Abstract
In this work, hydrated TiO2 spheres (HTS) of the submicron order have been developed. Normally, the group of amines, such as dodecylamine, hexadecylamine, methylamine, and NH3, had been the main structure-directing agents (SDAs) used in the sol–gel process to obtain monodisperse hydrated TiO2 spheres. Even though progress has been made in the synthesis of HTS, it is crucial to include new SDAs capable of synthesizing monodisperse HTS with improved or new properties for practical applications. In this work, for the first time we demonstrate that a thiol, 3-mercaptopropionic acid (MPA), can be used as an effective SDA to synthesize monodisperse hydrous TiO2 spheres with a controllable particle diameter between 150 to 950 nm. Experimental preparation parameters such as Ti concentration, [MPA]/[Ti] and [Water]/[Ti] molar ratio in the precursor solution (Titanium (IV) butoxide—MPA—ethanol—water) were thoroughly optimized to get both high yield and high monodispersity. Remarkably, a wide range in the [Water]/[Ti] molar ratio, 17 to 118, was achieved, which is much wider than the typical Rw range of the amines group of 2 to 16, thus giving more control for choosing the HTS final size. The controlled growth of hydrated TiO2 nanospheres was explained according to the LaMer and DVLO theory. To demonstrate the applicability of the HTS synthesized using MPA as SDA, the development of efficient dye-sensitized solar cells getting an energy conversion greater than 9% as well as of an effective photocatalytic degradation process of the analgesic acetaminophen under concentrated solar radiation was conducted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hydrous TiO2 nanospheres with diameters in the range from 0.10 to 1.0 microns are a remarkably material typically used as a self-template for the synthesis of mesoporous TiO2 spheres with tunable composition, crystallographic phases, and internal structures suitable for a variety of applications, including environmental photocatalysis [1, 2], solar fuel synthesis [3, 4], lithium and lithium-ion batteries [5,6,7,8,9,10], smart coating materials for antibacterial and self-cleaning applications [11], and the construction of third generation solar cells photoelectrodes [12, 13].
The first highly monodispersed hydrous TiO2 spheres were prepared by hydrolysis and condensation reactions of dilute ethanolic solutions of titanium tetraethoxide (TTIP) with controlled quantities of water, measured as the molar ratio of water and Ti precursor (Rw = [H2O]/[Ti]) [14, 15]. TiO2 spheres with diameters from 310 to 500 nm with a standard deviation of 1.09 were obtained, changing the Rw from 7 to 3. High monodispersity of the TiO2 spheres can be attributed to the use of dilute solutions of titanium tetraethoxide, precise control of Rw parameter, purity of reactives, and that the synthesis was performed inside a glove box to prevent water vapor prior to the controlled addition of liquid water to the system. Further advancement in the synthesis of hydrous TiO2 spheres was achieved by introducing structural directing agents (SDA), which are molecules that improve self-assembly, monodispersity and reproducibility of the hydrous TiO2 spheres, and allow the synthesis to be made in ambient conditions.
In one of the first reports investigating the use of ammonia, methylamine and dimethylamine as structural directing agents [16], methylamine gave TiO2 spheres with the highest monodispersion (lower coefficient of variation) and hydrous TiO2 spheres with diameters between 500 to 200 nm controlling the Rw from 1.6 to 10 in a dilute solution of Ti at 0.03 M. Other reports have shown the use of amines as effective SDAs, as well as tetrabutylamine [17], hexadexylamine [18], and dodecylamine [19]. Other molecules confirmed as SDAs are organic acids [20, 21], salts [22], polymers [22, 23] and ethylenglycol [24, 25]. Among these SDAs, amines are the most reported for the synthesis of hydrous TiO2 spheres, which are then used as self-templates to fabricate mesoporous TiO2 anatase spheres with several applications. Size of hydrous TiO2 spheres has been mainly controlled by Rw. For example, NH3 has shown a diameter control from 1000 to 300 nm by varying Rw from 2 to 15 [26]. With hexadecylamine and a Rw of 3 to 10 (0.076 M), a diameter control of 1150 nm to 320 nm was achieved [18] and with dodecylamine a diameter control of 900 nm to 300 nm varying Rw from 0.5 to 12 [19]. In the case of carboxylic acids as SDAs, specifically n-butyric acid (C4H8O2), n-valeric acid (C5H10O2), heptanoic acid (C7H14O2), capric acid (C10H20O2) and dodecanoic acid (C12H24O2), these showed a narrower range over the average diameter control, usually from 50 to 300 nm with Rw from 550 to 1400 [21]. The concentration of Ti precursor, the SDA to Ti molar ratio, and temperature are parameters that have demonstrated to influence the final diameter size of the hydrous TiO2 spheres, but they do not exhibit the same degree of change and control as Rw makes [19].
In this paper, we show that a cationic thiol surfactant, 3-mercaptopropionic acid (MPA) with the chemical formula (C3H6O2S); can act as an effective SDA for the sol–gel synthesis of hydrous TiO2 spheres at 24 °C. The synthesis parameters such as concentration of the precursor [Ti], SDA to Ti [MPA]/[Ti] and water to Ti (Rw) molar ratios were optimized, since these parameters have shown the greatest effect in controlling the size and morphology of the hydrous TiO2 spheres in previous works. All these parameters allowed to get an effective size control in the hydrous TiO2 sphere diameter from 200 to 950 nm with a wide Rw window from 17 to 118 and a low coefficient of variation (< 10%), showing a high monodispersity of the spheres. These values of Rw taken in this work are wider than the ones reported in the group of amines and organic acids, thus giving a higher degree of control and being less sensitive to water content.
Literature research shows that SDAs are still used by the scientific community, especially those based on the amino group, while the use of MPA as SDA represents the novelty of this study. Therefore, the results of this work remain current and relevant to the community.
To analyze the applications of the hydrous TiO2 spheres synthesized with MPA as a structure directing agent, dye sensitized solar cells (DSSCs) and photocatalytic degradation were chosen as proof of concept. Initially, a solvothermal process was performed in the hydrous TiO2 spheres with an average diameter of 259 nm to obtain mesoporous TiO2 spheres. For the DSSCs with a double layer film architecture made of a transparent film of anatase TiO2 nanoparticles with an average diameter of 25 nm and a reflective film of anatase mesoporous TiO2 spheres was compared against a single layer film architecture made only of the transparent film. The DSSCs with the double layer film architecture provided a maximum power conversion efficiency (PCE) of 9.31%. DSSCs with the single layer film architecture with the same thickness achieve a PCE of 7.82%. Finally, mesoporous TiO2 spheres were used for the photocatalytic degradation of the analgesic acetaminophen under concentrated solar radiation.
2 Experimental section
2.1 Materials and reagents
The synthesis of hydrous TiO2 spheres was done by employing titanium (IV) butoxide (TBT, 97% Sigma-Aldrich), 3-mercaptopropionic acid (MPA, ≥ 99% Sigma-Aldrich), absolute ethanol (99.9%) from Fermont, and deionized water from Milli 85-Q/RIO. Terpineol (mixture of isomers anhydrous, Sigma-Aldrich) and ethyl cellulose (viscosity 46 cP, 5% in toluene/ethanol 80:20 (lit.) Sigma-Aldrich) were employed for the paste preparation.
For the fabrication of the DSSC solar cells SnO2:F (FTO) of 15 Ω/sq and 8 Ω/sq (Pilkington), titanium (IV) isopropoxide (97%, Sigma-Aldrich), 18 NR-T transparent titania paste (Greatcell Solar Materials), ruthenium dye N719 (Ruthenizer 535-bis TBA Solaronix), Surlyn polymer (Meltonix 1170–60, Solaronix), platinum paste (PT-1, Dyesol) and silver paste (elcosil S-L/SP, Solaronix) were used. For the electrolyte 1-methyl-3-propylimidazolium iodide (99%) and guanidine thiocyanate (> 98%) were acquired from Merck while 4-tert-butylpyridine (96%), iodine (99.999% trace metals), lithium iodide (99.9% trace metals), and acetonitrile (99%) were acquired from Sigma Aldrich. Finally, a vac’n’fill Syringe (Solaronix) was used to introduce the electrolyte in the solar cell.
2.2 Hydrous TiO2 spheres synthesis
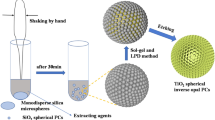
Hydrous TiO2 spheres were prepared using a sol–gel synthesis. In a general procedure, 1.6 mL of tetrabutyl titanate (4.7 mmol) and 0.112 mL of MPA were dissolved in 56 mL of ethanol at 24 °C with 320 rpm of agitation for 30 min. Then, a specific amount of previously weighed deionized water was rapidly added to the mixture, and an abrupt transformation from transparent to milky white was observed. The mixture was continuously stirred for 12 h. The mixture was then centrifuged and rinsed twice with ethanol before being stored for further characterization. Figure 1 shows a schematic drawing that describes each of the steps performed during the preparation of the material: a) sol–gel synthesis of the hydrated TiO2 spheres, b) preparation of microspheres through the solvothermal process and c) thermal processes at temperatures of 450–480 °C for different applications.
