Abstract
The need to limit anthropogenic CO2 emissions and lower the atmospheric CO2 concentration makes CO2 conversion an imminent requirement. Availability of suitable facilities and prior understanding how electro and thermal catalysis work renders them as appealing platforms for conversion of CO2. Catalysts play a crucial part in the conversion of CO2 to chemicals in both processes. Catalysis is a process initiated by the interaction of reactants, intermediates, and products produced on the catalyst’s surface. Generally, higher temperatures in thermo-catalytic process or electrical potentials in electrocatalytic process are used to increase the reaction rate to get the desired results and to overcome the kinetic barrier. Several studies have been reported in both the processes with a desire to decrease the atmospheric CO2 concentration by stopping CO2 emissions at the site of generation itself. The viability of catalytic performance in both situations for the large-scale conversion of CO2 is still up for debate. In this review, we intend to focus on recent developments in CO2 conversion aided by diverse catalysts by analyzing and comparing proof-of-principle investigations on applied conditions, catalyst activity and stability for thermocatalytic and electrocatalytic CO2 conversions. The most common catalyst synthesis techniques employed in both experiments were analyzed. Primary goal of this review is to draw connections between the two fields in order to generate fresh insights that will lead to a more efficient and integrated CO2 conversion process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
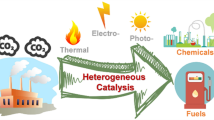
The atmospheric CO2 emissions after the industrial revolution and the rapid expansion of fossil fuel based system made it a prominent cause of global warming [1, 2]. Currently, the majority of energy utilized worldwide is produced by burning hydrocarbon fuels, which have low energy conversion efficiency and produce a lot of CO2 emissions [3, 4]. The reliance on fossil fuels has economic and geopolitical implications. Simultaneously, alternative, sustainable sources of energy cannot meet the fast increasing demand. However, predicting the precise future implications of anthropogenic climate change is challenging, the scientific community agrees that limiting CO2 emissions is very desirable [5]. We emphasize that using CO2 to make fuels and chemicals does not remove any net CO2 from the atmosphere, but it can replace the use of fossil fuels and could potentially lower net CO2 emissions. The carbon that is used to create chemicals and fuels can be thought of as coming from CO2, which is the eco-friendliest source. Between 1 and 4.2 gigatons of CO2 could potentially be used as fuels each year, according to estimates [6]. With the rising demand for fossil fuels and chemicals made from fossil feedstock’s, CO2 capture and utilization could therefore be important for assisting in meeting global emission targets. Currently, most CO2 chemical conversion research is focused on thermocatalytic [7, 8] and electrocatalytic reactions [9, 10]. CO2 electrocatalytic conversions are most frequently carried out on a smaller scale, which is preferable for the limited CO2 conversion to fine compounds. Whereas thermocatalytic conversions are more viable for large scale applicability due of its simple process equipment, minimal expense and ease of industrial expansion [11]. Since using captured CO2 to create synthetic chemicals and fuels is an appealing way to cut CO2 emissions, numerous research groups throughout the world are interested in this subject [12]. Figure 1 shows the possible products that could be generated through the conversion of CO2 via electrocatalysis and thermocatalysis. Carbon dioxide (CO2) is electrochemically converted into a wide range of products, such as formic acid (HCOOH), methanol (CH3OH), carbonmonoxide (CO), and methane (CH4), in aqueous conditions by the use of H2O as an H2 source has been the subject of extensive investigation [13,14,15,16,17]. This strategy seems appealing because it simply requires water, CO2 and electricity as an energy source. However, the CO2 conversion process via electrochemistry uses electricity inefficiently [18]. Low CO2 solubility in water and significant diffusion constraints place restrictions on this process [19]. Thermocatalytic conversion is an alternate strategy that combines a heterogeneous catalyst and high temperatures together to provide quick reaction rates and, as a result, enable high volume production [20, 21]. But it was found from the study that in order to obtain the requisite kinetics and thermodynamics for thermocatalytic CO2 conversion on conventional catalysts, high temperatures are needed, which has a substantial impact on equipment and operating expenses [22]. For the thermocatalytic process, a H2 source is required for the CO2 conversion into fuels and chemicals.11 Methane-dry-reforming (MDR), reverse-water–gas-shift (RWGS), CO2 methanation, and CO2 hydrogenation are a few thermocatalytic techniques that can be used to convert CO2.8 Syngas (predominantly H2 and CO in combination) is produced when methane (CH4) reacts with CO2 in the process of methane-dry -reforming (MDR) [23,24,25]. The reverse-water–gas-shift-process (RWGS) [26,27,28,29], a potential path for CO2 hydrogenation, is another method of producing syngas. Syngas is a valuable feedstock for chemicals that can be employed in Fischer–Tropsch synthesis (FTS) to further convert to fuels [30, 31]. CO2 hydrogenation is a method for transforming CO2 to methanol and other hydrocarbons like lower olefins, aromatics, gasoline, and petroleum gas [32,33,34]. Another method for converting CO2 into CH4 with the help of hydrogen (H2) is CO2 methanation [35,36,37]. The thermocatalytic method for producing dimethyl carbonate (DMC) from CO2 and methanol with a novel designed catalyst is an effective way to reduce CO2 emissions [1]. There are a number of benefits to electrocatalytic CO2 conversion, including the ability to create desired reaction products. By modifying the catalyst's structure and applied voltage, Hori et al. were able to report around twelve products from electrochemical CO2 reduction on copper-based catalyst [38]. But in the case of copper catalyst, the system becomes complex due to the synthesis of numerous products. However, researchers were able to successfully produce selective product synthesis of ethylene (gas) and ethanol (liquid) on copper catalyst by considering the additional factors such as electrolyte pH, membrane, electrochemical reactor, catalyst design, and environment [39]. Numerous investigations have shown the making of a single HCOOH product through electrochemical CO2 reduction, utilizing suitable catalysts such as zinc (Zn), tin (Sn), and lead (Pb) [40,41,42]. The prospective technique is the production of HCOOH as the sole product of CO2 electroreduction because the heavy demand in pulp, paper, and pharmaceutical industries [43]. We can produce any of the above products of our interest in the case of electrocatalysis, however, there are advantages and disadvantages to both thermocatalysis and electrocatalysis for CO2 conversion. In order to make connections between the two fields and produce new ideas that will result in a more effective CO2 conversion process, we will have to compare and contrast the benefits and drawbacks of thermocatalysis and electrocatalysis for a CO2 conversion, which is explored in this review along with a discussion on the reactors design in both the catalytic systems.
