Abstract
Introduction
Because of the substantial and widespread usage of drugs for both human and animal health care, drug residues are now pervasive in the environment. During the wastewater treatment process, removing drug residues could not be done entirely, resulting in accumulation in the aquatic environment, potentially impacting biodiversity, ecosystem functioning, and public health. This environmental issue has attracted particular attention and prompted scientists to investigate the removal of drug residues in wastewater.
Methods
The utilization of activated carbon from mango seeds to remove paracetamol drug from water is described in this study. Scanning Electron Microscopy and Fourier Transform Infrared Spectroscopy were used to examine the properties of mango seeds activated carbon (MSAC) that had been chemically treated with phosphoric acid. The effects of initial paracetamol concentration, adsorbent dosage, and contact time on the optimal removal of paracetamol using MSAC were examined through the central composite design.
Results and analysis
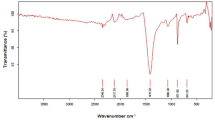
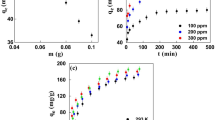
Carboxylic acid, amines, alkenes, ketones, ethers, phosphine oxides, aromatics, and alkyl halides were discovered as functional groups in the mesopore and macropore structures of MSAC. The removal efficiency of 94.01?2.16% paracetamol from the water was recorded at optimal conditions:150 ppm initial paracetamol concentration, 1.95 g of MSAC as adsorbent, and 64 min contact time. Freundlich was the best-fitting isotherm model, with a high correlation coefficient (R2=0.9718), showing multilayer development of adsorbate on the heterogeneous MSAC surface. Using kinetic analysis, the most appropriate kinetic model was pseudo-firstorder (R2 = 0.9435), indicating that physisorption is the best mechanism to explain paracetamol adsorption onto 10.1007/s42250-023-00597-4 MSAC.
Conclusion
Overall, the MSAC has a 7.23 mg/g adsorption capacity for paracetamol in water, indicating that it has a lot of potential as a waste-based source of activated carbon for wastewater treatment applications.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study can be available upon request. You may contact the corresponding author for a soft copy of the data if needed.
References
Shraim A, Diab A, Alsuhaimi A et al (2017) Analysis of some pharmaceuticals in municipal wastewater of Almadinah Almunawarah. Arab J Chem 10:S719–S729. doi: https://doi.org/10.1016/j.arabjc.2012.11.014
Küster A, Adler N (2014) Pharmaceuticals in the environment: scientific evidence of risks and its regulation. Philos Trans R Soc B Biol Sci doi. https://doi.org/10.1098/rstb.2013.0587
Yusoff NA, Ngadi N, Alias H, Jusoh M (2017) Chemically treated chicken bone waste as an efficient adsorbent for removal of acetaminophen. The Italian Association of Chemical Engineering 56:925–930. doi: https://doi.org/10.3303/CET1756155
Chtourou M (2018) Pharmaceutical and personal care products removal by advanced treatment technologies. Water Science and Technology 1–200
Adeleye AP (2016) Perfluorinated compounds, bishenol a and acetaminophen in selected waste water treatment plants in and around Cape town, South Africa. Applied Sciences 1–134
Wu S, Zhang L, Chen J (2012) Paracetamol in the environment and its degradation by microorganisms. Appl Microbiol Biotechnol 96:875–884. doi: https://doi.org/10.1007/s00253-012-4414-4
Mezzelani M, Gorbi S, Fattorini D et al (2016) Transcriptional and cellular effects of non-steroidal anti-inflammatory drugs (NSAIDs) in experimentally exposed mussels, Mytilus galloprovincialis. Aquat Toxicol 180:306–319. doi: https://doi.org/10.1016/j.aquatox.2016.10.006
Rovani S, Censi MT Jr SLP et al (2014) Development of a new adsorbent from agro-industrial waste and its potential use in endocrine disruptor compound removal. J Hazard Mater 271:311–320. doi: https://doi.org/10.1016/j.jhazmat.2014.02.004
Henderson BE, Ross RK, Pike MC, Casagrande JT (1982) Endogenous hormones as a major factor in human cancer. Cancer Res 42(8):3232–3239
Chen WY (2008) Exogenous and endogenous hormones and breast cancer. Best Pract Res Clin Endocrinol Metabolism 22(4):573–585. doi: https://doi.org/10.1016/j.beem.2008.08.001
Mandaric L, Kalogianni E, Skoulikidis N et al (2019) Contamination patterns and attenuation of pharmaceuticals in a temporary Mediterranean river. Sci Total Environ 647:561–569. doi: https://doi.org/10.1016/j.scitotenv.2018.07.308
Chavoshani A, Hashemi M, Mehdi Amin M, Ameta SC (2020) Pharmaceuticals as emerging micropollutants in aquatic environments. Micropollutants and Challenges. Royal Society Publishing, pp 35–90
Balaram V (2016) Recent advances in the determination of elemental impurities in pharmaceuticals - status, challenges and moving frontiers. Trends Anal Chem 80:83–95. doi: https://doi.org/10.1016/j.trac.2016.02.001
Bu Q, Shi X, Yu G et al (2016) Assessing the persistence of pharmaceuticals in the aquatic environment: Challenges and needs. Emerg Contaminants 2:145–147. doi: https://doi.org/10.1016/j.emcon.2016.05.003
Rahman MF, Yanful EK, Jasim SY (2009) Occurrences of endocrine disrupting compounds and pharmaceuticals in the aquatic environment and their removal from drinking water: Challenges in the context of the developing world. Desalination 248:578–585. doi: https://doi.org/10.1016/j.desal.2008.05.105
Yusuf MK, Shehu MS (2013) Kinetic modeling and equilibrium studies of selected endocrine disrupting chemicals on to low cost adsorbent. Adv Appl Sci Res 4(2):331–341
Chang K, Hsieh J, Ou B et al (2012) Adsorption studies on the removal of an endocrine- disrupting compound (Bisphenol A) using activated carbon from rice straw agricultural waste. Sep Sci Technol 47:37–41. doi: https://doi.org/10.1080/01496395.2011.647212
Fauziah S, Draman S, Mohd N (2015) Removal of paracetamol from aqueous solution by dried cellulose and activated carbon. J Eng Appl Sci 10:9544–9548
Chang H, Choo K, Lee B, Choi S (2009) The methods of identification, analysis, and removal of endocrine disrupting compounds (EDCs) in water. J Hazard Mater 172:1–12. doi: https://doi.org/10.1016/j.jhazmat.2009.06.135
Jodeh S, Abdelwahab F, Jaradat N et al (2016) Adsorption of Diclofenac from aqueous solution using activated carbon prepared from olive stones. Int J Hydrog Energy 41(24):10380–10390. doi: https://doi.org/10.1016/j.ijhydene.2016.01.096
Bensghaier R, Tlili I, Latrous L, Megriche A (2022) A new date stone biochar for effective solid phase extraction of non-steroidal anti-inflammatory drugs in water. Chemistry Africa 1–14. doi: https://doi.org/10.1007/s42250-022-00405-5
Maneewong Y, Chaemchuen S, Verpoort F, Klomkliang N (2022) Paracetamol removal from water using N-doped activated carbon derived from coconut shell: kinetics, equilibrium, cost analysis, heat contributions, and molecular-level insight. Chem Eng Res Des 185:163–175. doi: https://doi.org/10.1016/j.cherd.2022.07.007
Idris NN, Hamidon TS, Abdullah NS et al (2022) Potential of oil palm frond cellulose nanocrystals-activated carbon hydrogel beads for the removal of paracetamol from aqueous media. Cellulose 29:1583–1607. doi: https://doi.org/10.1007/s10570-021-04379-4
Sellaoui L, Gómez-Avilés A, Dhaouadi F et al (2023) Adsorption of emerging pollutants on lignin-based activated carbon: analysis of adsorption mechanism via characterization, kinetics and equilibrium studies. Chem Eng J 452:139399. doi: https://doi.org/10.1016/j.cej.2022.139399
Capistrano AJR, Labadan RJD, Viernes JEB et al (2022) Ibuprofen removal using activated carbon from acid-modified Acacia sawdust. Energy, Ecology and Environment 1–12. doi: https://doi.org/10.1007/s40974-022-00264-3