Schematic drawing that describes each of the steps performed during the preparation of the material: (a) sol–gel synthesis of the hydrated TiO2 spheres, (b) preparation of microspheres through the solvothermal process and (c) thermal processes at temperatures of 450–480 °C for different applications
2.3 Synthesis of mesoporous TiO2 spheres for the manufacture of reflective layer
Based on a solvothermal process, hydrous TiO2 spheres obtained after the sol–gel process with an average diameter of 259 nm were redissolved in ethanol to a final concentration of 0.08 M. This solution was then transferred to an autoclave and heated to 200 °C for 12 h at a pressure of 54 atm to obtain mesoporous TiO2 spheres [27]. The autoclave was allowed to cool down to room temperature, and the solution resulting from the solvothermal process was centrifuged at 5000 rpm for 15 min. Subsequently, the obtained mesoporous TiO2 spheres were washed twice in anhydrous ethanol and then centrifuged at 5000 rpm for 15 min. The washing process was repeated twice. Finally, the precipitated was used to elaborate the screen-printing paste.
In a quantum confinement study carried out on TiO2 nanoparticles (nanocrystals), during crystal growth as a function of pressure and temperature, Sánchez García et al. managed to prepare mesoporous films of TiO2 nanoparticles with anatase phase through a hydrothermal process at temperatures between 127 and 210 °C for 12 h [27]. Higher temperatures were not recommended because the autoclave's Teflon O-ring will melt. The authors found that as the temperature increases, the pressure also increases inside the autoclave and at the same time, the crystal size increases and the value of energy bandgap Eg directly depends on the crystal size. Specifically, the sample prepared at 200 °C had a crystal size of 12 nm, and when it was treated at 530 °C, its crystal size changed slightly to 12.4 nm. Considering that the crystal structure of anatase has an indirect energy band gap, it decreases from 3.52 to 3.34 eV when the crystal size increases from 10.8 to 14.1 nm. For a bulk crystal, the anatase phase of TiO2 has an energy bandgap of 3.2 eV. A bulk crystal means a large crystal. On the other hand, in a crystal constructed with N primitive cells, each primitive cell contributes exactly one value of the wave vector k to each energy band E(k) [28]. Therefore, the larger the crystal, the greater the number of primitive cells and the greater the number of electron levels in each energy band (valence and conduction band).
Considering the photoresponse measurements carried out by the same authors on TiO2 films, they also found that the film with a TiO2 crystallite size of around 12.4 nm showed the highest photoresponse measurements and therefore the highest electrical photoconductivity. This means that it is necessary to have a minimum crystal size to build sufficient electron levels in both the conduction band and the valence band that favor both the photogeneration of electron–hole pairs (e−—h+) as well as the transport of electrical charge.
A TiO2 screen printing paste based on the mesoporous TiO2 spheres of an average diameter size of 248 nm was fabricated. In a vial, 0.5 g of mesoporous TiO2 spheres, 2 g of terpineol, 2.5 g of 10% wt. ethyl cellulose in ethanol, and 8 mL of ethanol were completely combined for 10 min, sonicated for 15 min, and then mixed for an additional 10 min. The mixture was inserted into a rotary evaporator for 25 min at 60 °C to remove ethanol. A viscous white paste with a weight percentage of 18% TiO2 spheres, 9% ethyl Cellulose and 73% terpineol was obtained with which later was prepared the reflective layer by screen printing.
2.4 DSSC fabrication process
A double layer film architecture was used for the DSSC photoelectrodes, FTO (15 Ω/sq)/c-TiO2/m-TiO2/r-TiO2/N-719/Electrolyte/Pt-FTO (8 Ω/sq), where c-TiO2 is the compact or blocking TiO2 layer, m-TiO2 is the mesoporous or transparent TiO2 layer, r-TiO2 is the reflective or scattering layer made with mesoporous TiO2 spheres, and Pt-FTO is a layer of platinum over a FTO. FTO (15 Ω/sq) for the photoelectrodes and FTO (8 Ω/sq) for the counter electrodes were cut to a size of 2 cm × 2 cm. For the counter-electrodes substrates, one hole was drilled for the injection of the electrolyte. For reference, a single layer film architecture that does not include the r-TiO2 layer, i.e., FTO/c-TiO2/m-TiO2/N-719/Electrolyte/Pt-FTO, was also fabricated. Counter-electrodes were fabricated by screen printing platinum paste over FTO (8 Ω/sq), then these were heated to 450 °C for 75 min and then allowed to cool to ambient temperature. Electrolyte consisted of 0.60 M 1-methyl-3-propylimidazolium iodide, 0.50 M 4-tert-butylpyridine, 0.05 M iodine, 0.10 M lithium iodide and 0.10 M guanidine thiocyanate in acetonitrile.
The c-TiO2 layer for both photoelectrodes was fabricated by spin coating a sol–gel solution of TiO2 at 3000 rpm for 30 s on a FTO (15 Ω/sq) substrate. TiO2 sol–gel solution was prepared by adding 0.60 mL of titanium isopropoxide and 0.20 mL of HCl in 10 mL of isopropyl alcohol and sonicated for 10 min. FTO/c-TiO2 substrates were heated to 480 °C for 75 min and then allowed to cool down to ambient temperature. Figure 2 shows a schematic drawing of a) a single layer film architecture and b) a double layer film architecture both including dye 719, electrolyte and Pt counter electrode.
Over FTO/c-TiO2 substrates, an ATMA AT-45PA machine was used to screen-print a mesoporous layer m-TiO2 using 18 NR-T transparent titania paste and a reflective layer r-TiO2 using mesoporous TiO2 spheres paste. FTO/c-TiO2/m-TiO2/r-TiO2 substrates were heated to 480 °C for 75 min and at 60–50 °C all substrates were removed from the muffle and submerged in a fresh solution of 0.50 mM N719 with 5 mM chenoxolic acid in absolute ethanol for 24 h. After this time, TiO2 photoelectrodes were rinsed with absolute ethanol and dried with N2. Silver contacts were screen printed on the photoelectrodes and counter-electrodes and treated at 100 °C for 10 min. To assemble the DSSC solar cell, a TiO2 photoelectrode and a counter electrode were sandwiched between a Surlyn frame and hot-pressed to seal them. Electrolyte was injected through the hole in the counter electrode by using a vac'n'fill syringe (Solaronix) until completed filled and that no air bubbles were observed. Finally, a Surlyn square and coverslip were placed on top of the hole and hot-pressed to seal the cell. Photovoltaic measurements were then performed.
2.5 Experimental set up
The morphology and size of hydrous TiO2 spheres were measured from SEM images obtained using a field emission scanning electron microscope (FE-SEM Hitachi S-5500). To determine the average diameter of hydrous TiO2 spheres, the free image processing software ImageJ was used. The diameter of 50 observable hydrous TiO2 spheres in the SEM images was traced and recorded. Subsequently, the average diameter of hydrous TiO2 spheres was calculated. To measure the distribution size of the sample, the percentage coefficient of variation (% CV) was calculated according to the formula % CV = σ/µ, where σ is the standard deviation of the sample of 50 spheres, and µ is the average diameter size of the sample.
Diffraction patterns of hydrous TiO2 spheres without and with a solvothermal process were measured using a Rigaku DMAX-2200 equipment with a wavelength λo = 1.5406 Å. TiO2 sphere samples were prepared by drop casting 100 µL of TiO2 spheres in ethanol in a corning glass. r-TiO2 films with ~ 4 µm thickness were prepared from the mesoporous TiO2 sphere paste and screen printed in a corning glass. The optical diffuse reflectance and transmittance of the r-TiO2 films printed on Corning glass were obtained using a Shimadzu UV-3101 spectrophotometer. To test the solar cell performance of the DSSCs, current versus voltage graphs (J-V curves) were measured with an Autolab PGSTAT302N potentiostat/galvanostat coupled to a Sciencetech SLB 150A solar simulator equipped with a UXL-150S0 short arc xenon lamp and an AM 1.5 air mass filter.
For the measurement of J-V curves, a working area of 0.14 cm2 was chosen for all the solar cells. Five different solar cells were measured for each condition. To perform the photocatalytic degradation of the pharmaceutical product Acetaminophen, a photocatalytic reactor integrated with a 1-sol solar Compound Parabolic Collector (CPC) was used. Photodegradation processes were conducted under the following experimental conditions: 7 L reactor volume, 40 mg/L pollutant concentration, 100 mg/L powdered photocatalyst concentration, 1 mL/L H2O2 oxidizing agent, and a pH of 5.