2 Thermocatalysis and electrocatalysis of CO2 conversion
In a practical situation, catalysts decrease the energy required for conversion of an unreactive CO2 into a suitable product by chemically activating it. The process of changing the rate of a chemical reaction by introducing a catalyst is called catalysis. The two most useful catalytic CO2 conversion processes are electrocatalysis, which involves the CO2 dissolved in electrolytes reacts on a surface of electrode at ambient conditions and applied voltage, and thermocatalysis, which involves passing gases (CO2 and other gases) over a catalyst at adequate pressures and temperatures [44]. In Fig. 2, we outline the salient features of the electrocatalytic and thermocatalytic CO2 conversion technologies, highlighting the variations in the reaction pathways, end products, advantages, and disadvantages of each approach.
When CO2 is converted via electrocatalysis, the electrode (catalyst) is subjected to an applied voltage that generates electrons & ions, which are considered as reactants in the electrochemical reaction alongside CO2 [45]. The electrochemical system needs an electrolyte to move ions, conductors to move electrons. To achieve a high yield and desired product selectivity, the catalyst selection, voltage, and electrolyte are all essential. In electrocatalysis, the reaction occurs at the catalyst-electrolyte interface of two different electrode surfaces; initially, oxidation takes place at the anode-catalyst surface, and then ions are transferred from the anode to the cathode surface, where a second reaction occurs that produces the desired products [46, 47]. In thermocatalytic conversions, high temperatures are required for the reaction and there are multiple approaches for the CO2 conversion with high product outputs considered as a best conversion technique when compared with electrocatalytic process [44]. However, the process has drawbacks such as excessive energy consumption, poor selectivity, instability, and coke production [48]. The variety of possible products, some of which may be thermodynamically favorable but exhibit slow kinetics, such as the Sabatier reaction (methanation) or the production of methanol and dimethyl ether (DME), or thermodynamically not favorable for partial reduction to CO, makes thermocatalytic CO2 reduction very challenging. In general, high reaction temperatures are required for a high CO2 activation barrier, whereas high H2 pressures are necessary for a H2 activation; both of these factors reduce energy efficiency. Additional difficulties with material design include: The co-existence of enough active sites for each reactant is necessary for CO2 reduction because it necessitates molecular hydrogen breakdown into H* adatoms close to an activated COx species [49]. The advantages of the electrocatalytic method are the ability to conduct at ambient temperature, high yield, and selectivity while requiring less initial investment. The drawbacks of this method include limited CO2 adsorption, strong hydrogen evolution with significant over potential, and the generation of numerous products [6]. In the electrocatalytic CO2 conversion process, it is challenging to activate CO2 molecule, which is a chemically inert molecule with linear chemical linkages. It is difficult to electrocatalytically transform CO2 into desirable products. High over potential, low selectivity, and unable to endure intense catalytic activity for an extended period of time are common issues with electrocatalytic CO2 conversion. Additionally, it is necessary to stop the hydrogen evolution reaction (HER), a side reaction. Despite being a tremendously important energy source, in order to exploit the production of energy-demanding carbon-based fuels, the applied potential energy for the reaction must be used for a CO2 conversion reaction rather than for H2 evolution reaction. To increase the selectivity, stability and decrease over potential, it is crucial to use the right electrocatalysts [50]. Selectivity is a constant problem in both processes, and a number of methods have been developed to address these issues by changing the catalytic system's intrinsic or extrinsic characteristics in order to control reaction activity and selectivity. For example, changes to the electrocatalysts surface structure, particle size, roughness factor, and composition are all part of the intrinsic properties of the catalysts that are being monitored [4,5,6]. Similar to this, the scientific community has determined extrinsic factors that affect the reactions activity and selectivity. These variables include the electrolyte cation, molecular additives, electrode voltage, and pH in case of electrocatalytic process and temperature, reactants concentration, flowrate, pressure in thermocatalytic process [6]. Separation is a big problem in the electrocatalytic process since some catalysts generate several products. However, researchers have started working on single product yields on specific catalysts, such as Sn, Pb, and Zn catalysts, in an attempt to tackle the problem [16, 41,42,43]. Because a mixture of hydrocarbons and oxygenates is generated depending on the catalyst, the Fischer–Tropsch synthesis of hydrocarbons from CO2 suffers from very poor yields of any one product. Here, separation is a problem that is resolved by choosing a certain catalyst for high product selectivity and to limit the other side reactions [6]. Numerous research has focused on the yield of electrocatalytic reaction of a targeted product, although efforts are underway to scale up the process [9, 10, 51].
2.1 Various catalysts synthesis techniques in thermocatalytic CO2 conversion
In thermocatalytic conversion, supported catalysts are essential. When making catalysts for the thermocatalytic pathway, the preparation process is a crucial factor to take into account. The crystal structure, metal dispersion, catalytic activity, and allowed metal loading can all be impacted by the methods utilized to combine metal with its support. Catalysts for thermocatalytic CO2 conversion processes were made using a various synthetic approaches [52]. The following is a list of some of the methods for synthesizing catalysts. In our discussions, some selected metals (e.g., Cu and Zn) are frequently used to represent the active sites; similarly, specific oxides (e.g., Al2O3 and SiO2) are used as support powders, however the methods can be treated as general and extend to synthesize other metals/oxide systems as well.
3 Precipitation method
Nitrate salts of active metals (e.g. copper (Cu) and zinc (Zn)) are dissolved in deionized water and mixed vigorously with support (e.g., Al2O3 and SiO2) powders in the precipitation process [53, 54], the NaOH-containing aqueous solution is added drop by drop. The pH was maintained at a typical level of 9.0 throughout the precipitation. Finally, the product is aged at room temperature, while being stirred; then filtered, followed by washing with deionized water, and thereafter the filter-cakes are being dried overnight. The obtained catalysts are subjected to a calcination process before to catalytic runs [53]. A general synthesis of metal catalyst by a precipitation method is schematically show in Fig. 3a.
4 Impregnation method
The impregnation method is one of the most frequently used method to produce heterogeneous catalysts. Because of its inexpensive instrumentation requirements, simple to follow technical steps, and minimal waste, this preparation method has been applied for a diverse set of catalytic systems [55]. Figure 3b illustrates the impregnation method procedure for making metal catalysts. The Cu and Zn cation containing salt solution is added to the Al2O3 and SiO2 supports during the impregnation method, then the mix is vigorously stirred for hours at room temperature. After that, water is evaporated using a rotating evaporator with low pressure. The prepared catalysts are sent for a calcination prior to performing catalytic reaction [53].
5 Micro-emulsion method
The micro emulsion method has been regarded as the best way to create very thermally stable organic and inorganic nanomaterial catalysts [56]. The catalyst produced using this technique has been reported to enhance CO2 methanation by incorporating a large surface area and extremely high metal phase dispersion [57]. This approach involves the use of liquid solutions that are isotropic and comprise oil, water, and a combination of co- and surfactants. Metal salts and other substances make up the majority of the aqueous phase, the oil is mainly a blend of olefins & hydrocarbons. Both water-in-oil and oil-in-water micro emulsions come into one of two categories, with the solute coming under the first category and the solvent/dispersion medium coming under the second [52]. As an example, highly dispersed aggregate of magnesium and palladium in silica are reported to act as a catalyst was prepared by means of a reverse micro emulsion synthesis [58]. Catalyst synthesis using the Microemulsion method is shown schematically in Fig. 4a.
6 Sol–gel method
Numerous nanostructures, in particular metal oxide nanoparticles, have been produced using the sol–gel method [59]. Figure 4b illustrates the Sol–gel method procedure for making metal catalysts. This method entails the dissolving of molecular precursor (metal alkoxide) in water/ROH, heated, and stirred until it gels [60]. Due to the gel’s wet/moist nature produced during the hydrolysis or alcohololysis process, it must be dried correctly based on the gel's intended use and desired properties. Further, the produced gels are crushed and then calcined [60].
7 Combustion method
The solution combustion method (SCM) has been widely used to prepare nanocrystalline materials and catalysts because of being an easy, cost-effective technology [61]. The combustion approach has been applied to produce an array of metal/metal oxide (Ni/ZrO2) catalysts with metal loading and various combustion methods, including glycerol, urea, ethanol, glycol, and n-propanol [62]. Metal ion nitrate salts in combination with a deionized water are combined and agitated for half an hour at room temperature, the mixture thereafter placed in a quartz tank. Later, the mix is heated to 550 °C in a tube furnace and kept there for 4 h before being cooled to room temperature to get a Ni/ZrO2 catalysts [62]. Schematic of Metal catalyst synthesis using a combustion method was shown in Fig. 4c.
7.1 Various catalysts synthesis techniques in electrocatalytic CO2 conversion
A significant and crucial part of electrocatalytic CO2 conversion is played by catalysts acting as electrodes. There are a many number of metallic catalysts that can catalyze the CO2 conversion reaction [63]. Different methods of synthesis have been applied for producing catalysts for electrocatalytic CO2 conversion systems. Some of the methods employed to synthesize the catalysts are listed below.
8 Electrodeposition method
By allowing catalysts to grow directly on conductive substrates, numerous techniques have been used for making electrodes to date. For widespread application, electrodeposition stands out among them as being inexpensive and environmentally friendly [64]. An externally applied potential forces current to flow as a result of the movement of ions (positive & negative) in the electrolyte solution, and a coating film is formed on the electrode by the redox reaction of electron gain. This process is known as electrochemical deposition [16]. Figure 5a illustrates the electrodeposition method procedure for the metal catalysts synthesis. In this process, the anode metal can be oxidized to generate a metal ion in the solution, and a metal coating layer on the cathode may result from the reduction of metal ions. The metal ion reacts with the electron that is produced via oxidation to cause the deposition on the cathode. The current flowing through the electrodes will balance the overall charge [42, 65]. Numerous studies have been published on the electrodeposition method of producing catalysts for electrocatalytic CO2 conversion [16, 65, 66]. Replacing the solar energy with electrical energy for the catalyst synthesis, it has been reported to synthesize Sn-based catalysts using the solar electrodeposition approach in order to convert CO2 to HCOOH electrocatalytically [67].
9 Sputtering method
Sputtering is the process of high-energy plasma or gas particles striking solid surfaces. Sputtering is believed to be a successful method for creating thin nanomaterial films [68]. Sputtering commonly takes place in an evacuated chamber that has been injected with sputtering gas as shown in Fig. 5b. Gas ions are generated when gas and free electrons collide while being exposed to a high-voltage cathode. The cathode target is continuously struck by the strongly accelerating ions in an electric field that are positively charged, causing the atoms to be ejected from the surface of the target [69]. To convert CO2 electrocatalytically, Cu and Cu-C, two distinct Cu-based cathode catalysts have been reported to be prepared by means of a sputtering method [70].
10 Hydrothermal synthesis
The hydrothermal synthesis is a good method for producing large amounts of catalyst with high purity [71]. The metal salt is initially dissolved in an aqueous water/ethylene glycol solution while stirring continuously at higher temperatures for a longer period of time to produce a metal salt solution [72]. Then the molar concentration of precursor is prepared under vigorous stirring. By adding the precursor solution drop by drop to the metal salt solution while stirring continuously for an extended period to obtain a transparent solution. After that, the mixture is placed into an autoclave and maintained for at a higher temperature, and the powder is further separated from the solution by centrifugation. The product is dried in a vacuum oven to generate the required metal catalyst [71, 72]. Figure 5c illustrates the catalysts synthesis by a hydrothermal method. The authors reported the hydrothermal synthesis of copper-based catalysts for the electrocatalytic conversion of CO2 into CO and CH4 products [71, 73].
11 Comparison of several CO2 conversion catalysts through thermo- and electrocatalysis
Catalysts play a significant part in the thermocatalytic and electrocatalytic conversion reactions. Several catalysts have been employed to convert CO2 via thermocatalytic and electrocatalytic processes has been reported in a number of studies [6, 46, 52]. Certain catalysts for CO2 conversion have been discovered to be effective in both thermocatalysis and electrocatalysis [6]. In order to convert CO2 to CH3OH, copper is generally regarded as the best electrocatalyst and thermocatalyst. The CO2 gives numerous products on the copper catalyst with higher CH3OH efficiency in the electrocatalytic process [74], whereas CO2 hydrogenation in thermocatalysis yields CH3OH as a primary product with high efficiency [75]. Similarly, in both catalysis processes, the Pd alloy catalyst is thought to be the most effective in converting CO2 to HCOOH; nevertheless, the thermocatalytic method exhibits better efficiency than the electrocatalytic approach [4, 76]. The production of CO was frequently achieved by the Au alloy in both catalysis process [6, 77]. The possibility of highly desirable methods for recycling CO2 into various chemicals is made possible by the thermocatalytic conversion of CO2 to produce multiple products. Methane, carbon monoxide, formic acid and methanol are just a few examples of the many products that can be formed based on the catalyst choice and the reaction conditions. The findings on many catalysts for thermocatalytic CO2 conversion are shown in Table 1, and offer proof-of-concept support for the effectiveness of these catalysts in selectivity to various products [6]. Numerous electrocatalysts for the electrocatalytic CO2 reduction process have been reported to make a range of products.
The mechanism for the thermocatalytic hydrogenation of CO2 to produce several products, such as CH3OH, CO, and HCOOH on thermo catalyst, was illustrated in Fig. 6. The mechanism showed that formate route favors formation of products from the conversion of CO2 [1, 8]. Adsorbed CO2 radical absorbs protons and transforms them into formate ions, which then absorb H + and create HCOOH or further splits into CO and OH ions, taking a proton to make CO and stripping away H2O, and CO desorbs into gaseous form. or CO absorbs protons to create COH*, which then undergoes hydrogenation to produce HCOH* and H2COH*. The methoxy (H2COH*) with proton generated CH3OH in the form of gas. [8]. Further CO2 can react with CH3OH to form dimethyl carbonate [1].
A review of some of the most effective and selective electrocatalysts for a particular product may be found in Table 2. CO and HCOOH are produced electrochemically by the two-electron transfer reaction on diverse electrocatalysts with remarkable faradaic efficiencies and low over potentials, whereas in the case of CO2 conversion to methane, alcohols and ethylene exhibit significantly larger over potentials and lower selectivity’s [95]. Carbonates and bicarbonates of potassium & sodium are most frequently used liquid electrolytes in the reaction along with K2SO4, Nafion, KBr and KCl respectively [4]. Most experiments show higher faradaic efficiencies and selectivity; hydrocarbons were primarily detected in the presence of copper electrocatalyst [5]. Figure 7 showed the mechanism for the electrocatalytic reduction of CO2 to yield a number of products, including CH3OH, HCHO, and HCOOH, as well as C2H5OH on copper catalyst [65]. The primary causes of the formation of different products from the electrocatalytic CO2 conversion are the type of catalyst and addition of protons and electrons to the intermediate steps [42]. The reduction of CO2 is shown by the mechanism, which begins with the acceptance of an electron from Cu2O and adsorbing on it to generate a CO2 free radical. Next, a electron from Cu2O is accepted and certain internal arrangements are made to form COads/CO. As a result, CO is released from the cathode's surface. By absorbing protons and electrons from the anode, COads will further contribute to the process and create various products, primarily ethanol [65].
12 Comparison of catalyst deactivation and mitigation in thermocatalytic and electrocatalytic CO2 conversion
The commercialization of a catalytic process depends heavily on catalyst longevity, and which affects overall sustainability, process design, reactors choice, and economic viability [49]. Though theoretically catalysts in their reaction environment are not expected to change, however, over some intervals of time, catalyst deactivation always becomes apparent, frequently preventing commercial viability [117].
12.1 Catalyst deactivation and mitigation in thermocatalytic CO2 conversion
Deterioration of the metallic constituent due to sintering/coking/poisoning and/or oxidation, as well as physical & chemical alterations in the supporting materials are the most frequent causes of thermocatalytic reaction deactivation [49, 118]. For catalytic applications, the tiny metal catalysts are typically loaded on carbon/metal oxide supports. The congregation of the metals in these applications can also exhibit some degree of motilities, which is commonly referred to as the sintering of small metals in thermocatalysis [119]. Overall, sintering refers to the process of metal particle growth in a reactive environment, which can happen by particle migration or agglomeration [49]. Decreasing the catalytic reaction temperature slows down the formation kinetics of nano metals by lowering their diffusion coefficient of metallic components, which is a strategy for preventing sintering [120]. Another reason for deactivation in thermocatalysis involving carbon-containing reactants is coking, in which the tightly bonded carbon poisons the metal's surface. Low temperature operation, pore hierarchy, and oxygen/steam introduction can significantly reduce coking. In some cases, the high catalyst activity made from crystalline frameworks allows for lower temperature CO2 reduction, which could lessen coking [49]. Impurities in feeds have historically been linked to poisoning, and common instances include the sulfur/carbon-monoxide poisoning of metals [117]. Controlling the poisoning of metals by intermediates, reversibly bound reactants, or products is challenging. Developing multifunction thermocatalyst that combine catalytic sites for CO2 reduction with the selective binding and molecular sieving properties of crystalline frameworks for eliminating SOx and NOx poisons can be an effective strategy [49]. Another deactivation issue in thermocatalysis is oxidation, where the oxidation of a metal might even completely oxidize the nanoparticle, changing its structure and efficiency. The optimal reaction temperature must be chosen for the best possible combination of catalyst activity and stability, since thermocatalysis uses higher temperatures, which promotes rapid oxidation [119].118 The ongoing involvement of the catalysts for metal organic frameworks in a thermocatalytic reaction modifies the physical–chemical features of a catalyst elements [49]. Catalysts deactivate as a result of such modifications to their characteristics. Higher reaction temperatures impact a catalyst's physical and chemical characteristics, which affects the activity and selectivity of the catalyst [120]. A careful selection of suitable metal catalysts with a high melting point is necessary for greater activity and stability. The creation of an exterior, metal oxide-based ''shell,'' which shields the particle from the harsh environment, is another technique reported for stabilizing metal nanoparticles [118].
12.2 Catalyst deactivation and mitigation in electrocatalytic CO2 conversion
It is well-known that during electrochemical CO2 reduction, the catalyst deactivates [121]. Impurities in the input stream or electrolyte are the primary reasons for catalyst deactivation. Electrochemical cell performance is influenced by the electrode, electrolyte, CO2 concentration, and operating conditions [16]. However, the catalyst utilized in the reaction significantly influences the selectivity and activity [6]. The onset reduction potential and the Faradaic efficiency are typically taken into account when evaluating catalytic activity, while changes in performance of catalyst with increasing electrolysis time are typically used to evaluate catalyst stability [122]. Regarding the catalyst’s stability, the problem of deactivation has frequently been discussed; the primary causes are the development of harmful intermediates and the accumulation of inert constituents on electrode’s surface [123]. Hori et al. suggested a number of potential causes for catalyst deactivation, which are linked with heavy metal impurities found in reagent used for electrolyte solution, the organic compounds that may be present in water, and adsorption of intermediate poisoning species/products created during CO2 conversion on electrodes [124]. In addition to this, the mode and circumstance of electrolysis can impact catalyst stability [122, 125]. However, the appearance of the catalyst surface following electrochemical reaction is not common, which implies that deactivation also depends on the experimental strategy utilized by the various research groups and not necessarily entirely on the intermediates or products generated during the reaction [124]. Several studies reported that catalyst is poisoned by the deposition of contaminants that were initially present in the solution. However, in some cases, a pre-electrolysis of electrolyte solution before the electrochemical reaction prevents such deactivation in CO2 reduction [124, 126]. Consequently, the important factors need to be considered in order to mitigate the deterioration of catalyst stability/activity are the influence of catalyst type, structure, composition, and operating conditions [122].
13 Integrating thermo- and electrocatalysis for effective CO2 conversion
It is difficult to distinguish between reactions that are triggered by high temperatures or electric potential because certain materials used as catalysts behave differently in thermo- and electrocatalytic conditions. Although there are instances when knowledge is shared between the two platforms, the individual scientific endeavors largely function independently. In electrocatalytic processes, the electro-metal-solution interface may modify the reaction conditions affecting the reaction kinetics and that usually is not well understood in the case in thermo-catalytic reactions [22]. Nonetheless, with the advent of advanced tools like in-situ TEM and near ambient pressure XPS, it is anticipated that the local conditions near catalyst surface-reactant interface vary considerably during the course of reaction resulting in change in catalyst surface along with near-surface environment. Various Cu–Zn based catalysts have been studied for the CO2 conversion in both thermal CO2 hydrogenation and electrochemical CO2 reduction reactions [127,128,129,130]. Cu-Zr based composites employed in the synthesis of CH3OH in the hydrogenation reaction and HCOOH in the electrochemical reaction [131, 132]. The electrocatalytic approach that creates HCOOH and the hydrogenation process that produces CO are studied using Bi-In based catalysts [133, 134]. Because the aforementioned catalysts exhibit distinct product distributions in both reaction settings, it is challenging to directly compare the two approaches. For instance, even though multiple common catalysts have been used but at least one different supported catalyst (CuZn/C, CuZnO/Al2O3) was used in both processes [130, 135]. Investigations using identical reaction conditions and a common catalyst material can provide useful information for revealing commonalities and contrasts about the two systems reaction kinetics and mechanism. Koshy et al. examined the Ni-C based catalyst in both reaction techniques, although different reactors and applied conditions were utilized, it was observed that the catalyst is effective for the CO generation in both the techniques, whereas a higher CO formation rate was noticed in electrocatalytic method than the thermocatalytic method [22]. A systematic study utilizing similar catalysts in both the catalytic methods (thermal and electro) can provide deeper insights for quantitative comparison of the two reaction settings.
14 Summary and perspective
This review provides a thorough summary of decades of history and current trends in the catalysis of CO2 reduction to aid in the study and advancement of CO2 thermo-electroreduction. Thermo- or electrocatalysis can offer an appealing and sustainable solution to the challenge of combating climate change associated with CO2 emissions by converting it to various chemicals and fuels. Although slow kinetics or thermal equilibrium can restrict certain reactions, the thermocatalytic method can easily be applied at a larger scale to achieve industrially significant results. In comparison, the electrocatalytic method of CO2 reduction is far less developed but has certain benefits, including low temperature and pressure, the use of water as a hydrogen source. A thorough study has been conducted on catalysts, impact of reactor setup, and other applied conditions for CO2 thermo-electroreduction. The generation of various products by CO2 reduction has often seen thermocatalytic methods advance more than electrocatalytic methods. Although many developed catalysts have been utilized to accelerate the production of various products and achieve high selectivity, it is difficult to meet the demands of commercial applications due to high over potential, low current density, low stability in electrocatalysis, modifications in the crystalline structure during reaction, sintering, coking, poisoning, oxidation and low-stability in thermocatalysis. Various catalysts explored and reported in the thermocatalytic and electrocatalytic CO2 conversion studies are summarized. To make it easier for readers to get the information they need for the catalyst synthesis, the most commonly used catalyst synthesis methods from both the studies have been summarized and presented in schematic forms. Furthermore, we presented the reaction conditions, selectivity, products and reactors employed together with the results of several catalysts for thermo- and electrocatalytic CO2 reduction in tables. In both studies, a summary of the various catalyst deactivation causes and methods to mitigate the deactivation along with studies on integrating the two approaches for efficient CO2 conversion are also provided.
Data availability
The manuscript contains all the necessary data.
References
S. Chaemchuen, O.V. Semyonov, J. Dingemans, W. Xu, S. Zhuiykov, A. Khan, F. Verpoort, Chem. Afr. 2, 533–549 (2019). https://doi.org/10.1007/s42250-019-00082-x
W. Xi, P. Yang, M. Jiang, X. Wang, H. Zhou, J. Duan, M. Ratova, D. Wu, Appl. Catal. B Environ. 341, 123291 (2024). https://doi.org/10.1016/j.apcatb.2023.123291
A. Ashok, A. Kumar, M. A. S. Saad, M. J. Al-Marri, J. CO2 Util 53, 101749 (2021). https://doi.org/10.1016/j.jcou.2021.101749
P. Duarah, D. Haldar, V.S.K. Yadav, M.K. Purkait, J. Environ. Chem. Eng. 9(6), 106394 (2021). https://doi.org/10.1016/j.jece.2021.106394
A.R. Woldu, Z. Huang, P. Zhao, L. Hu, D. Astruc, Coord. Chem. Rev. 454, 214340 (2022). https://doi.org/10.1016/j.ccr.2021.214340
S. Das, J. Perez-Ramirez, J. Gong, N. Dewangan, K. Hidajat, B.C. Gates, S. Kawi, Chem. Soc. Rev. 49(10), 2937–3004 (2020). https://doi.org/10.1039/c9cs00713j
P. Ebrahimi, A. Kumar, M. Khraisheh, Int. J. Hydrogen Energ. 47(97), 41259–41267 (2022). https://doi.org/10.1016/j.ijhydene.2021.12.142
M.I. Alam, R. Cheula, G. Moroni, L. Nardi, M. Maestri, Catal. Sci. Techno. 11(20), 6601–6629 (2021). https://doi.org/10.1039/d1cy00922b
X. Yan, C. Duan, S. Yu, B. Dai, C. Sun, H. Chu, Renew. Sust. Energ. Rev. 190, 114086 (2024). https://doi.org/10.1021/acscatal.0c04887
L. Fan, C. Xia, P. Zhu, Y. Lu, W. Haotin, Nat. Commun. 11, 3633 (2020). https://doi.org/10.1038/s41467-020-17403-1
J. Zhang, Z. Li, Z. Zhang, K. Feng, B. Yan, Appl. Energy 281, 116076 (2021). https://doi.org/10.1016/j.apenergy.2020.116076
B. Kumar, J.P. Brian, V. Atla, S. Kumari, K.A. Bertram, R.T. White, J.M. Spurgeon, Catal. Today 270, 19–30 (2016). https://doi.org/10.1016/j.cattod.2016.02.006
J. Agarwal, T.W. Shaw, H.F. Schaefer, A.B. Bocarsly, Inorg. Chem. 54(11), 5285–5294 (2015). https://doi.org/10.1021/acs.inorgchem.5b00233
Y. Linghu, T. Tong, C. Wu, J. Physic. Chem. C 127(31), 15035–15042 (2023). https://doi.org/10.1021/acs.jpcc.3c02034
I. Ganesh, Renew. Sust. Energ. Rev. 31, 221–257 (2014). https://doi.org/10.1016/j.rser.2013.11.045
V.S.K. Yadav, M.K. Purkait, New J. Chem. 39(9), 7348–7354 (2015). https://doi.org/10.1039/c5nj01182e
C.A. Obasanjo, G. Gao, J. Crane, V. Golovanova, F.P. Garcia de Arquer, C.T. Dinh, Nat. Commun. 14(1), 3176 (2023). https://doi.org/10.1038/s41467-023-38963-y
M. Gattrell, N. Gupta, A. Co, Energy Convers. Manag. 48(4), 1255–1265 (2007). https://doi.org/10.1016/j.enconman.2006.09.019
X. Lu, D.Y.C. Leung, H. Wang, M.K.H. Leung, J. Xuan, ChemElectroChem 1(5), 836–849 (2014). https://doi.org/10.1002/celc.201300206
C.H. Vo, C. Mondelli, H. Hamedi, J. Perez-Ramirez, S. Farooq, I.A. Karimi, A.C.S. Sustain, Chem. Eng. 9(31), 10591–10600 (2021). https://doi.org/10.1021/acssuschemeng.1c02805
D. S. A. Simakov, (Springer Briefs in Energy, 2017), pp 1-75, https://doi.org/10.1007/978-3-319-61112-9
D.M. Koshy, S.S. Nathan, A.S. Asundi, A.M. Abdellah, S.M. Dull, D.A. Cullen, D. Higgins, Z. Bao, S.F. Bent, T.F. Jaramillo, Angew. Chem. Int. Ed. 60(32), 17472–17480 (2021). https://doi.org/10.1002/anie.202101326
Q. Zhu, H. Zhou, L. Wang, L. Wang, C. Wang, H. Wang, W. Fang, M. He, Q. Wu, F.S. Xiao, Nat. Catal. 5(11), 1030–1037 (2022). https://doi.org/10.1038/s41929-022-00870-8
E. le Sache, T.R. Reina, Prog. Energy Combust. Sci. 89, 100970 (2022). https://doi.org/10.1016/j.pecs.2021.100970
V. Danghyan, A. Kumar, A. Mukasyan, E.E. Wolf, Appl. Catal. B: Environmental 273, 119056 (2020). https://doi.org/10.1016/j.apcatb.2020.119056
Q. Zhang, M. Bown, L., Pastor-Perez, M. S., Duyar and T. R. Reina, Ind. Eng. Chem. Res. 61(34), 12857–12865 (2022). https://doi.org/10.1021/acs.iecr.2c00305
M. Zhu, Q. Ge, X. Zhu, Trans. Tianjin Univ. 26(3), 172–187 (2020). https://doi.org/10.1007/s12209-020-00246-8
Y. A., Daza and J. N. Kuhn, RSC Adv. 6(55), 49675–49691 (2016). https://doi.org/10.1039/c6ra05414e
M. Gonzalez-Castano, B. Dorneanu, H. Arellano-Garcia, React. Chem. Eng. 6(6), 954–976 (2021). https://doi.org/10.1039/d0re00478b
Y.H. Choi, Y.J. Jang, H. Park, W.Y. Kim, Y.H. Lee, S.H. Choi, J.S. Lee, Appl. Catal. B Environ. 202, 605–610 (2017). https://doi.org/10.1016/j.apcatb.2016.09.072
A.D.N. Kamkeng, M. Wang, Chem. Eng. J. 462, 142048 (2023). https://doi.org/10.1016/j.cej.2023.142048
P. Sharma, J. Sebastian, S. Ghosh, D. Creaser, L. Olsson, Catal. Sci. Tech. 11(5), 1665–1697 (2021). https://doi.org/10.1039/d0cy01913e
M. Bowker, ChemCatChem 11(17), 4238–4246 (2019). https://doi.org/10.1002/cctc.201900401
G. Leonzio, E. Zondervan, P.U. Foscolo, Int. J. Hydrog. Energy 44(16), 7915–7933 (2019). https://doi.org/10.1016/j.ijhydene.2019.02.056
D. Schmider, L. Maier, O. Deutschmann, Ind. Eng. Chem. Res. 60(16), 5792–5805 (2021). https://doi.org/10.1021/acs.iecr.1c00389
A. Tripodi, F. Conte, I. Rossetti, Energ. Fuel 34(6), 7242–7256 (2020). https://doi.org/10.1021/acs.energyfuels.0c00580
J. Ashok, S. Pati, P. Hongmanorom, Z. Tianxi, C. Junmei, S. Kawi, Catal. Today 356, 471–489 (2020). https://doi.org/10.1016/j.cattod.2020.07.023
Y. Hori, I. Takahashi, O. Koga, N. Hoshi, J. Mol. Catal. A: Chem. 199(1–2), 39–47 (2003). https://doi.org/10.1016/S1381-1169(03)00016-5
L. Zaza, K. Rossi, R. Buonsanti, ACS Energy Lett. 7(4), 1284–1291 (2022). https://doi.org/10.1021/acsenergylett.2c00035
J. Tian, M. Wang, M. Shen, X. Ma, Z. Hua, L. Zhang, J. Shi, Chemsuschem 13(23), 6442–6448 (2020). https://doi.org/10.1002/cssc.202002184
V.S.K. Yadav, M.K. Purkait, RSC Adv. 6(47), 40916–40922 (2016). https://doi.org/10.1039/c6ra04549a
V.S.K. Yadav, M.K. Purkait, RSC Adv. 5(50), 40414–40421 (2015). https://doi.org/10.1039/c5ra05899f
V.S.K. Yadav, M.K. Purkait, Energ. Fuel 30(4), 3340–3346 (2016). https://doi.org/10.1021/acs.energyfuels.6b00047
B.M. Tackett, E. Gomez, J.G. Chen, Nat. Catal. 2(5), 381–386 (2019). https://doi.org/10.1038/s41929-019-0266-y
J. Gao, S. Choo Sze Shiong and Y. Liu, Chem. Eng. J. 472, 145033 (2023). https://doi.org/10.1016/j.cej.2023.145033
G. Wang, J. Chen, Y. Ding, P. Cai, L. Yi, Y. Li, C. Tu, Y. Hou, Z. Wen, L. Dai, Chem. Soc. Rev. 50(8), 4993–5061 (2021). https://doi.org/10.1039/d0cs00071j
J. Hao, W. Shi, Chinese. J. Catal. 39(7), 1157–1166 (2018). https://doi.org/10.1016/S1872-2067(18)63073-6
W. Zhang, D. Ma, J. Perez-Ramirez, Z. Chen, Adv. Energ. Sustain. Res. 3(2), 2100169 (2022). https://doi.org/10.1002/aesr.202100169
S. Mehla, A.E. Kandjani, R. Babarao, A.F. Lee, S. Periasamy, K. Wilson, S. Ramakrishna, S.K. Bhargava, Energy Environ. Sci. 14(1), 320–352 (2021). https://doi.org/10.1039/d0ee01882a
F. Yu, K. Deng, M. Du, W. Wang, F. Liu, D. Liang, Carbon Capture. Sci. Technol. 6, 100081 (2023). https://doi.org/10.1016/j.ccst.2022.100081
V.S.K., Yadav, M.K. Purkait, RSC Adv. 5(84), 68551–68557 (2015). https://doi.org/10.1039/c5ra12369k
M. Younas, L. Loong Kong, M.J.K. Bashir, H. Nadeem, A. Shehzad, S. Sethupathi, Energ. Fuel. 30(11), 8815–8831 (2016). https://doi.org/10.1021/acs.energyfuels.6b01723
O. Tursunov, L. Kustov, Z. Tilyabaev, J. Taiwan, Inst. Chem. Eng. 78, 416–422 (2017). https://doi.org/10.1016/j.jtice.2017.06.049
Y. Wu, T. Wang, H. Wang, X. Wang, X. Dai, F. Shi, Nat. Commun. 10(1), 2599 (2019). https://doi.org/10.1038/s41467-019-10633-y
P. Munnik, P.E. de Jongh, K.P. de Jong, Chem. Rev. 115(14), 6687–6718 (2015). https://doi.org/10.1021/cr500486u
M.A. Malik, M.Y. Wani, M.A. Hashim, Arab. J. Chem. 5(4), 397–417 (2012). https://doi.org/10.1016/j.arabjc.2010.09.027
Y. Yu, S. Mottaghi-Tabar, M.W. Iqbal, A. Yu, D.S.A. Simakov, Catal. Today 379, 250–261 (2021). https://doi.org/10.1016/j.cattod.2020.08.017
J.N. Park, E.W. McFarland, J. Catal. 266(1), 92–97 (2009). https://doi.org/10.1016/j.jcat.2009.05.018
F. Adam, T.S. Chew, J. Andas, J. SolGel Sci. and Technol. 59(3), 580–583 (2011). https://doi.org/10.1007/s10971-011-2531-7
D. Bokov, A. Turki Jalil, S. Chupradit, W. Suksatan, M. Javed Ansari, I.H. Shewael, G.H. Valiev, E. Kianfar, Adv. Mater. Sci. Eng. 2021, 21 (2021). https://doi.org/10.1155/2021/5102014
A.S. Prakash, C. Shivakumara and M.S. Hegde, Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 139(1), 55–61 (2007). https://doi.org/10.1016/j.mseb.2007.01.034
K. Zhao, W. Wang, Z. Li, J. CO2 Util. 16, 236–244 (2016). https://doi.org/10.1016/j.jcou.2016.07.010
D.L.T. Nguyen, M.S. Jee, D.H. Won, H. Jung, H.S. Oh, B.K. Min, Y.J. Hwang, A.C.S. Sustain, Chem. Eng. 5(12), 11377–11386 (2017). https://doi.org/10.1021/acssuschemeng.7b02460
J. Liu, P. Li, J. Bi, Q. Zhu, B. Han, Chem. Eur. J. 28(31), e202200242 (2022). https://doi.org/10.1002/chem.202200242
V.S.K. Yadav, M.K. Purkait, Energ. Fuel. 29(10), 6670–6677 (2015). https://doi.org/10.1021/acs.energyfuels.5b01656
L.M. Aeshala, R.G. Uppaluri, A. Verma, J. CO2 Util., 3–4, 49–55 (2013). https://doi.org/10.1016/j.jcou.2013.09.004
V.S.K. Yadav, Y. Noh, H. Han, W.B. Kim, Catal. Today 303, 276–281 (2018). https://doi.org/10.1016/j.cattod.2017.09.015
H. Wender, R.V. Goncalves, A.F. Feil, P. Migowski, F.S. Poletto, A.R. Pohlmann, J. Dupont, S.R. Teixeira, J. Phys. Chem. C 115(33), 16362–16367 (2011). https://doi.org/10.1021/jp205390d
N. Baig, I. Kammakakam, W. Falath, I. Kammakakam, Mater. Adv. 2(6), 1821–1871 (2021). https://doi.org/10.1039/d0ma00807a
N. Gutierrez-Guerra, J.A. Gonzalez, J.C, Serrano-Ruiz, E. Lopez-Fernandez, J.L. Valverde, A. de Lucas-Consuegra, J. Energy Chem. 31, 46–53 (2019). https://doi.org/10.1016/j.jechem.2018.05.005
Y. Gao, Y. Guo, Y. Zou, W. Liu, Y. Luo, B. Liu, C. Zhao, A.C.S. Appl, Energy Mater. 6(3), 1340–1354 (2023). https://doi.org/10.1021/acsaem.2c03131
M. Saranya, R. Ramachandran, E.J.J. Samuel, S.K. Jeong, A.N. Grace, Powder Technol. 279, 209–220 (2015). https://doi.org/10.1016/j.powtec.2015.03.041
Z. Zhao, X. Peng, X. Liu, X. Sun, J. Shi, L. Han, G. Li, J. Luo, J. Mater. Chem. A 5(38), 20239–20243 (2017). https://doi.org/10.1039/c7ta05507b
A.P. Periasamy, R. Ravindranath, S.M. Senthil Kumar, W.P. Wu, T.R. Jian, H.T. Chang, Nanoscale 10(25), 11869–11880 (2018). https://doi.org/10.1039/c8nr02117a
P. Gao, L. Zhong, L. Zhang, H. Wang, N. Zhao, W. Wei, Sun, Y. Catal. Sci. Technol., 5(9), 4365–4377 (2015). https://doi.org/10.1039/c5cy00372e
Z. Zhang, L. Zhang, S. Yao, X. Song, W. Huang, M.J. Hulsey, N. Yan, J. Catal. 376, 57–67 (2019). https://doi.org/10.1016/j.jcat.2019.06.048
W. Zhu, Y.J. Zhang, H. Zhang, H. Lv, Q. Li, R. Michalsky, A.A. Peterson, S. Sun, J. Am. Chem. Soc. 136(46), 16132–16135 (2014). https://doi.org/10.1021/ja5095099
X. Xiao, J. Gao, S. Xi, S.H. Lim, A.K.W. Png, A. Borgna, W. Chu, Y. Liu, Appl. Catal. B Environ. 309, 121239 (2022). https://doi.org/10.1016/j.apcatb.2022.121239
Z. Xu, N.D. Mcnamara, G.T. Neumann, W.F. Schneider, J.C. Hicks, ChemCatChem 5(7), 1769–1771 (2013). https://doi.org/10.1002/cctc.201200839
S. Yao, L. Lin, W. Liao, N. Rui, N. Li, Z. Liu, J. Cen, F. Zhang, X. Li, L. Song, L. Betancourt De Leon, D. Su, S.D. Senanayake, P. Liu, D. Ma, J.G. Chen, J.A. Rodriguez, ACS Catal. 9(10), 9087–9097 (2019). https://doi.org/10.1021/acscatal.9b01945
C. Wang, E. Guan, L. Wang, X. Chu, Z. Wu, J. Zhang, Z. Yang, Y. Jiang, L. Zhang, X. Meng, B.C. Gates, F.S. Xiao, J. Am. Chem. Soc. 141(21), 8482–8488 (2019). https://doi.org/10.1021/jacs.9b01555
A. Goguet, S.O. Shekhtman, R. Burch, C. Hardacre, F.C. Meunier, G.S. Yablonsky, J. Catal. 237(1), 102–110 (2006). https://doi.org/10.1016/j.jcat.2005.10.020
H. Song, J. Yang, J. Zhao, L. Chou, Chinese. J. Catal. 31(1), 21–23 (2010). https://doi.org/10.1016/s1872-2067(09)60036-x
J. Liu, C. Li, F. Wang, S. He, H. Chen, Y. Zhao, M. Wei, D.G. Evans, X. Duan, Catal. Sci. Technol. 3(10), 2627–2633 (2013). https://doi.org/10.1039/c3cy00355h
F. Wang, S. He, H. Chen, B. Wang, L. Zheng, M. Wei, D.G. Evans, X. Duan, J. Am. Chem. Soc. 138(19), 6298–6305 (2016). https://doi.org/10.1021/jacs.6b02762
X. Lu, F. Gu, Q. Liu, J. Gao, Y. Liu, H. Li, L. Jia, G. Xu, Z. Zhong, F. Su, Fuel Processing Technol. 135, 34–46 (2015). https://doi.org/10.1016/j.fuproc.2014.10.009
A. Beuls, C. Swalus, M. Jacquemin, G. Heyen, A. Karelovic, P. Ruiz, Appl. Catal. B Environ. 113–114, 2–10 (2012). https://doi.org/10.1016/j.apcatb.2011.02.033
Y. Yin, B. Hu, G. Liu, X. Zhou, X. Hong, Wuli Huaxue Xuebao/ Acta Phys. – Chim. Sin. 35(3), 327–336 (2019). https://doi.org/10.3866/PKU.WHXB201803212
C. Tisseraud, C. Comminges, S. Pronier, Y. Pouilloux, A. le Valant, J. Catal. 343, 106–114 (2016). https://doi.org/10.1016/j.jcat.2015.12.005
J. Diez-Ramirez, J.A. Diaz, P. Sanchez, F. Dorado, J. CO2 Util. 22, 71–80 (2017). https://doi.org/10.1016/j.jcou.2017.09.012
I. Sharafutdinov, C.F. Elkjær, H.W.P. de Carvalho, D. Gardini, G.L. Chiarello, C.D. Damsgaard, J.B. Wagner, J.D. Grunwaldt, S. Dahl, I. Chorkendorff, J. Catal. 320(1), 77–88 (2014). https://doi.org/10.1016/j.jcat.2014.09.025
Y. Chen, S. Choi, L.T. Thompson, J. Catal. 343, 147–156 (2016). https://doi.org/10.1016/j.jcat.2016.01.016
Y. Hartadi, D. Widmann, R.J. Behm, J. Catal. 333, 238–250 (2016). https://doi.org/10.1016/j.jcat.2015.11.002
B. An, J. Zhang, K. Cheng, P. Ji, C. Wang, W. Lin, J. Am. Chem. Soc. 139(10), 3834–3840 (2017). https://doi.org/10.1021/jacs.7b00058
Y.Y. Birdja, E. Perez-Gallent, M.C. Figueiredo, A.J. Gottle, F. Calle-Vallejo, M.T.M. Koper, Nat. Energy 4(9), 732–745 (2019). https://doi.org/10.1038/s41560-019-0450-y
H. Zhong, Y. Qiu, X. Li, L. Pan, H. Zhang, J. Energy Chem. 55, 236–243 (2021). https://doi.org/10.1016/j.jechem.2020.06.058
Y. Qiu, J. Du, W. Dong, C. Dai and C. Tao, J. CO2 Util. 20, 328–335 (2017). https://doi.org/10.1016/j.jcou.2017.05.024
W. Luc, C. Collins, S. Wang, H. Xin, K. He, Y. Kang, F. Jiao, J. Am. Chem. Soc. 139(5), 1885–1893 (2017). https://doi.org/10.1021/jacs.6b10435
Y. Kwon, J. Lee, Electrocatalysis 1(2–3), 108–115 (2010). https://doi.org/10.1007/s12678-010-0017-y
X. Bai, W. Chen, C. Zhao, S. Li, Y. Song, R. Ge, W. Wei, Y. Sun, Angew. Chem., 129(40), 12387–12391 (2017). https://doi.org/10.1002/ange.201707098
S. Sarfraz, A.T. Garcia-Esparza, A. Jedidi, L. Cavallo, K. Takanabe, ACS Catal. 6(5), 2842–2851 (2016). https://doi.org/10.1021/acscatal.6b00269
F. Cai, D. Gao, H. Zhou, G. Wang, T. He, H. Gong, S. Miao, F. Yang, J. Wang, X. Bao, Chem. Sci. 8(4), 2569–2573 (2017). https://doi.org/10.1039/c6sc04966d
J. Wu, M. Liu, P.P. Sharma, R.M. Yadav, L. Ma, Y. Yang, X. Zou, X.D. Zhou, R. Vajtai, B.I. Yakobson, J. Lou, P.M. Ajayan, Nano Lett. 16(1), 466–470 (2016). https://doi.org/10.1021/acs.nanolett.5b04123
L. Fu, Z. