery-of-mass-elephant-die-off-age-of-extinction-aoe#:~:text=Hundreds%20of%20elephants%20died%20in,of%20another%20mass%20die%2Doff
Christoffersen K, Kaas H (2000) Toxic cyanobacteria in water. A guide to their public health consequences, monitoring, and management. Limnol Oceanograp. https://doi.org/10.4319/lo.2000.45.5.1212
Niedermeyer THJ (2014) Microcystin congeners described in the literature. Dataset. https://doi.org/10.6084/M9.FIGSHARE.880756
Altaner S, Puddick J, Wood SA, Dietrich DR, D’Anglada LV, Hilborn ED, Backer LC (2017) Adsorption of ten microcystin congeners to common laboratory-ware is solvent and surface dependent. Toxins 9(129):1–15. https://doi.org/10.3390/toxins9040129
Chorus I, Fastner J, Welker M (2021) Cyanobacteria and cyanotoxins in a changing environment: concepts, controversies. Challenges Water 13(18):2463–2463. https://doi.org/10.3390/W13182463
Lone Y, Bhide M, Koiri RK (2016) Microcystin-LR induced immunotoxicity in mammals. J Toxicol 2016:8048125. https://doi.org/10.1155/2016/8048125
Svirčev Z, Drobac D, Tokodi N, Lužanin Z, Munjas AM, Nikolin B, Meriluoto J (2014) Epidemiology of cancers in Serbia and possible connection with cyanobacterial blooms. J Environ Sci Health, Part C 32(4):319–337. https://doi.org/10.1080/10590501.2014.967053
Chen J, Xie P, Li L, Xu J (2009) First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicol Sci 108(1):81–89. https://doi.org/10.1093/toxsci/kfp009
Chia MA, Ameh I, George KC, Balogun EO, Akinyemi SA, Lorenzi AS (2022) Genetic diversity of microcystin producers (cyanobacteria) and microcystin congeners in aquatic resources across africa: a review paper. Toxics 10(12):772
Fischer E (1894) Influence of configuration on the action of enzymes. Ber 27:2985–2993
Mosbach K (2006) The promise of molecular imprinting. Sci Am 295:86–91
Pichon V, Chapuis-Hugon F (2008) Role of molecularly imprinted polymers for selective determination of environmental pollutants—a review. Analytica Chimica Acta 622:48–61
Owens PK, Karlsson L, Lutz ESM, Andersson LI (1999) Molecular imprinting for bio- and pharmaceutical analysis. Trends Anal Chem 18(3):146–154. https://doi.org/10.1016/S0165-9936(98)00092-2
Svenson J, Nicholls IA (2001) On the thermal and chemical stability of molecularly imprinted polymers
Haupt K, Mosbach K (2000) Molecularly imprinted polymers and their use in biomimetic sensors. Chem Rev. https://doi.org/10.1021/cr990099w
Chianella I, Lotierzo M, Piletsky SA, Tothill IE, Chen B, Karim K, Turner APF (2002) Rational design of a polymer specific for microcystin-LR using a computational approach. Anal Chem 74(6):1288–1293. https://doi.org/10.1021/ac010840b
Figueiredo, L., Erny, G. L., Santos, L., & Alves, A. (2016). Applications of molecularly imprinted polymers to the analysis and removal of personal care products: A review (Vol. 146, pp. 754–765).