3 Results and discussion
3.1 Hydrous TiO2 spheres
A series of sol–gel experiments were performed to determine parameters of Ti precursor concentration and MPA/Ti molar ratio for the system of Ti butoxide, 3- mercaptopropionic acid (cationic surfactant), water, and ethanol. A series of experiments were also made to determine the highest concentration of Ti precursor that can obtain highly monodisperse hydrous TiO2 spheres. The highest concentration of Ti was explored because of the use of hydrous TiO2 spheres as self templates to mesoporous TiO2 spheres that are of great importance in several applications. Ti precursor concentrations of 0.20 M, 0.16 M, 0.12 M and 0.08 M were prepared at a fixed ratio of MPA/Ti of 1 and a Rw of 20 in order to grow TiO2 spheres whose experimental results are shown in Fig. 3. The average diameter of the hydrated TiO2 spheres formed by agglomeration and nucleation processes during the sol–gel stage shown in Fig. 3 by varying the concentration of the Ti precursor reagent is shown in part A of Table 1. A 24 °C temperature was chosen for the sol–gel reaction process. Higher temperatures promote aggregative self-assembly, thus HTS with smaller size can be formed due to more nucleation centers, faster reaction kinetics, lower solubility, and shorter induction time for self-assembly. But if temperatures are higher than 40 °C, this can lead to disassembly of the spheres due to enhanced thermal motion [21].
The role of temperature in controlling the size of the hydrous TiO2 spheres has not been extensively reported in the literature. Of the several reports cited in this paper, only the work of Zhao et al. discusses the role of temperature in the properties of the hydrous TiO2 spheres [21]. They prepared HTS with n-valeric acid as the SDAs at three different reaction temperatures: -40 °C, -5 °C and 25 °C. They obtained HTS with average diameter sizes of 312, 257 and 101 nm, respectively, and with a coefficient of variation of 9.4, 7.9 and 5.8%. Normally, a greater number of nucleation centers/nuclei are associated with lower temperatures [29, 30]. In the case of sol–gel reactions of HTS prepared with an SDA, increasing the reaction temperature and Rw accelerates the hydrolysis of TTIP with the generation of a greater number of nuclei once triggered. Hydrolysis also suppresses the dissolution of the formed nuclei, so smaller sized HTS can be formed due to more nucleation centers, faster reaction kinetics, lower solubility, and shorter induction time for self-assembly.
From Table 1 is easy to observe a drastic increase of the particle diameter of TiO2 spheres (0.60, 1.00, 1.22 and 2.50 microns) when the concentration of the Ti precursor reagent is also increased (0.08, 0.12, 0.16 and 0.20 M, respectively) during the sol–gel process whose growth mechanism can be described by the eqs. (1) to (3). At a concentration of 0.20 M of the Ti precursor, no HTS are observed, but rather large amorphous particles of sizes between 1 to 2.5 µm and smaller particles with diameters of less than 1 µm.
Production of hydrous TiO2 spheres is observed at concentrations of 0.16 M Ti precursor, but also the presence of a severe agglomeration and a high size dispersion were detected. At 0.12 M, formation of spheres with less agglomeration and a more homogenized size is also seen. At 0.08 M, highly monodisperse spheres with an average diameter of 0.65 microns and with a low coefficient variation of 6% are observed. A value of 0.08 M of Ti is a high concentration compared to other reports using carboxylic acids as SDAs with Ti precursor concentrations from 0.008 M [21] and 0.0178 M [20]. Amines as SDAs have been also reported at Ti precursor concentrations between 0.018 M to 0.067 M [16, 17, 19, 26]. With hexadecylamine at a Ti precursor concentration of 0.074 M [18], this is one of the highest concentrations that has been reported.
Molar ratios of MPA/Ti with values of 1, 0.75, 0.6, and 0.3 were also studied, maintaining a Ti concentration of 0.08 M and a Rw of 82, whose results are shown in part B of Table 1 and in the Online Resource 2. The difference in these molar ratios of MPA/Ti did not change the average diameter size of the spheres and gave an average diameter around ~ 200 nm and a low coefficient variation of less than 12%. At the MPA/Ti = 0.6, higher monodispersity (% CV of 8.5) and specially a reduced presence of dimers and trimers is seen. With a lower MPA/Ti molar ratio than 0.30, a higher agglomeration of spheres is observed, showing that not enough SDAs have been used. With a lower MPA/Ti molar ratio than 0.30, a higher agglomeration of spheres is observed, showing that not enough SDAs have been used.
At low Rw values between 5.91 and 11.82, amorphous spheres of hydrous TiO2 with diameter sizes from 1 to 4 μm are obtained as well as a high agglomeration of spheres, remarkably the spherical shape is still observed (see Online Resource 1 in the supplementary information). Hydrous TiO2 spheres with average diameter sizes of 150, 178, 259, 329, 412, 561, 641, 804 and 950 nm were obtained with Rw of 118, 82, 54, 35.50, 29, 23.60, 20.70, 18.90 and 17.70, respectively, setting the values to 0.08 M for the Ti concentration and MPA/Ti at 0.60. A low agglomeration degree is observed, especially for Rw values between 17.70 and 18.90, then a high monodispersity is observed on samples with Rw in the range of 23.6 to 82. The average diameter size of the HTS and their % CV as a function of the Rw value are shown in Fig. 4. The general trend shown in the graph of Fig. 5 is an exponential type of decay in the average size of the diameter of the TiO2 spheres with an increase in the ratio Rw ([H2O]/[Ti]).
To explain the results shown in Figs. 1 and 2 and Online Resource 1 and 2, several models reported in the literature should be considered. A formation mechanism model of hydrous TiO2 spheres has been recently explained by a LaMer model in the first stage and a self-assembly model in a second stage [31]. In the first stage sol–gel reactions occur when a Ti(OR)4 alkoxide gets in contact with water, starting a series of reactions of: (1) hydrolysis, (2) condensation-polymerization and (3) condensation—self-dehydration [21, 32].
The self-assembly driving forces proposed by LaMer for the TiO2 nuclei are the Van der Waals attractions, electrostatic repulsion and steric forces present in colloidal systems which are better described by the DLVO (Derjaguin, Landau, Verwey and Overbeek) theory [33].
In the DLVO model, the presence of charged particles in a colloidal solution leads to electrostatic interactions, where the interaction energy (electrostatic potential) between two colloidal particles is a function of the distance d between them [34]. The electrical charge on a particle can arise from dissociated charge groups, adsorbed ions, or surfactants. The electrical charge on particles leads to the formation of an electrical double layer in solution. Predominantly, the overlap of these electrical double layers first leads to a repulsive force created by counterions between two particles and secondly to an attractive force between particles, which is caused by the Van der Waals attraction.
If the distance between colloidal particles is large, firstly the electrostatic interaction can be described through a weak attraction which can be represented through a minimum of potential energy called a second minimum. This second energy minimum may or may not exist depending on the composition of the solution. For example, a high concentration of salts in solution would give rise to such a minimum. If the interaction energy of this second minimum is of the order of the thermal energy, then the colloidal particles can interact, aggregate and form flocks. However, the interaction between colloidal particles in this second minimum is weak and said aggregation is reversible [35].
As the distance between colloidal particles decreases, both particles experience a potential energy barrier that prevents them from coming into contact. 3-Mercaptonic acid is a bifunctional molecule composed of a carboxylic acid group and a thiol group. MPA carboxylic acid group bonds with the Ti alkoxide reducing the hydrolysis rate, while the -SH groups stay at the surface of the hydrous TiO2 spheres recently formed, thus leading to the stabilization of the solution because of electrostatic repulsion between the thiols groups.
The particles will bounce after each interaction and remain dispersed throughout the medium, as long as the height of the barrier is greater than the thermal energy of the colloidal system [36, 37]. If by some means the potential barrier is crossed, then the interaction between particles would be attractive and, as a result, the particles would aggregate. For a colloidal system, thermodynamic equilibrium can be reached when the particles are in the primary potential well. In this state, the particles coagulate, and this process is non-reversible [38]. Final size of the particles is reached when the agglomeration and repulsive interaction tendencies are balanced, and the growth tendencies caused by agglomeration stop and short-range repulsive interactions among other neighbor spheres increase [39].
The main effects of SDA on the morphology of hydrated TiO2 spheres can be explained by the bond strength of the Ti alkoxide with the functional group of the SDA and the length of its hydrocarbon chain [20, 21]. SDAs with too strong a bond between the Ti alkoxide and its functional group will experience too slow a hydrolysis reaction. With a weak bond between the Ti alkoxide and the SDA functional group, a very rapid hydrolysis reaction will be experienced. Both a hydrolysis reaction that is too fast and a reaction that is too slow would lead to non-uniform growth of HTS. The hydrocarbon chain length of the SDA is related to the strength of the bond between the Ti alkoxide and the functional group bond, while short chain lengths lead to stronger bonds and longer chains lead to weaker bonds. Only SDAs with suitable bonding strength and hydrocarbon chain length will form monodisperse hydrated TiO2 spheres [20].
