Abstract
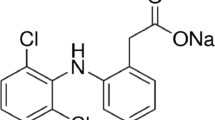
In this research, the adsorptive ability of a novel activated carbon (CWAC) prepared from citrus industrial processing waste (CW) via phosphoric acid activation in decontaminating synthetically contaminated water with diclofenac (DCF), selected as a high toxicity model drug, was examined in detail. Optimum adsorption conditions were decided by analyzing the impacts of effective experimental parameters such as CWAC amount, DCF concentration, adsorption time, and ambient temperature. Experimental results were evaluated in kinetic, isothermal, thermodynamic, and intra-particle diffusion models to characterize the CWAC-DCF adsorption system. The kinetic and equilibrium isotherm data conformed the Lagergren and Langmuir models, respectively. The maximum amount of DCF adsorbed by CWAC was determined to be 185.19 mg/g under the specified optimum operating conditions. The values of the calculated thermodynamic parameters indicated that the process was endothermic and spontaneity increased at high temperatures. In addition, the CWAC was observed to maintain its high adsorption capacity even after five regeneration cycles. The adsorption mechanism was clarified by SEM and FT-IR spectroscopic examinations. The results of this research demonstrated the applicability of CWAC as a highly effective and reusable alternative adsorbent for decontamination of DCF-contaminated water.






Similar content being viewed by others
References
Ouyang J, Zhou L, Liu Z, Heng JY, Chen W (2020) Biomass-derived activated carbons for the removal of pharmaceutical micro-pollutants from wastewater: a review. Sep Purif Technol 253:117536. https://doi.org/10.1016/j.seppur.2020.117536
Changotra R, Rajput H, Guin JP, Varshney L, Dhir A (2019) Hybrid coagulation, gamma irradiation and biological treatment of real pharmaceutical wastewater. Chem Eng J 370:595–605. https://doi.org/10.1016/j.cej.2019.03.256
Shirani Z, Song H, Bhatnagar A (2020) Efficient removal of diclofenac and cephalexin from aqueous solution using Anthriscus sylvestris-derived activated biochar. Sci Total Environ 745:140789. https://doi.org/10.1016/j.scitotenv.2020.140789
Van Tran T, Nguyen DTC, Le HT, Vo DVN, Nanda S, Nguyen TD (2020) Optimization, equilibrium, adsorption behavior and role of surface functional groups on graphene oxide-based nanocomposite towards diclofenac drug. J Environ Sci 93:137–150. https://doi.org/10.1016/j.jes.2020.02.007
Lonappan L, Rouissi T, Brar SK, Verma M, Surampalli RY (2018) An insight into the adsorption of diclofenac on different biochars: mechanisms, surface chemistry, and thermodynamics. Bioresour Technol 249:386–394. https://doi.org/10.1016/j.biortech.2017.10.039
de Franco MAE, de Carvalho CB, Bonetto MM, de Pelegrini SR, Féris LA (2018) Diclofenac removal from water by adsorption using activated carbon in batch mode and fixed-bed column: isotherms, thermodynamic study and breakthrough curves modeling. J Clean Prod 181:145–154
Hiew BYZ, Lee LY, Lee XJ, Gan S, Thangalazhy-Gopakumar S, Lim SS, Pan GT, Yang TCK (2019) Adsorptive removal of diclofenac by graphene oxide: optimization, equilibrium, kinetic and thermodynamic studies. J Taiwan Inst Chem Eng 98:150–162. https://doi.org/10.1016/j.jtice.2018.07.034
Acuña V, Ginebreda A, Mor JR, Petrovic M, Sabater S, Sumpter J, Barceló D (2015) Balancing the health benefits and environmental risks of pharmaceuticals: diclofenac as an example. Environ Int 85:327–333. https://doi.org/10.1016/j.envint.2015.09.023
Patel M, Kumar R, Kishor K, Mlsna T, Pittman CU Jr, Mohan D (2019) Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem Rev 119:3510–3673. https://doi.org/10.1021/acs.chemrev.8b00299
Xiong T, Yuan X, Wang H, Wu Z, Jiang L, Leng L, Xia K, Cao X, Zeng G (2019) Highly efficient removal of diclofenac sodium from medical wastewater by Mg/Al layered double hydroxide-poly (m-phenylenediamine) composite. Chem Eng J 366:83–91. https://doi.org/10.1016/j.cej.2019.02.069
Xu H, Zhu S, Xia M, Wang F (2021) Rapid and efficient removal of diclofenac sodium from aqueous solution via ternary core-shell CS@PANI@LDH composite: experimental and adsorption mechanism study. J Hazard Mater 402:123815. https://doi.org/10.1016/j.jhazmat.2020.123815