Singh M, Singh S, Singh SP, Patel SS (2020) Recent advancement of carbon nanomaterials engrained molecular imprinted polymer for environmental matrix. Trends Environ Analyt Chem 27:e00092
Azizi A, Bottaro CS (2020) A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples. J Chromatograp A. https://doi.org/10.1016/j.chroma.2019.460603
Nicholls IA (1995) Thermodynamic considerations for the design of and ligand recognition by molecularly imprinted polymers. Chem Lett 24(11):1035–1036. https://doi.org/10.1246/CL.1995.1035
Sullivan MV, Dennison SR, Archontis G, Reddy SM, Hayes JM (2019) toward rational design of selective molecularly imprinted polymers (MIPs) for proteins: computational and experimental studies of acrylamide based polymers for myoglobin. J Phys Chem B 123(26):5432–5443. https://doi.org/10.1021/ACS.JPCB.9B03091/SUPPL_FILE/JP9B03091_SI_001.PDF
Pan S-D, Chen X-H, Li X-P, Cai M-Q, Shen H-Y, Zhao Y-G, Jin M-C (2015) In situ controllable synthesis of graphene oxide-based ternary magnetic molecularly imprinted polymer hybrid for efficient enrichment and detection of eight microcystins. J Mater Chem A 3(45):23042–23052. https://doi.org/10.1039/C5TA05840F
Pan SD, Chen XH, Li XP, Cai MQ, Shen HY, Zhao YG, Jin MC (2015) In situ controllable synthesis of graphene oxide-based ternary magnetic molecularly imprinted polymer hybrid for efficient enrichment and detection of eight microcystins. J Mater Chem A. https://doi.org/10.1039/c5ta05840f
Li Y, You J, He Y, Ge Y, Song G, Zhou J (2020) Two-fragment-dummy-template molecularly imprinted polymers Mn Doped ZnS quantum dots based room-temperature phosphorescene probing for hepatotoxic homologues of microcystin. ChemistrySelect 5(38):12028–12033. https://doi.org/10.1002/slct.202002715
Mbukwa E, Msagati AMT, Mamba B, Boussiba S (2015) Toxic Microcystis novacekii from Phakalane Ponds, Botswana PCR Amplifications of Microcystin Synthetase (mcy) Genes Extraction and LCESI-MS Identification of Microcystins. J Anal Toxicol, doi: https://doi.org/10.4172/2161-0525.s7-010
Häder DP, Villafañe VE, Helbling EW (2014) Productivity of aquatic primary producers under global climate change. Photochem Photobiol Sci 13(10):1370–1392
Metcalf JS, Richer R, Cox PA, Codd GA (2012) Cyanotoxins in desert environments may present a risk to human health. Sci Total Environ 421–422:118–123. https://doi.org/10.1016/j.scitotenv.2012.01.053
World Health Organization & International Programme on Chemical Safety (1996) Guidelines for drinking-water quality. vol 2, Health criteria and other supporting information, 2nd edn. World Health Organization. https://apps.who.int/iris/handle/10665/38551
Lebogang L, Mattiasson B, Hedström M (2014) Capacitive sensing of microcystin variants of Microcystis aeruginosa using a gold immunoelectrode modified with antibodies, gold nanoparticles and polytyramine. Microchimica Acta. https://doi.org/10.1007/s00604-014-1199-4
Ballot A, Sandvik M, Rundberget T, Botha CJ, Miles CO, Ballot A, Miles CO (2013) Diversity of cyanobacteria and cyanotoxins in Hartbeespoort Dam South Africa. Marine Freshwater Res 65(2):175–189. https://doi.org/10.1071/MF13153
Eguzozie K, Mavumengwana V, Nkosi D, Kayitesi E, Nnabuo-Eguzozie EC (2016) Bioaccumulation and Quantitative variations of microcystins in the Swartspruit river, South Africa. Arch Environ Contaminat Toxicol. https://doi.org/10.1007/s00244-016-0269-5
Tilahun S, Kifle D, Zewde TW, Johansen JA, Demissie TB, Hansen JH (2019) Temporal dynamics of intra-and extra-cellular microcystins concentrations in Koka reservoir (Ethiopia): Implications for public health risk. Toxicon 168:83–92. https://doi.org/10.1016/j.toxicon.2019.06.217
Zewde TW, Johansen JA, Kifle D, Demissie TB, Hansen JH, Tadesse Z (2018) Concentrations of microcystins in the muscle and liver tissues of fish species from Koka reservoir, Ethiopia: a potential threat to public health. Toxicon 153:85–95. https://doi.org/10.1016/j.toxicon.2018.08.013
Zewde TW, Kifle D, Johansen JA, Demissie TB, Hansen JH, Tadesse Z (2020) Cyanobacterial abundance and microcystins in water, seston and fish tissues in Lake Hora-Arsedi (Ethiopia). Afr J Aquat Sci 475–485. https://doi.org/10.2989/16085914.2020.1723485
Major Y, Kifle D, Spoof L, Meriluoto J (2018) Cyanobacteria and microcystins in Koka reservoir (Ethiopia). Environ Sci Pollut Res 25(27):26861–26873. https://doi.org/10.1007/s11356-018-2727-2
Kaggwa MN, Straubinger-Gansberger N, Schagerl M (2018) Cyanotoxins in small artificial dams in Kenya utilised for cage fish farming–a threat to local people? Afr J Aquat Sci. https://doi.org/10.2989/16085914.2018.1470084
Miruka JB, Getabu A, Sitoki L, James O, Mwamburi J, George O, Chrisphine N, Odoli C (2021) Water quality, phytoplankton composition and microcystin concentrations in Kisumu Bay (Kenya) of Lake Victoria after a prolonged water hyacinth infestation period. Lakes Reserv: Sci Policy Manage Sustain Use 26(4):e12380. https://doi.org/10.1111/lre.12380
Simiyu BM, Oduor SO, Rohrlack T, Sitoki L, Kurmayer R (2018) Microcystin content in phytoplankton and in small fish from eutrophic nyanza gulf lake Victoria Kenya. Toxins. https://doi.org/10.3390/TOXINS10070275
Mbonde AS, Sitoki L, Kurmayer R (2015) Phytoplankton composition and microcystin concentrations in open and closed bays of Lake Victoria. Tanzania Aquatic Ecosyst Health Manag 18(2):212–220. https://doi.org/10.1080/14634988.2015.1011030
Nonga HE, Mdegela RH, Sandvik M, Lie E, Miles CO, Skaare JU (2017) Cyanobacteria and cyanobacterial toxins in the alkaline-saline Lakes Natron and Momela. Tanzania Tanzania Veterinary J 32(1):108–116. https://doi.org/10.4314/tvj.v32i1
Addico GND, Hardege JD, Kohoutek J, Degraft-Johnson KAA, Babica P (2017) Cyanobacteria and microcystin contamination in untreated and treated drinking water in Ghana. Adv Oceanograp Limnol. https://doi.org/10.4081/aiol.2017.6323
Addico GND, Lawton L, Edwards C (2017) Hepatotoxic-microcystins in two drinking water reservoirs in the central region of Ghana. Toxicol Forens Med 2(1):1–11. https://doi.org/10.17140/TFMOJ-2-111
Chia MA (2015) Kwaghe MJ (2015) Microcystins contamination of surface water supply sources in Zaria-Nigeria. Environ Monit Assessm 187(10):1–12. https://doi.org/10.1007/S10661-015-4829-3
Chia MA, Abdulwahab R, Ameh I, Balogun JK, Auta J (2021) Farmed tilapia as an exposure route to microcystins in Zaria-Nigeria: a seasonal investigation. Environ Pollut 271:116366–116366. https://doi.org/10.1016/J.ENVPOL.2020.116366
Abdullahi H, Tanimu Y, Akinyemi SA, do Carmo Bittencourt-Oliveira M, Chia MA (2022) Assessment of microcystins in surface water and irrigated vegetables in Kwaru stream, Hayin Danmani, Kaduna-Nigeria. Environ Sci Pollut Res 29(52):78303–78313. https://doi.org/10.1007/s11356-022-21381-w
Amrani A, Nasri H, Azzouz A, Kadi Y, Bouaic̈ha, N. (2014) Variation in cyanobacterial hepatotoxin (microcystin) content of water samples and two species of fishes collected from a shallow lake in Algeria. Arch Environ Contam Toxicol 66(3):379–389. https://doi.org/10.1007/S00244-013-9993-2