In the specific case of this work, the discussion of Fig. 1 will be given first. During preparing the precursor solution, tetrabutyl titanate and MPA (a cationic surfactant) were dissolved in ethanol and the water was added in a controlled manner. The sudden content of water in contact with Ti(OR)4 induced a sharp increase in the particle concentration above the nucleation threshold, rapidly forming amorphous hydrous TiO2 nuclei, as shown by eqs. (1) to (3). At high concentrations of precursors (i.e. 0.20 M), the water content must increase to have a Rw of 20. This high content in water induces a rapid hydrolysis and the buildup time for the self-assembly of hydrous TiO2 spheres shortens. Due to this high concentration of water hydrous TiO2 spheres with very large diameters (1–2.5 microns) and undefined geometries are observed.
On the contrary, when a low concentration of precursors is used (0.08 M), the water content also drops and the hydrolysis reaction rate decreases, then the buildup time for the self-assembly increases which results in hydrous TiO2 spheres with high monodispersity as seen in Fig. 1. Nevertheless, hydrous TiO2 spheres are amorphous, and no crystal structure is observed (see Figure XRD data) due to this fact a smooth surface is observed by SEM as nanocrystals have not formed yet due to a low temperature process. Only when the HTS have been submitted to a solvothermal treatment of 200 C, nanocrystals of anatase appears, the presence of solvent avoids serious crystal fusion, and the SDA keeps the morphology of the spheres [1].
Following only a diffusion-controlled growth mechanism, these TiO2 nuclei cannot grow up to sub-micrometer sizes (0.10 to 1 µm). Instead, these amorphous hydrous TiO2 nuclei have a high surface energy and spontaneously agglomerate and self-assembly to sub micrometer spheres, whose spatial configuration is an isotropic and thermodynamically stable structure [31]. Under these conditions, the particle diameter increases as the concentration of the Ti precursor reagent increases, as described in Table 1 Part B. According to the DLVO theory, the large agglomeration of TiO2 amorphous hydrated particles is largely due to the Van der Waals attraction experienced by these particles in the Ti precursor solution, which allows them to reach average particle diameters up to 2.50 \(\mathrm{\mu m}\).
Regarding the results shown in Online Resource 2, the growth of TiO2 particles was studied, varying the molar ratios of MPA/Ti in values of 1, 0.75, 0.60, and 0.30 for whose precursor solution a Ti concentration of 0.08 M and a Rw = [H2O]/[TI] = 82 were taken. The decrease in the concentration of the Ti precursor and the use of 3-mercaptopropionic acid (MPA) as a structure directing agent helped to increase the repulsive forces between particles and to synthesize monodisperse hydrated TiO2 spheres with a controllable particle diameter. At MPA/Ti = 0.60, the highest monodispersity was obtained (% CV of 8.50).
Concerning the results shown in Fig. 4, the SEM images show hydrous TiO2 spheres with different average diameter sizes by varying Rw in the range of Rw ([H2O]/[Ti]) from 17 to 118. For this series of experiments, fixed synthesis parameters were used, such as 0.08 M for Ti and 0.6 for the MPA/Ti ratio. A high agglomeration degree is observed especially for Rw values between 17.7 and 18, but at high values of Rw, homogeneous particles are observed whose TiO2 diameter decays below 200 nm. A high monodispersity (% CV) of particles is also observed on samples with Rw above 23. The general trend shown in the graph of Fig. 5 is an exponential type of decay in the average size of the diameter of the TiO2 spheres with an increase in the ratio Rw ([H2O]/[Ti]). Considering that the concentration [Ti] remains fixed, this means that an increase in the concentration of H2O also diminishes the concentration of [Ti] leading to a decrease in the average diameter of hydrous TiO2 spheres.
In summary, the following tendencies have been observed: a) an increase in the molar concentration of Ti increases the average TiO2 particle size through an agglomeration process of colloidal particles. b) A molar ratio MPA/Ti with values of 1, 0.75, 0.60, and 0.30 provides a high monodispersity of TiO2 particles and delivers a small average particle diameter of around ~ 200 nm. Structure directing agents, such as MPA, improve the reproducibility, quality, and uniformity of the self-assembled hydrous TiO2 spheres. SDAs have been proposed as precipitation prompters and as morphological controllers [31]. SDAs function as precipitation prompters accelerating the hydrolysis and condensation reactions of Ti(OR)4 alkoxides with water whose evidence of this behavior is the shortening of the induction time. c) The higher Rw = [H2O]/[Ti], the smaller the particle size due to an increase in the concentration of H2O also diminishes the concentration of [Ti] leading to a decrease in the average diameter of hydrous TiO2 spheres.
3.2 Crystal structure of hydrous TiO2 spheres after solvothermal treatment
Hydrous TiO2 spheres have been used as self templates for the synthesis of mesoporous TiO2 spheres, which have several applications. One of them is as an active reflective layer of photoelectrodes in solar cells. TiO2 spheres have a double function as a highly reflective layer and as a dye loading surface [40]. Mesoporous TiO2 spheres can be used to fabricate highly efficient DSSCs using a double layer film architecture, one layer of mesoporous transparent TiO2 nanoparticles (typically around 20 nm) with a high surface area for dye absorption and a second additional layer of highly light scattering TiO2 particles.
Hydrous TiO2 spheres with an average diameter of 250 nm were used as a self-template to synthesize mesoporous TiO2 spheres by a solvothermal route. The x-ray diffraction pattern of hydrous TiO2 spheres, and mesoporous TiO2 spheres after the solvothermal treatment at 200 °C is shown in Fig. 6. Hydrous TiO2 spheres are amorphous because they do not show clear diffraction peaks, then there is not present a crystallographic arrangement of atoms. In contrast, mesoporous TiO2 spheres show clear peaks that correspond to an anatase crystal structure of TiO2 (PDF-21–1276) at 2ϴ degree of 25.30, 37.77, 48.01, 55.10 and 62.64 which match to the planes (101), (004), (200), (211) and (204), respectively. As peak diffraction is observed, apparent crystallite sizes of the TiO2 nanocrystals were calculated from the Scherrer equation [41]:
where D is the average crystallite size, β is the full width at half maximum (FWHM) of the peak diffraction (101) in radians. Ɵ is the half diffraction angle, λ is the X-ray wavelength equal to 1.5406 Å and K is a dimensionless shape factor equal to 0.90. Following Eq. 4, the calculated average crystallite size of the TiO2 is 11.49 nm.
SEM images of the precursor HTS and of the same sample after the solvothermal process to give mesoporous TiO2 spheres are shown in Fig. 7. The average diameter of mesoporous TiO2 spheres is 248 nm, with a standard deviation of 16.8 and a % CV of 6.16. Comparing the size of mesoporous TiO2 spheres (248 nm) to HTS with an average size of 259 nm, the spheres had a 4.20 shrinkage percentage. A mesoporous TiO2 sphere is formed by several TiO2 nanocrystals, as observed in Fig. 7 d). Considering that the diameter of 50 nanocrystals was measured with the software ImageJ, an average crystal size of 25 nm with a standard deviation of 7% was calculated for the TiO2 nanocrystals with anatase phase. The reflective r–TiO2 layer made with TiO2 nanoparticles had a BET surface area of 119 m2/g.
3.3 TiO2 spheres for application in the development of DSSC and as photocatalyst
Considering the quantum confinement found in the nanocrystals with anatase crystalline structure that build the TiO2 spheres, and that an anatase phase is a very stable crystalline structure, it is possible to affirm that it retains all the semiconductor properties described in Section 2.3. Anatase TiO2 nanocrystals were prepared using the same chemical reagents under the same sol–gel/solvothermal method and thermally treated in air at temperatures of 453 °C and 480 °C for use in photocatalysis or DSSC development, respectively. Table 2 describes the experimental preparation conditions and structural properties of the TiO2 spheres for both applications. From Table 2 it is easy to see that it is possible to prepare anatase crystallites with specific properties for special applications (in DSSC or as a photocatalytic material). It is worth mentioning that the values of the experimental parameters described in Table 3 follow the behavior shown in Fig. 5 between the average particle diameter and Rw.
In general, the anatase phase of TiO2 is an n-type semiconductor whose electrical conductivity σ is proportional to the electron density n (e) in the conduction band (e being the electron charge) and the hole density p(h+) in the valence band (h+ being the hole charge). In this case, it is possible to assume that n(e) > > p(h).
Another experimental study on electrical photoconductivity reported results obtained on TiO2 films assembled with anatase TiO2 nanoparticles and having a crystallite size of 16.3 nm. The films, thermally treated at 450 °C, were illuminated with photons of energy higher than Eg, causing the electrons in the valence band of TiO2 to be excited to the conduction band. Below 350 °C of heat treatment, TiO2 films are not photoconductive. This experiment allowed us to determine the electrical photoconductivity of this n-type semiconductor. Under illumination, the electrical photoconductivity of TiO2 films increases dramatically from 1 × 10–7 to 1 × 10–3 [Ohm-cm]−1 [42].
Therefore, all the experimental characterization carried out on the TiO2 spheres allowed us to affirm that the nanocrystals that assemble the TiO2 spheres retain almost the same structural, morphological and optoelectronic properties of an anatase phase, consequently, it is possible to use them in several applications, specifically, in the development of DSSC and as a photocatalyst.
3.3.1 TiO2 spheres for DSSC
A terpineol based paste was fabricated with the mesoporous TiO2 spheres and then used as a reflective TiO2 layer by screen printing, which later was used to fabricate DSSC cells. The Fig. 8 a) shows the SEM image of the cross section of a 4 µm thick TiO2 film with a spherical configuration on a Corning glass. The diffuse reflectance on the r-TiO2 films and that made with the TiO2 18 NR-T paste (m-TiO2 layer) was measured as shown in Fig. 8 b). For the r-TiO2 layer, a high percentage of diffuse reflection of 78% at 380 nm is observed, which decreases to 69% at 800 nm, while a maximum diffuse reflection of 80% is observed at 400 nm. In the case of the m-TiO2 layer, a low diffuse reflection is observed with a maximum of 16% diffuse reflection at 400 nm. The r-TiO2 layer made of the mesoporous TiO2 spheres has a BET surface area of 119 m2/g with a mean pore size of 12.6 nm as shown in Table 2. A high reflective TiO2 layer is important as it reflects the photons that were not absorbed at the first trial by the dye molecules attached to the mesoporous transparent TiO2 layer covered with the dye, this is the main advantage of a double layer architecture [43].
3.3.2 TiO2 spheres used in photocatalysis
Table 3 describes the BET surface area, total pore volume and average diameter values of hydrated TiO2 spheres in its TiO2 M5 modifications. For comparison purposes, the values of surface area of the commercial Hombikat and Degussa P25 photocatalysts are 202 and 48.6 m2/g, respectively, and their total pore volume are 0.343 and 0.176 cm3/gr. It is observed that the photocatalyst with the smallest surface area is Degussa P25 and the one with the largest surface area is the Hombikat photocatalyst while in the medium range belong to the TiO2 M5.
As shown in Table 3, the diameter classification of TiO2 sample M5 lies in the range of mesoporous materials (2–50 nm). Meso- and macroporous materials have a considerably accessible surface for molecular diffusion and adsorption, so the photochemical properties can be improved due to the increase in the accessible surface and to the acceleration of mass transport efficiency [45]. In photocatalysis processes, mesoporous materials present good light absorption regardless of the irradiation direction of the light source [1]. Regarding the sphere diameter, it is found that spheres with a diameter around 450 nm show the best light scattering effect [45]. In a photocatalytic reaction, the oxidation process can occur both on the surface and inside the photocatalyst since the reactants diffuse into the internal structure and are adsorbed on the internal surface where the oxidation reactions are also produced [46].
3.4 DSSC solar cells
To prove the mesoporous TiO2 spheres synthesized with MPA as a structure directing agent as an effective photoactive reflective layer, dye sensitized solar cells (DSSCs) were chosen as a proof of concept. DSSCs were fabricated using two types of photoelectrodes. The first photoelectrode has the following architecture: FTO/c-TiO2/m-TiO2, and the second type: FTO/c-TiO2/m-TiO2/r-TiO2 where both photoelectrodes were treated in air at 480 °C for one hour with the purpose to achieve optimal electrical and optical properties. The difference being that the second one structure has a photoactive reflective layer (r-TiO2) made of mesoporous TiO2 spheres with a diameter of ~ 250 nm, this structure is typically named as a double layer film architecture. Both types of photoelectrodes have the same thickness measured with a profilometer (~ 14 µm). DSSCs are typically composed of conductive transparent glass (FTO), TiO2 particles sensitized by dye molecules, a liquid electrolyte and a counter electrode.
Dye molecules absorb electromagnetic radiation when the DSSC cell is illuminated, and for each photon absorbed, an electronic transition is generated from the dye's basic state (So) toward its excited state (S*), the electron moves from HOMO to LUMO level in the dye. From this state, the electron is injected into the conduction band of the transparent layer of TiO2, creating an electron vacancy or a hole in the dye. In order to renew the dye, an electron must be removed from iodide (I−) in the electrolytic solution, resulting in its oxidation producing triiodide (I3−), which is reduced by removing electrons from the counter electrode returning to its original state (I−) [47].
This flow of electrons and holes generates the electric current in the solar cell. The overall power conversion efficiency (PCE) of the solar cell was calculated from the J-V curves by:
Pin is the input incident light irradiance power, which is 0.1W/cm2 at 1.5 AM. Voc is the open circuit voltage, which is the maximum voltage created by the cell when there is no current flow. Jsc is the short circuit current density, which corresponds to the highest photocurrent produced by the cell at zero voltage. FF is the fill factor, which is the ratio of the actual maximum power generated by the cell to its ideal maximum power with no series resistance and infinite shunt resistance. FF is calculated by:
VMP and JMP are the voltage and current density of the cell at maximum power, respectively.
The best current density–voltage (J-V) curves of a single (FTO/c-TiO2/m-TiO2) and a double (FTO/c-TiO2/m-TiO2/r-TiO2) layer DSSCs architecture under 1 sun illumination are shown in Fig. 9, and their average photovoltaic parameters, standard deviation and best efficiency are shown in Table 4. Average short circuit current (Jsc), open-circuit voltage (Voc), fill factor (FF) and PCE of the TiO2 single layer DSSCs architecture were 17.27 mA/cm2, 702 mV, 64% and 7.72%, and for the double layer DSSCs architecture were 19.00 mA/cm2, 690 mV, 69% and 9.17%, respectively. The main difference in the photovoltaic parameters was for the average Jsc and PCE with a percentage difference of 9.10% and 15.6% respectively. A higher current Jsc is because the TiO2 spheres reflect photons that were not absorbed on their first path of its travel. Research has been made related to the optimal size of mesoporous TiO2 spheres used for a double layer film architecture in DSSC [48, 49]. Yu et al. studied mesoporous TiO2 spheres with diameter sizes of 260, 350, 450, 560, 800 and 980 nm, their results showed that the highest light-scattering efficiency among these TiO2 spheres was with a 450 nm diameter, followed by 260 and 800 nm [48]. Nevertheless, the difference in power conversion efficiency among the DSSC made with 450 nm (9.04%), 260 nm (8.74%) and 800 nm (8.77%) was only 3.30%, where the main difference was between using TiO2 spheres and not using them with a PCE of 6.92%.
The maximum reported power conversion efficiencies range between 9 to 10% for dye sensitized solar cells with a double layer film architecture and sensitized with the dye N-719 [17, 48, 49]. More recent reports about the use of mesoporous TiO2 spheres synthesized via a microwave route, graphene loaded TiO2 spheres or a blend of TiO2 nanoparticles and spheres have shown PCEs below 7.5% [50,51,52].
Surface area, spheres diameter and pore size play an important role in the performance of each application of mesoporous materials. In DSSC, mesoporous TiO2 spheres have a high accessible surface area and adequate porosity for dye molecules diffusion and absorption. Chen et al. showed that mesoporous TiO2 spheres with an average diameter of 300 nm, a surface area of 90 m2/g and a pore size of 23 nm were able to support a N-719 dye loading in an amount of 1.91 × 10–5 mol/g [12]. When average diameter of mesoporous TiO2 spheres increased from 500 to 800 nm, having both samples a surface area of 90 m2/g and a pore size of 23 nm, they also measured a slightly decrease in the dye loading to amounts of 1.67 × 10–5 and 1.47 × 10–5 mol/g, respectively. In summary, the light scattering effect of mesoporous TiO2 spheres had a bigger influence on the performance of the DSSC cells than the dye loading amount as long as the surface area were similar.
Taleb et al. demonstrated that the aggregation of particles into TiO2 nanospheres strongly influences the performance of DSSC. The highest energy conversion efficiency in their DSSC was achieved using photoelectrodes assembled with TiO2 spheres with a diameter of approximately 90 nm, a crystallite size of 22 nm, a surface area of 90 m2/g and a pore size of 9 nm. The authors concluded that the highest conversion efficiency was achieved due to an optimized balance between dye loading (large surface area), higher light scattering, better connectivity for charge transport, and reduced recombination processes [53].
3.5 Photocatalytic degradation of acetaminophen
The chemical reagents that were used for the synthesis of the TiO2 spheres through the sol–gel/solvothermal process were titanium butoxide at a concentration of 0.08 M, a MPA/Ti ratio of 0.60 and a Rw = [H2O]/[Ti] of 25. After preparation of the sol–gel solution, it was placed in an autoclave at 200 °C for 12 h and then heated at 450 °C in air for 75 min. For the photocatalytic degradation process of analgesic acetaminophen, hydrated TiO2 spheres were used as a photocatalyst, in powder form and with a particle diameter of 459 nm with a CV of 9%. The degradation process of acetaminophen required concentrated solar radiation provided by a 1-sun CPC collector integrated into the photocatalytic reactor.
In order to evaluate the performance of the TiO2 catalyst with spherical geometry, certain reference measurements were included such as: exposure of the contaminant in aqueous phase in dark conditions and under UV illumination, photolysis, degradation with and without oxidizing agent (H2O2), with catalyst in darkness and lighting, and with catalyst, H2O2 and UV radiation. For a better estimation of the performance of the TiO2 M5 spheres, as a frame of reference, the contaminant has been also degraded using the commercial photocatalyst Hombikat (HKM) distributed by the Merck company.
Figure 10(A) shows the degradation of paracetamol as a function of exposure time under different experimental conditions: a) UV irradiation, b) H2O2/UV, c) TiO2 HMK/UV, d) TiO2 M5/UV, e) HKM TiO2/H2O2/UV and f) TiO2 M5/H2O2/UV. For all these cases in which TiO2 M5 nanospheres or the commercial TiO2 Hombikat catalyst are used, during the first 20 min of the process, the contaminant was first adsorbed on the surface of both photocatalysts, so the degradation of the contaminant was negligible. This event is most notable for the Hombikat catalyst. After adsorption, degradation of the contaminant begins. Under illumination, the TiO2 HKM/UV process is slightly better than the TiO2 M5/UV. However, by adding the oxidizing agent, the TiO2 M5/H2O2/UV process is much better than TiO2 HKM/H2O2/UV under the same conditions.
Table 5 shows the final contaminant degradation values in darkness and under UV illumination of all the cases explained above after 3 h of exposure. From Table 5 it is observed that in the dark and under any condition of both the catalyst and the oxidizing agent, practically no degradation occurs. Significant cases of degradation appear when the system to be degraded is illuminated and it is during the photolysis of the contaminant that elimination levels of just 4% are reached.
For all these cases in which TiO2 M5 nanospheres or the commercial TiO2 Hombikat catalyst are used, during the first 20 min of the process, the contaminant was first adsorbed on the surface of both photocatalysts, so the degradation of the contaminant was negligible. This event is most notable for the Hombikat catalyst. After adsorption, degradation of the contaminant begins. Under illumination, the TiO2 HKM/UV process is slightly better than the TiO2 M5/UV. However, by adding the oxidizing agent, the TiO2 M5/H2O2/UV process is much better than TiO2 HKM/H2O2/UV under the same conditions.
When TiO2 M5 nanospheres are used, degradation levels of approximately 88% are achieved in the next 50 min and final levels of 93% after 180 min, while for the TiO2 HKM/H2O2/UV process in the next 50 min. Only an elimination of 60% is achieved and after 180 min final degradation levels of 83% are reached. Another fact to highlight is the final degradation levels determined by the chemical oxygen demand (COD) and total organic carbon (TOC), which in the case of the TiO2 M5/H2O2/UV process were 96% and 72%, while in the TiO2 process HKM/H2O2/UV process were 84 and 67%, respectively.
The addition of the oxidizing agent in the photocatalyst mixture contributes to increasing the photodegradation rate of organic contaminants in most cases, reducing the recombination of e−—h+ pairs and increasing the concentration of hydroxyl radicals to achieve higher degradation levels of organic pollutants [54]. The next step would be to slightly increase the concentration of the oxidizing agent to reach degradation levels higher than 95%. Lin et al. reported the enhanced photocatalytic degradation of acetaminophen at a concentration of 50 mg/L acetaminophen solution using hollow mesoporous TiO2 microspheres TSS and THS and TiO2 Degussa P25 finding that the photocatalytic activities of TSS, and THS were better than that of P25 TiO2, that means, the achieved degradation levels were: P25 82%, TSS 90% and HTS 92% [55].
Taking into account a global first-order kinetics for the degradation processes of Fig. 10 A), during the first 50 min of exposure to radiation of the contaminant, Fig. 10 (B) shows the ln (Co/C) plots) versus time through which it is possible to determine the reaction constant k for each case, which turned out to be: a) UV; 0.00013, b) H2O2/UV; 0.009, c) TiO2 HBK/UV; 0.002, d) TiO2 M5/UV; 0.005 and e) TiO2 HBK/H2O2/UV; 0.017 and f) TiO2 M5/H2O2/UV 0.041 min−1.
Based on the reaction kinetic constants obtained, it is possible to affirm that the TiO2 M5 photocatalyst with spherical geometry made it possible to achieve the fastest reaction rates and the highest levels of acetaminophen degradation, even higher than those corresponding to the commercial Hombikat TiO2 catalyst under the same oxidant agent and solar radiation conditions and verified by the analytical techniques of UV–Vis Spectrophotometry, COD and TOC.
In non-porous materials, mass transfer takes place only in the boundary layer and on the surface of the catalyst, while in porous materials, the photocatalytic reaction can occur both on the surface and inside the photocatalyst increasing the probability of interaction of the contaminant molecules with the photocatalytic surface. Additionally, the contaminant molecules diffuse into the internal structure and adsorb on the internal surface where photodegradation reactions also occur [46]. Mesoporous TiO2 spheres have a high accessible surface area for molecular diffusion and adsorption, so the photochemical degradation of acetaminophen can be improved due to the increase in the accessible surface and the acceleration of mass transport efficiency [45].
4 Conclusions
A thiol molecule, 3-mercaptopropionic acid, has been used as an effective structure directing agent for the synthesis of monodisperse hydrous TiO2 spheres with a controllable particle diameter of 150, 178, 259, 329, 412, 561, 641, 804 and 950 nm and high monodispersity (% CV < 10%). This means that the thiol molecule, 3-mercaptopropionic acid, increases the repulsion forces between colloidal particles in the precursor solution of Ti according to the DLVO theory. The group of thiol molecules can be added to the list of chemical species adequate for the synthesis of hydrous TiO2 spheres that offer other complementary capabilities different from other SDAs such as amines, organic acids, salts, polymers and ethylenglycol. It is important to note that each of these SDA chemical species has its own optimal synthesis conditions, the main ones being the concentration of the Ti precursor, the range of [SDA]/[Ti] and Rw = [H2O]/[Ti] molar ratios. Each SDA has a unique influence in the physical–chemical properties of the HTS, such as the range of diameter sizes, monodispersity (percentage of coefficient variation), degree of size control by the Rw parameter, among others.
To prove the application of the synthesized HTS by 3-mercaptopropionic acid, HTSs of ~ 259 nm diameter size was used as a template for the synthesis of mesoporous TiO2 spheres and a reflective screen paste was made to fabricate dye sensitized solar cells. DSSCs provided a maximum power conversion efficiency (PCE) of 9.31% fabricated with a reflective layer made of the mesoporous TiO2 spheres. With no reflective layer of mesoporous TiO2 spheres, a DSSCs with the same thickness achieves a PCE of 7.82%. Finally, hydrous TiO2 spheres were used as photocatalysts for the photocatalytic degradation of the analgesic Acetaminophen in aqueous solution under concentrated solar radiation, achieving degradation levels of 92%.
Data availability
Authors declare that all data supporting the findings of this study are available within the article and on the supplementary material section. Additional information is available upon request in the notebook of the laboratory experiment log.
References
J.H. Pan, Z. Cai, Y. Yu, X.S. Zhao, Controllable synthesis of mesoporous F-TiO2 spheres for effective photocatalysis. J. Mater. Chem. 21, 11430–11438 (2011). https://doi.org/10.1039/c1jm11326g
T. Lu, Y. Wang, Y. Wang, L. Zhou, X. Yang, Y. Su, Synthesis of Mesoporous Anatase TiO 2 Sphere with High Surface Area and Enhanced Photocatalytic Activity. J. Mater. Sci. Technol. 33, 300–304 (2017). https://doi.org/10.1016/j.jmst.2016.03.019
N. Lakshminarasimhan, E. Bae, W. Choi, Enhanced Photocatalytic Production of H2 on Mesoporous TiO 2 Prepared by Template-Free Method: Role of Interparticle Charge Transfer. J. Phys. Chem. C 111, 15244–15250 (2007). https://doi.org/10.1021/jp0752724
X. Shi, Z. Lou, P. Zhang, M. Fujitsuka, T. Majima, 3D-Array of Au–TiO 2 Yolk-Shell as Plasmonic Photocatalyst Boosting Multi-Scattering with Enhanced Hydrogen Evolution. ACS Appl. Mater. Interfaces. 8, 31738–31745 (2016). https://doi.org/10.1021/acsami.6b12940
Y.G. Guo, Y.S. Hu, J. Maier, Synthesis of hierarchically mesoporous anatase spheres and their application in lithium batteries. Chem. Commun. 2783–2785 (2006). https://doi.org/10.1039/b605090e
H. Wei, E.F. Rodriguez, A.F. Hollenkamp, A.I. Bhatt, D. Chen, R.A. Caruso, High Reversible Pseudocapacity in Mesoporous Yolk-Shell Anatase TiO 2 /TiO 2 (B) Microspheres Used as Anodes for Li-Ion Batteries. Adv. Func. Mater. 27, 1703270 (2017). https://doi.org/10.1002/adfm.201703270
Z. Hao, M. Tian, Y. Ren, W. Dai, M. Wang, W. Chen, G.Q. Xu, 3D-Assembled rutile TiO 2 spheres with c -channels for efficient lithium-ion storage. Nanoscale 13, 11104–11111 (2021). https://doi.org/10.1039/D1NR02064A
Z. Li, Y. Peng, X. Zhang, Y. Ren, W. Chen, F. Xu, N. Wang, C. Liu, L. Mo, Y. Ding, L. Hu, D. Ji, G. Cao, Oxygen Vacancies Enhance Lithium-Ion Storage Properties of TiO 2 Hierarchical Spheres. Batter. Supercaps 4, 1874–1880 (2021). https://doi.org/10.1002/batt.202100157
N. Al-Ansi, A. Salah, J. Lin, G.-D. Yang, H.-Z. Sun, L. Zhao, Fabrication and synergistic control of ternary TiO2/MoO2@NC hollow spheres for high-performance lithium/sodium-ion batteries anodes. Appl. Energy 334, 120691 (2023). https://doi.org/10.1016/j.apenergy.2023.120691
T.S. Munonde, M.C. Raphulu, Review on titanium dioxide nanostructured electrode materials for high-performance lithium batteries. J. Energy Storage 78, 110064 (2024). https://doi.org/10.1016/j.est.2023.110064
L. Cao, D. Chen, W. Wu, J.Z.Y. Tan, R.A. Caruso, Monodisperse anatase titania microspheres with high-thermal stability and large pore size (∼80 nm) as efficient photocatalysts. J. Mater. Chem. A 5, 3645–3654 (2017). https://doi.org/10.1039/C6TA08981J
D. Chen, F. Huang, Y.-B. Cheng, R.A. Caruso, Mesoporous Anatase TiO 2 Beads with High Surface Areas and Controllable Pore Sizes: A Superior Candidate for High-Performance Dye-Sensitized Solar Cells. Adv. Mater. 21, 2206–2210 (2009). https://doi.org/10.1002/adma.200802603
S. Velázquez-Martínez, S. Silva-Martínez, A.E. Jiménez-González, A. Maldonado Álvarez, Synthesis of Mesoporous TiO 2 Spheres via the Solvothermal Process and Its Application in the Development of DSSC. Adv. Mater. Sci. Eng. 2019, 1–15 (2019). https://doi.org/10.1155/2019/9504198
E.A. Barringer, H.K. Bowen, Formation, Packing, and Sintering of Monodisperse TiO2 Powders. J. Am. Ceram. Soc. 65, C-199-C–201 (1982). https://doi.org/10.1111/j.1151-2916.1982.tb09948.x
E.A. Barringer, H.K. Bowen, High-purity, monodisperse TiO2 powders by hydrolysis of titanium tetraethoxide. 1. Synthesis and physical properties. Langmuir 1, 414–420 (1985). https://doi.org/10.1021/la00064a005
E. Mine, M. Hirose, D. Nagao, Y. Kobayashi, M. Konno, Synthesis of submicrometer-sized titania spherical particles with a sol–gel method and their application to colloidal photonic crystals. J. Colloid Interface Sci. 291, 162–168 (2005). https://doi.org/10.1016/j.jcis.2005.04.077
S. Yu, J.S. You, I.S. Yang, P. Kang, S.B. Rawal, S.D. Sung, W.I. Lee, Tailoring of nanoporous TiO2 spheres with 100–200 nm sizes for efficient dye-sensitized solar cells. J. Power Sources 325, 7–14 (2016). https://doi.org/10.1016/j.jpowsour.2016.06.018
D. Chen, L. Cao, F. Huang, P. Imperia, Y.-B. Cheng, R.A. Caruso, Synthesis of Monodisperse Mesoporous Titania Beads with Controllable Diameter, High Surface Areas, and Variable Pore Diameters (14–23 nm). J. Am. Chem. Soc. 132, 4438–4444 (2010). https://doi.org/10.1021/ja100040p
S. Tanaka, D. Nogami, N. Tsuda, Y. Miyake, Synthesis of highly-monodisperse spherical titania particles with diameters in the submicron range. J. Colloid Interface Sci. 334, 188–194 (2009). https://doi.org/10.1016/j.jcis.2009.02.060
S. Liu, G. Han, M. Shu, L. Han, S. Che, Monodispersed inorganic/organic hybrid spherical colloids: Versatile synthesis and their gas-triggered reversibly switchable wettability. J. Mater. Chem. 20, 10001 (2010). https://doi.org/10.1039/c0jm02101f
T. Zhao, R. Qian, Y. Tang, J. Yang, Y. Dai, W.I. Lee, J.H. Pan, Controllable Synthesis and Crystallization of Nanoporous TiO2 Deep-Submicrospheres and Nanospheres via an Organic Acid-Mediated Sol-Gel Process. Langmuir: ACS J. Surf. Colloids 36, 7447–7455 (2020). https://doi.org/10.1021/acs.langmuir.0c01008
S. Eiden-Assmann, J. Widoniak, G. Maret, Synthesis and Characterization of Porous and Nonporous Monodisperse Colloidal TiO 2 Particles. Chem. Mater. 16, 6–11 (2004). https://doi.org/10.1021/cm0348949
L. Yang, Y. Lin, J. Jia, X. Li, X. Xiao, X. Zhou, Cauliflower-like TiO2 rough spheres: Synthesis and applications in dye sensitized solar cells. Microporous Mesoporous Mater. 112, 45–52 (2008). https://doi.org/10.1016/j.micromeso.2007.09.011
X. Jiang, T. Herricks, Y. Xia, Monodispersed Spherical Colloids of Titania: Synthesis, Characterization, and Crystallization. Adv. Mater. 15, 1205–1209 (2003). https://doi.org/10.1002/adma.200305105
M. Pal, J. García Serrano, P. Santiago, U. Pal, Size-Controlled Synthesis of Spherical TiO2 Nanoparticles: Morphology, Crystallization, and Phase Transition. J. Phys. Chem. C 111, 96–102 (2007). https://doi.org/10.1021/jp0618173
J.H. Pan, X.Z. Wang, Q. Huang, C. Shen, Z.Y. Koh, Q. Wang, A. Engel, D.W. Bahnemann, Large-scale Synthesis of Urchin-like Mesoporous TiO 2 Hollow Spheres by Targeted Etching and Their Photoelectrochemical Properties. Adv. Funct. Mater. 24, 95–104 (2014). https://doi.org/10.1002/adfm.201300946
M.A. Sánchez-García, X. Bokhimi, A. Maldonado-Álvarez, A.E. Jiménez-González, Effect of Anatase Synthesis on the Performance of Dye-Sensitized Solar Cells. Nanoscale Res. Lett. 10, 306 (2015). https://doi.org/10.1186/s11671-015-0991-3
C. Kittel, Introduction to solid state physics, 8th edn. (Wiley, Hoboken, NJ, 2005)
J.W.P. Schmelzer, A.S. Abyzov, V.M. Fokin, C. Schick, E.D. Zanotto, Crystallization of glass-forming liquids: Maxima of nucleation, growth, and overall crystallization rates. J. Non-Cryst. Solids 429, 24–32 (2015). https://doi.org/10.1016/j.jnoncrysol.2015.08.023
V.M. Fokin, E.D. Zanotto, N.S. Yuritsyn, J.W.P. Schmelzer, Homogeneous crystal nucleation in silicate glasses: A 40 years perspective. J. Non-Cryst. Solids 352, 2681–2714 (2006). https://doi.org/10.1016/j.jnoncrysol.2006.02.074
D. Ma, J. Schneider, W.I. Lee, J.H. Pan, Controllable synthesis and self-template phase transition of hydrous TiO2 colloidal spheres for photo/electrochemical applications. Adv. Coll. Interface. Sci. 295, 102493 (2021). https://doi.org/10.1016/j.cis.2021.102493
J.H. Pan, Q. Wang, D.W. Bahnemann, Hydrous TiO2 spheres: An excellent platform for the rational design of mesoporous anatase spheres for photoelectrochemical applications. Catal. Today 230, 197–204 (2014). https://doi.org/10.1016/j.cattod.2013.08.007
T. Cosgrove, Colloid science: principles, methods and applications, 2nd edn. (Wiley, Chichester, U.K., 2010)
H. Ohshima, Electrostatic interaction between soft particles. J. Colloid Interface Sci. 328, 3–9 (2008). https://doi.org/10.1016/j.jcis.2008.08.009
S.G. Reed, M.T. Orr, C.B. Fox, Key roles of adjuvants in modern vaccines. Nat. Med. 19, 1597–1608 (2013). https://doi.org/10.1038/nm.3409
J.-D. Masson, M. Thibaudon, L. Bélec, G. Crépeaux, Calcium phosphate: a substitute for aluminum adjuvants? Expert Rev. Vaccines 16, 289–299 (2017). https://doi.org/10.1080/14760584.2017.1244484
A.T. Glenny, C.G. Pope, H. Waddington, U. Wallace, Immunological notes. XVII-XXIV. J. Pathol. 29, 31–40 (1926). https://doi.org/10.1002/path.1700290106
W. Wang, M. Singh, Selection of Adjuvants for Enhanced Vaccine Potency. WJV 01, 33–78 (2011). https://doi.org/10.4236/wjv.2011.12007
J.-L. Look, C.F. Zukoski, Colloidal Stability and Titania Precipitate Morphology: Influence of Short-Range Repulsions. J Am. Ceram. Soc. 78, 21–32 (1995). https://doi.org/10.1111/j.1151-2916.1995.tb08356.x
F. Huang, D. Chen, X.L. Zhang, R.A. Caruso, Y.-B. Cheng, Dual-Function Scattering Layer of Submicrometer-Sized Mesoporous TiO 2 Beads for High-Efficiency Dye-Sensitized Solar Cells. Adv. Func. Mater. 20, 1301–1305 (2010). https://doi.org/10.1002/adfm.200902218
B.D. Cullity, S.R. Stock, Elements of X-Ray Diffraction: Pearson New International Edition (Pearson Education, Limited, 2014)
A.E.J. González, S.G. Santiago, Structural and optoelectronic characterization of TiO 2 films prepared using the sol–gel technique. Semicond. Sci. Technol. 22, 709–716 (2007). https://doi.org/10.1088/0268-1242/22/7/006
Z.S. Wang, H. Kawauchi, T. Kashima, H. Arakawa, Significant influence of TiO2 photoelectrode morphology on the energy conversion efficiency of N719 dye-sensitized solar cell. Coord. Chem. Rev. 248, 1381–1389 (2004). https://doi.org/10.1016/j.ccr.2004.03.006
M.J. Hernández Rodríguez, E. Pulido Melián, O. González Díaz, J. Araña, M. Macías, A. González Orive, J.M. Doña Rodríguez, Comparison of supported TiO2 catalysts in the photocatalytic degradation of NOx. J. Mol. Catal. A Chem. 413, 56–66 (2016). https://doi.org/10.1016/j.molcata.2015.12.007
Y. Ding, I.S. Yang, Z. Li, X. Xia, W.I. Lee, S. Dai, D.W. Bahnemann, J.H. Pan, Nanoporous TiO2 spheres with tailored textural properties: Controllable synthesis, formation mechanism, and photochemical applications. Prog. Mater Sci. 109, 100620 (2020). https://doi.org/10.1016/j.pmatsci.2019.100620
V.K.H. Bui, T.N. Nguyen, V. Van Tran, J. Hur, I.T. Kim, D. Park, Y.-C. Lee, Photocatalytic materials for indoor air purification systems: An updated mini-review. Environ. Technol. Innov. 22, 101471 (2021). https://doi.org/10.1016/j.eti.2021.101471
M.A. Sánchez-García, X. Bokhimi, A. Maldonado-Álvarez, A.E. Jiménez-González, Effect of Anatase Synthesis on the Performance of Dye-Sensitized Solar Cells. Nanoscale Res. Let. 10, (2015). https://doi.org/10.1186/s11671-015-0991-3
I.G. Yu, Y.J. Kim, H.J. Kim, C. Lee, W.I. Lee, Size-dependent light-scattering effects of nanoporous TiO2 spheres in dye-sensitized solar cells. J. Mater. Chem. 21, 532–538 (2011). https://doi.org/10.1039/c0jm02606a
Y. Chen, F. Huang, D. Chen, L. Cao, X.L. Zhang, R.A. Caruso, Y.B. Cheng, Effect of mesoporous TiO2 bead diameter in working electrodes on the efficiency of dye-sensitized solar cells. Chemsuschem 4, 1498–1503 (2011). https://doi.org/10.1002/cssc.201100060
B. Vasanth, R. Govindaraj, P. Ramasamy, Microwave-assisted hydrothermal synthesis and characterization of TiO2 microspheres for efficient dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 33, 17660–17667 (2022). https://doi.org/10.1007/s10854-022-08629-4
M.U. Shahid, N.M. Mohamed, A.S. Muhsan, S.N. Azella Zaine, M. Khatani, A. Yar, W. Ahmad, M.B. Hussain, A.A. Alothman, M.S. Saleh Mushab, Graphene loaded TiO2 submicron spheres scattering layer for efficient dye-sensitized solar cell. Chemosphere 138009, (2023). https://doi.org/10.1016/j.chemosphere.2023.138009.
M.U. Shahid, N.M. Mohamed, A.S. Muhsan, S.N.A. Zaine, A. Yar, W. Ahmad, M.I. Irshad, M.B. Hussain, High-yield TiO2 submicron sphere/nanoparticle-blended scattering layer for efficient and scalable dye-sensitized solar cells. Emergent Mater. 6, 671–679 (2023). https://doi.org/10.1007/s42247-023-00467-2
A. Taleb, F. Mesguich, A. Hérissan, C. Colbeau-Justin, X. Yanpeng, P. Dubot, Optimized TiO2 nanoparticle packing for DSSC photovoltaic applications. Sol. Energy Mater. Sol. Cells 148, 52–59 (2016). https://doi.org/10.1016/j.solmat.2015.09.010
H.K. Singh, M. Saquib, M.M. Haque, M. Muneer, D.W. Bahnemann, Titanium dioxide mediated photocatalysed degradation of phenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid, in aqueous suspensions. J. Mol. Catal. A: Chem. 264, 66–72 (2007). https://doi.org/10.1016/j.molcata.2006.08.088
C.J. Lin, W.-T. Yang, C.-Y. Chou, S.Y.H. Liou, Hollow mesoporous TiO2 microspheres for enhanced photocatalytic degradation of acetaminophen in water. Chemosphere 152, 490–495 (2016). https://doi.org/10.1016/j.chemosphere.2016.03.017
Acknowledgements
The authors of this paper appreciate the financial support granted through the research projects DGAPA-PAPIIT-IT100821, CONAHCYT CF 2023 - I - 775, and LNC 2023-33 to cover the expenses required in the experimental part of this article. C. Rosiles Pérez and M. Ocampo Gaspar thank CONAHCYT for the postdoctoral fellowship. The authors thank R. Morán Elvira and M. L. Ramón García from IER-UNAM for the technical support provided to perform the FE-SEM and X-Ray Diffraction measurements, respectively.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design and development of this research article. The preparation of materials, data collection, analysis of results and support in writing the original draft was in charge of Rosiles-Pérez. The preparation of semiconductor materials and the design of the methodology was carried out by Maribel Ocampo Gaspar, Oscar de Jesús Padilla González, and Luis Fernando Román Flores. Conceptualization of the research strategy, analysis of results, proposal of models, obtaining funding, project administration, writing, review and editing of the manuscript by Antonio E. Jiménez-González. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
42247_2024_721_MOESM1_ESM.docx
Supplementary file1 (DOCX 856 KB) Online Resource 1. SEM images of hydrated TiO2 spheres synthesized at low values of Rw (a) 5.91 and b) 11.82) and at fixed values of 0.08 M Ti and MPA/Ti at 0.6 which is supplemental to that for hydrous TiO2 spheres obtained in the range of Rw from 17 to 118. Online Resource 2. SEM images of hydrous TiO2 spheres synthesized at a Ti precursor concentration of 0.08 M and a Rw of 82 with molar relations of MPA/Ti of a) 1, b) 0.75, c) 0.60 and d) 0.30. Scale bars: 2 µm.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rosiles-Perez, C., Ocampo Gaspar, M., Padilla González, O.J. et al. Size controlled synthesis of hydrous TiO2 spheres by a thiol structure directing agent and its application in photocatalysis and efficient DSSC cells. emergent mater. (2024). https://doi.org/10.1007/s42247-024-00721-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42247-024-00721-1