de Luna MD, Murniati, Budianta W et al (2017) Removal of sodium diclofenac from aqueous solution by adsorbents derived from cocoa pod husks. J Environ Chem Eng 5(2):1465–1474. doi: https://doi.org/10.1016/j.jece.2017.02.018
Ugonabo VI, Ezeh EM, Onukwuli OD et al (2022) Remediation of pharmaceutical industrial wastewater using activated carbon from seeds of Mangifera indica and husks of Treculia africana: Optimization, kinetic, thermodynamic and adsorption studies. Chem Afr 1–18. doi: https://doi.org/10.1007/s42250-022-00450-0
Kumar BGP, Gulyani BB, Vijaikumar B, Miranda LR (1977) Chemical activation of mango seed coat (Mangifera indica L.) preparation and characterization.Chemical Engineering Department 357–361
Kanjilal T, Bhattacharjee C, Datta S (2014) Application of mango seed integuments as bio- adsorbent in lead removal from industrial effluent. Desalin Water Treat 984-996. doi: https://doi.org/10.1080/19443994.2014.950999
Yakout SM, El-deen GS (2016) Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab J Chem 9:S1155–S1162. doi: https://doi.org/10.1016/j.arabjc.2011.12.002
Bui TX, Choi H (2009) Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J Hazard Mater 168(2–3):602–608. doi: https://doi.org/10.1016/j.jhazmat.2009.02.072
Dutta M, Das U, Mondal S (2015) Adsorption of acetaminophen by using tea waste derived activated carbon. Int J Environ Sci 6(2):270–281. doi: https://doi.org/10.6088/ijes.6031
Uddin T, Rahman A (2017) A potential low cost adsorbent for the removal of cationic dyes from aqueous solutions. Appl Water Sci 7(6):2831–2842. doi: https://doi.org/10.1007/s13201-017-0542-4
Das B, Mondal NK, Bhaumik R, Roy P (2014) Insight into adsorption equilibrium, kinetics and thermodynamics of lead onto alluvial soil. Int J Environ Sci Technol 11:1101–1114. doi: https://doi.org/10.1007/s13762-013-0279-z
Sajid M, Bari S, Saif Ur Rehman M et al (2022) Adsorption characteristics of paracetamol removal onto activated carbon prepared from Cannabis sativum Hemp. Alexandria Eng J 61:7203–7212. doi: https://doi.org/10.1016/j.aej.2021.12.060
Rey-Mafull CA, Tacoronte JE, Garcia R et al (2014) Comparative study of the adsorption of acetaminophen on activated carbons in simulated gastric fluid. SpringerPlus J 3:1–12. doi: https://doi.org/10.1186/2193-1801-3-48
Thakur A, Sharma N, Mann A (2020) Removal of ofloxacin hydrochloride and paracetamol from aqueous solutions: Binary mixtures and competitive adsorption. Materials Today: Proceedings 28 1514–1519. doi: https://doi.org/10.1016/j.matpr.2020.04.833
Phatai P, Utara S, Hatthapanit N (2015) Removal of methyl violet by adsorption onto activated carbon derived from coffee residues. Adv Mater Res 864–867:710–714. doi: https://doi.org/10.4028/www.scientific.net/AMR.864-867.710
Bursztyn Fuentes AL, Canevesi RLS, Gadonneix P et al (2020) Paracetamol removal by Kon-Tiki kiln-derived biochar and activated carbons. Ind Crops Prod 155:112740. doi: https://doi.org/10.1016/j.indcrop.2020.112740
Acknowledgements
The authors would like to thank the College of Engineering and Technology of the University of Science and Technology of Southern Philippines – Claveria for this study’s support. Special thanks to Amley Food Corporation for the waste materials used in the research.
Funding
This work is institutionally funded by the University of Science and Technology of Southern Philippines.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the study through conceptualization, laboratory experimentation, data gathering, analysis, and interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Preglo, A.R., Namata, J., Caculba, J. et al. Paracetamol Removal from Aqueous Solution Through Activated Carbon from Mango Seeds. Chemistry Africa 6, 699–710 (2023). https://doi.org/10.1007/s42250-023-00597-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00597-4