Bouhaddada R, Nélieu S, Nasri H, Delarue G, Bouaïcha N (2016) High diversity of microcystins in a Microcystis bloom from an Algerian lake. Environ Pollut 216:836–844. https://doi.org/10.1016/J.ENVPOL.2016.06.055
Mohamed Z, Ahmed Z, Bakr A, Hashem M, Alamri S (2020) Detection of free and bound microcystins in tilapia fish from Egyptian fishpond farms and its related public health risk assessment. J Verbraucherschutz Lebensmittelsicherh 15(1):37–47. https://doi.org/10.1007/S00003-019-01254-0
Douma M, Manaut N, Saqrane S, El Khalloufi F (2017) Toxicity assessment and detection of cyanobacterial toxins (Microcystins) in a Mediterranean natural lake (Dayete Aoua, Morocco). J Mater Environ Sci 8(9):3247–3251
Lynch KL (2017) Chapter 6 - toxicology: liquid chromatography mass spectrometry. In: Nair H, Clarke W (eds) Mass spectrometry for the Clinical Laboratory. Academic Press, San Diego, pp 109–130
He X, Stanford BD, Adams C, Rosenfeldt EJ, Wert EC (2018) Comparison of ELISA and LC-MS/MS analytical methods and validation of AWWA Hazen-adams CyanoTOX. J AWWA 110(10):62–67. https://doi.org/10.1002/awwa.1171
Abbas T, Kajjumba GW, Ejjada M, Masrura SU, Marti EJ, Khan E, Jones-Lepp TL (2020) Recent advancements in the removal of cyanotoxins from water using conventional and modified adsorbents—a contemporary review. Water 12(10):2756
Zhu S, Yin D, Gao N, Zhou S, Wang Z, Zhang Z (2016) Adsorption of two microcystins onto activated carbon: equilibrium, kinetic, and influential factors. Desalin Water Treat 57(50):23666–23674. https://doi.org/10.1080/19443994.2015.1137492
Bajracharya A, Liu Y-L, Lenhart JJ (2019) The influence of natural organic matter on the adsorption of microcystin-LR by powdered activated carbon. Environ Sci: Water Res Technol 5(2):256–267. https://doi.org/10.1039/C8EW00582F
Ren G, He X, Wu P, He Y, Zhang Y, Tang S, Yang F (2020) Biodegradation of microcystin-RR and nutrient pollutants using Sphingopyxis sp. YF1 immobilized activated carbon fibers-sodium alginate. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-07640-8
Arshad U, Mujahid A, Lieberzeit P, Afzal A, Bajwa SZ, Iqbal N, Roshan S (2020) Molecularly imprinted polymeric coatings for sensitive and selective gravimetric detection of artemether. RSC Adv 10(57):34355–34363. https://doi.org/10.1039/D0RA04785F
Mashile GP, Mpupa A, Dimpe MK, Nomngongo PN (2018) Magnetic activated carbon@ iron oxide@manganese oxide composite as an adsorbent for preconcentration of microcystin –LR in surface water, tap water, water and wastewater. Environ Nanotechnol Monitor Manag 10:199–205. https://doi.org/10.1016/j.enmm.2018.06.004
Chae S, Noeiaghaei T, Oh Y, Kim IS, Park JS (2019) Effective removal of emerging dissolved cyanotoxins from water using hybrid photocatalytic composites. Water Res 149:421–431. https://doi.org/10.1016/j.watres.2018.11.016
Wloch M, Datta J (2019) Chapter two synthesis and polymerisation techniques of molecularly imprinted polymers. Comprehens Analyt Chem 86:17–40
He J-X, Pan H-Y, Xu L, Tang R-Y (2020) Application of molecularly imprinted polymers for the separation and detection of aflatoxin. J Chem Res 45(5–6):400–410. https://doi.org/10.1177/1747519820980373
Krupadam RJ, Patel P, Balasubramanian R, Patel GP, Balasubramanian R (2012) Removal of cyanotoxins from surface water resources using reusable molecularly imprinted polymer adsorbents. Environ Sci Pollut Res 19:1841–1851. https://doi.org/10.1007/s11356-011-0703-1
Mbukwa EA, Msagati TAM, Mamba BB (2013) Preparation of guanidinium terminus-molecularly imprinted polymers for selective recognition and solid-phase extraction (SPE) of [arginine]- microcystins. Analyt Bioanalyt Chem. https://doi.org/10.1007/s00216-013-6791-7
Akhavan O (2011) Photocatalytic reduction of graphene oxides hybridized by ZnO nanoparticles in ethanol. Carbon 49(1):11–18. https://doi.org/10.1016/j.carbon.2010.08.030
Kumari P, Tripathi KM, Jangir LK, Gupta R, Awasthi K (2021) Recent advances in application of the graphene-based membrane for water purification. Mater Today Chem 22:100597. https://doi.org/10.1016/j.mtchem.2021.100597
Ahmed EA, El-Derany MO, Anwar AM, Saied EM, Magdeldin S (2023) Metabolomics and lipidomics screening reveal reprogrammed signaling pathways toward cancer development in non-alcoholic steatohepatitis. Int J Mol Sci 24(1):210
Chaudhary K, Kumar K, Venkatesu P, Masram DT (2021) Protein immobilization on graphene oxide or reduced graphene oxide surface and their applications: Influence over activity, structural and thermal stability of protein. Adv Colloid Interf Sci 289:102367. https://doi.org/10.1016/j.cis.2021.102367
Tian X, She C, Qi Z, Xu X (2019) Magnetic-graphene oxide based molecularly imprinted polymers for selective extraction of microsystin-LR prior to the determination by HPLC. Microchem J 146:1126–1133. https://doi.org/10.1016/J.MICROC.2019.02.033
Zhi K, Wang L, Zhang Y, Jiang Y, Zhang L, Yasin A (2018) Influence of size and shape of silica supports on the sol-gel surface molecularly imprinted polymers for selective adsorption of gossypol. Materials 11(5):777–777. https://doi.org/10.3390/MA11050777
Wu Z, He D, Cui B, Jin Z (2019) Ultrasensitive detection of microcystin-LR with gold immunochromatographic assay assisted by a molecular imprinting technique. Food Chem 283:517–521. https://doi.org/10.1016/J.FOODCHEM.2019.01.064
Garcia Y, Canfarotta F, Smolinska-Kempisty K, Piletsky SA, Pereira E (2019) Competitive pseudo-ELISA base on molecularly imprinted nanoparticles for microcystin-LR detection in water. Pure Appl Chem 91(10):1593–1604. https://doi.org/10.1515/PAC-2018-1207/PDF
Qi Z, Lu R, Wang S, Xiang C, Xie C, Zheng M, Xu X (2021) Selective fluorometric determination of microcystin-LR using a segment template molecularly imprinted by polymer-capped carbon quantum dots. Microchem J 161:105798. https://doi.org/10.1016/j.microc.2020.105798
Moreira Gonçalves L (2021) Electropolymerized molecularly imprinted polymers: perceptions based on recent literature for soon-to-be world-class scientists. Curr Opin Electrochem 25:100640. https://doi.org/10.1016/j.coelec.2020.09.007
Schweiger B, Kim J, Kim YJ, Ulbricht M (2015) Electropolymerized molecularly imprinted polypyrrole film for sensing of clofibric acid. Sensors 15(3):4870–4889
Sharma P, Sharma SK (2013) Microscopic investigations of Cu2O nanostructures. J Alloy Compd 557:152–159. https://doi.org/10.1016/j.jallcom.2012.12.082
Chen J, Gao P, Wang H, Han L, Zhang Y, Wang P, Jia N (2018) A PPy/Cu2O molecularly imprinted composite film-based visible light-responsive photoelectrochemical sensor for microcystin-LR. J Mater Chem C 6(15):3937–3944. https://doi.org/10.1039/C7TC05743A
De Middeleer G, Dubruel P, De Saeger S (2016) Characterization of MIP and MIP functionalized surfaces: current state-of-the-art. Trends Anal Chem 76:71–85. https://doi.org/10.1016/J.TRAC.2015.11.007
Matouke MM (2019) FTIR study of the binary effect of titanium dioxide nanoparticles (nTiO2) and copper (Cu2+) on the biochemical constituents of liver tissues of catfish (Clarias gariepinus). Toxicol Rep 6:1061–1070. https://doi.org/10.1016/j.toxrep.2019.10.002
Rinke-Kneapler CN, Sigman ME (2014) 15-applications of laser spectroscopy in forensic science. In: Baudelet M (ed) Laser spectroscopy for sensing. Woodhead Publishing, pp 461–495
Song M, Sun H, Yu J, Wang Y, Li M, Liu M, Zhao G (2021) Enzyme-free molecularly imprinted and graphene-functionalized photoelectrochemical sensor platform for pollutants. ACS Appl Mater Interf 13(31):37212–37222. https://doi.org/10.1021/acsami.1c10242
Goldstein JI, Newbury DE, Michael JR, Ritchie NWM, Scott JHJ, Joy DC (2018) Scanning Electron Microscopy and X-ray Microanalysis. Springer, New York
Liu Z, Xu Z, Liu H, Wang D, Yang Y, Duan Y, Lin T (2021) A review on molecularly imprinted polymers preparation by computational simulation-aided methods. Polymers 13(16):2657–2657. https://doi.org/10.3390/POLYM13162657
Tomoda BT, Yassue-Cordeiro PH, Ernesto JV, Lopes PS, Péres LO, da Silva CF, de Moraes MA (2020) Characterization of biopolymer membranes and films: physicochemical, mechanical, barrier, and biological properties. Biopoly Membran Films. https://doi.org/10.1016/B978-0-12-818134-8.00003-1
Nasrollahzadeh M, Atarod M, Sajjadi M, Sajadi SM, Issaabadi Z (2019) Chapter 6 - Plant-mediated green synthesis of nanostructures: mechanisms, characterization, and applications. In: Nasrollahzadeh M, Sajadi SM, Sajjadi M, Issaabadi Z, Atarod M (eds) Interface science and technology, vol 28. Elsevier, pp 199–322
Raja PB, Munusamy KR, Perumal V, Ibrahim MNM (2022) 5-characterization of nanomaterial used in nanobioremediation. In: Iqbal HMN, Bilal M, Nguyen TA (eds) Nano-bioremediation : fundamentals and applications. Elsevier, pp 57–83
Rajisha KR, Deepa B, Pothan LA, Thomas S (2011) 9 - Thermomechanical and spectroscopic characterization of natural fibre composites. In: Zafeiropoulos NE (ed) Interface engineering of natural fibre composites for maximum performance. Woodhead Publishing, pp 241–274
Dreher TW, Collart LP, Mueller RS, Halsey KH, Bildfell RJ, Schreder P, Ferry R (2019) Anabaena/Dolichospermum as the source of lethal microcystin levels responsible for a large cattle toxicosis event. Toxicon X 1:100003–100003. https://doi.org/10.1016/J.TOXCX.2018.100003
Naknaen A, Ratsameepakai W, Suttinun O, Sukpondma Y, Khan E, Pomwised R (2021) Microcystis Sp. co-producing microcystin and saxitoxin from Songkhla lake basin Thailand. Toxins 13(9):631–631. https://doi.org/10.3390/TOXINS13090631
Hasanah AN, Safitri N, Zulfa A, Neli N, Rahayu D (2021) Factors affecting preparation of molecularly imprinted polymer and methods on finding template-monomer interaction as the key of selective properties of the materials. Molecules 26(18):5612
Funding
Open access funding provided by University of Botswana. No funding was obtained for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Consent for Publication
No human participants were involved in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mbisana, M., Zewde, T.W., Mogopodi, D. et al. Molecularly Imprinted Polymers for the Selective Recognition of Microcystins: An African Perspective. Chemistry Africa 7, 13–33 (2024). https://doi.org/10.1007/s42250-023-00740-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00740-1