Bhadra BN, Seo PW, Jhung SH (2016) Adsorption of diclofenac sodium from water using oxidized activated carbon. Chem Eng J 301:27–34. https://doi.org/10.1016/j.cej.2016.04.143
Zhang S, Dong Y, Yang Z, Yang W, Wu J, Dong C (2016) Adsorption of pharmaceuticals on chitosan-based magnetic composite particles with core-brush topology. Chem Eng J 304:325–334. https://doi.org/10.1016/j.cej.2016.06.087
Mirzaee SA, Bayati B, Valizadeh MR, Gomes HT, Noorimotlagh Z (2021) Adsorption of diclofenac on mesoporous activated carbons: physical and chemical activation, modeling with genetic programming and molecular dynamic simulation. Chem Eng Res Des 167:116–128. https://doi.org/10.1016/j.cherd.2020.12.025
Shan D, Deng S, Zhao T, Wang B, Wang Y, Huang J, Yu G, Winglee J, Wiesner MR (2016) Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J Hazard Mater 305:156–163. https://doi.org/10.1016/j.jhazmat.2015.11.047
Kołtowski M, Hilber I, Bucheli TD, Charmas B, Skubiszewska-Zięba J, Oleszczuk P (2017) Activated biochars reduce the exposure of polycyclic aromatic hydrocarbons in industrially contaminated soils. Chem Eng J 310:33–40. https://doi.org/10.1016/j.cej.2016.10.065
Ahmed MJ, Islam MA, Asif M, Hameed BH (2017) Human hair-derived high surface area porous carbon material for the adsorption isotherm and kinetics of tetracycline antibiotics. Bioresour Technol 243:778–784. https://doi.org/10.1016/j.cej.2016.10.065
Tan KL, Hameed BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48. https://doi.org/10.1016/j.jtice.2017.01.024
de Luna MDG, Budianta W, Rivera KKP, Arazo RO (2017) Removal of sodium diclofenac from aqueous solution by adsorbents derived from cocoa pod husks. J Environ Chem Eng 5:1465–1474. https://doi.org/10.1016/j.jece.2017.02.018
El Naga AOA, El Saied M, Shaban SA, El Kady FY (2019) Fast removal of diclofenac sodium from aqueous solution using sugar cane bagasse-derived activated carbon. J Mol Liq 285:9–19. https://doi.org/10.1016/j.molliq.2019.04.062
Antunes M, Esteves VI, Guégan R, Crespo JS, Fernandes AN, Giovanela M (2012) Removal of diclofenac sodium from aqueous solution by Isabel grape bagasse. Chem Eng J 192:114–121. https://doi.org/10.1016/j.cej.2012.03.062
Larous S, Meniai AH (2016) Adsorption of diclofenac from aqueous solution using activated carbon prepared from olive stones. Int J Hydrogen Energ 41:10380–10390. https://doi.org/10.1016/j.ijhydene.2016.01.096
Delgado N, Capparelli A, Navarro A, Marino D (2019) Pharmaceutical emerging pollutants removal from water using powdered activated carbon: study of kinetics and adsorption equilibrium. J Environ Manage 236:301–308. https://doi.org/10.1016/j.jenvman.2019.01.116
Mokhati A, Benturki O, Bernardo M, Kecira Z, Matos I, Lapa N, Ventura M, Soares OSGP, Botelho do Rego AM, Fonseca IM, (2021) Nanoporous carbons prepared from argan nutshells as potential removal agents of diclofenac and paroxetine. J Mol Liq 326:115368. https://doi.org/10.1016/j.molliq.2021.115368
Xu D, Li Z, Wang P, Bai W, Wang H (2020) Aquatic plant-derived biochars produced in different pyrolytic conditions: spectroscopic studies and adsorption behavior of diclofenac sodium in water media. Sustain Chem Pharm 17:100275. https://doi.org/10.1016/j.scp.2020.100275
Ahmed M.J, Theydan S.K (2012) Adsorption of cephalexin onto activated carbons from Albizia lebbeck seed pods by microwave-induced KOH and K2CO3 activations. Chem Eng J 211-212:200-207. https://doi.org/10.1016/j.cej.2012.09.089
Khaled A, El Nemr A, El-Sikaily A, Abdelwahab O (2009) Removal of Direct N Blue-106 from artificial textile dye effluent using activated carbon from orange peel: adsorption isotherm and kinetic studies. J Hazard Mater 165:100–110. https://doi.org/10.1016/j.jhazmat.2008.09.122
Ahmed MJ, Okoye PU, Hummadi EH, Hameed BH (2019) High-performance porous biochar from the pyrolysis of natural and renewable seaweed (Gelidiella acerosa) and its application for the adsorption of methylene blue. Bioresour Technol 278:159-164. https://doi.org/10.1016/j.biortech.2019.01.054
Caner N, Kiran I, Ilhan S, Iscen CF (2009) Isotherm and kinetic studies of Burazol Blue ED dye biosorption by dried anaerobic sludge. J Hazard Mater 165:279–284. https://doi.org/10.1016/j.jhazmat.2008.09.108
Kostić M, Radović M, Velinov N, Najdanović S, Bojić D, Hurt A, Bojić A (2018) Synthesis of mesoporous triple-metal nanosorbent from layered double hydroxide as an efficient new sorbent for removal of dye from water and wastewater. Ecotoxicol Environ Saf 159:332–341. https://doi.org/10.1016/j.ecoenv.2018.05.015
Jauris IM, Matos CF, Saucier C, Lima EC, Zarbin AJG, Fagan SB, Machado FM, Zanella I (2016) Adsorption of sodium diclofenac on graphene: a combined experimental and theoretical study. Phys Chem Chem Phys 18:1526–1536
Gil A, Santamaría L, Korili SA (2018) Removal of caffeine and diclofenac from aqueous solution by adsorption on multiwalled carbon nanotubes. Colloid Interface Sci Commun 22:25–28. https://doi.org/10.1016/j.colcom.2017.11.007
Bernardo M, Rodrigues S, Lapa N, Matos I, Lemos F, Batista MKS, Carvalho AP, Fonseca I (2016) High efficacy on diclofenac removal by activated carbon produced from potato peel waste. Int J Environ Sci Technol 13:1989–2000. https://doi.org/10.1007/s13762-016-1030-3
Vedenyapina MD, Stopp P, Weichgrebe D, Vedenyapin AA (2016) Adsorption of diclofenac sodium from aqueous solutions on activated carbon. Solid Fuel Chem 50:46–50. https://doi.org/10.3103/S0361521916010109
Malhotra M, Suresh S, Garg A (2018) Tea waste derived activated carbon for the adsorption of sodium diclofenac from wastewater: adsorbent characteristics, adsorption isotherms, kinetics, and thermodynamics. Environ Sci Pollut Res 25:32210–32220. https://doi.org/10.1007/s11356-018-3148-y
Avcu T, Üner O, Geçgel Ü (2021) Adsorptive removal of diclofenac sodium from aqueous solution onto sycamore ball activated carbon–isotherms, kinetics, and thermodynamic study. Surf Interfaces 24:101097. https://doi.org/10.1016/j.surfin.2021.101097
Isa MH, Lang LS, Asaari FA, Aziz HA, Ramli NA, Dhas JPA (2007) Low cost removal of disperse dyes from aqueous solution using palm ash. Dyes Pigments 74:446–453. https://doi.org/10.1016/j.dyepig.2006.02.025
JiryaeiSharahi F, Shahbaz A (2017) Melamine-based dendrimer amine-modified magnetic nanoparticles as an efficient Pb(II) adsorbent for wastewater treatment: adsorption optimization by response surface methodology. Chemosphere 189:291–300. https://doi.org/10.1016/j.chemosphere.2017.09.050
El-Sayed GO (2011) Removal of methylene blue and crystal violet from aqueous solutions by palm kernel fiber. Desalination 272:225–232. https://doi.org/10.1016/j.desal.2011.01.025
Ji B, Wang J, Song H, Chen W (2019) Removal of methylene blue from aqueous solutions using biochar derived from a fallen leaf by slow pyrolysis: behavior and mechanism. J Environ Chem Eng 7:103036. https://doi.org/10.1016/j.jece.2019.103036
Gupta VK, Pathania D, Agarwal S, Sharma S (2012) De-coloration of hazardous dye from water system using chemically modified Ficus carica adsorbent. J Mol Liq 174:86-94. https://doi.org/10.1016/j.molliq.2012.07.017
Peng X, Huang D, Odoom-Wubah T, Fu D, Huang J, Qin Q (2014) Adsorption of anionic and cationic dyes on ferromagnetic ordered mesoporous carbon from aqueous solution: equilibrium, thermodynamic and kinetics. J Colloid Interface Sci 430:272–282. https://doi.org/10.1016/j.jcis.2014.05.035
Babić BM, Milonjić SK, Polovina MJ, Kaludierović BV (1999) Point of zero charge and intrinsic equilibrium constants of activated carbon cloth. Carbon 37:477–481. https://doi.org/10.1016/S0008-6223(98)00216-4
Funding
Financial support was provided by the Scientific Research Projects Coordinator of Dicle University (Project No: ZGEF-15–006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Güzel, F., Koyuncu, F. Adsorptive removal of diclofenac sodium from aqueous solution via industrial processed citrus solid waste–based activated carbon: optimization, kinetics, equilibrium, thermodynamic, and reusability analyses. Biomass Conv. Bioref. 13, 2401–2412 (2023). https://doi.org/10.1007/s13399-021-01969-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01969-x