Abstract
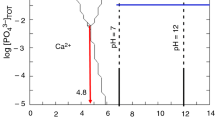
The experimental design strategy is applied to study and model the preparation of oxygenated apatite obtained by the dissolution-reprecipitation of hydroxyapatite in a hydrogen peroxide. The oxygenated apatite obtained is characterized by infrared spectroscopy (IR), X-ray diffraction (XRD) and scanning electron microscopy (SEM). The characterization by analyzes (IR, DRX, SEM) shows that the dissolution-repricipitation method gives better results for oxygenated apatite containing molecular oxygen. For this, we studied the influence of the four factors such as the temperature of the reaction medium, the pH, the concentration of hydrogen peroxide and the mass of the attacked hydroxyapatite. As well as their interactions whose goal is to increase the molecular oxygen insertion rate in apatitic tunnel. The orthogonal centered composite plane allowed us to establish an equation that links the insertion rate according to the factors studied, and we obtained an optimum of introduction of 5.06%.








Similar content being viewed by others
References
Elliot JC (1991) Structur and chemistry of the apatites and other calcium orthophosphates, vol 18, 1st edn. Elsevier Science, USA
Rey C, Trombe JC, Montel G (1978) Some features of the incorporation of oxygen in different oxidation states in the apatitic lattice -III Synthesis and properties of some oxygenated apatites. J inorg nucl Chem 40:27–30
Belouafa S, Faouzi F, Sallek B (2014) Etude de l’influence des paramètres de synthèse sur la composition chimique des apatites oxygénées carbonatées. Phosphorus Sulfur Silicon Relat Elem 189:1346–1353
Amjad Z (1998) Calcium phosphates in biological and industrial systems Springer; Softcover reprint of the original 1st ed. 1998 edition
Belouafa S, Chaair H, Loukili H, Digua K, Sallek B (2008) Characterization of antiseptic apatite powders prepared at biomimetics temperature and pH. Mater Res 11:93–96
Jerdioui S, Elansari L, Bouammali B (2015) Study of cobalt adsorption on an oxygenated apatite surface. J Mater Environ Sci 6:852–860
Yahyaoui R, Azzaoui K, Lamhamdi L, Mejdoubi E, Elabed S, Hammouti B (2014) Preparation of oxygenated apatite from hydrolysis of cured brushite cement in aqueous medium. Der Pharma Chem 6:133–138
Elgadi M, Mejdoubi E, Elansari L, Essaddek A, Abouricha S, Lamhamdi A (2005) Study of the chemical mechanisms of the reaction of neutralization of calcium hydroxide by phosphoric acid. J Phys IV 123:351–354
Lamhamdi A, Azzaouia K, Mejdoubia E, Hammouti B, ELansaria L, Jabria M, Berrabaha M, ELbali B (2015) Modeling and optimization of the synthesis of oxygenated apatite by hydrolysis of dicalcium phosphate dihydrate (DCPD) using the Box-Behnken design. Der Pharma Chem 7:46–52
Vandecandelaere N, Bosc F, Rey C, Drouet C (2014) Peroxide-doped apatites: preparation and effect of synthesis parameters. Powder Technol 255:3–9
Garcia AK (2019) Development of an apatite oxygen paleobarometer: experimental characterization of Sm3+-substituted apatite fluorescence as a function of oxygen availability. Precambrian Res. https://doi.org/10.1016/j.precamres.2019.105389
Azzaoui K, Lamhamdi A, MejdoubiE Berrabah M, ELidrissi A, Hammouti B, Zaoui S, Yahyaoui R (2013) Synthesis of nanostructured hydroxyapatite in presence of polyethylene glycol 1000. J Chem Pharm Res 5:1209–1216
Lamhamdi A(2013) Élaboration de nouvelles matrices phosphatées à usages odontologiques, environnementaux et industriels, en utilisant les techniques d’hydrolyse et de maturation contrôlées, Ph.D Thesis. Mohammed Premier University, Oujda, Morocco.
Trombe JC, Montel G (1978) Some features of the incorporation of oxygen in different oxidation states in the apatitic lattice-I on the existence of calcium and stronstium oxyapatites. J Inorg Nucl Chem 40:15–21
Yubao L, Klein LC, Xingdong Z, de Groot K (1994) Formation of a bone apatite-like layer on the surface of porous hydroxyapatite ceramics. Biomaterials 15:835–841
El Feki H, Khattech K, Jemal M, Rey C (1994) Thermal decomposition of carbonated containing sodium ions. Thermochim Acta 237:99–110
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naanaai, L., Azzaoui, K., Lamhamdi, A. et al. Study and Optimization of Oxygenated Apatite Obtained by Dissolution-Reprecipitation of Hydroxyapatite in a Solution of Hydrogen Peroxide. Chemistry Africa 3, 227–235 (2020). https://doi.org/10.1007/s42250-019-00100-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-019-00100-y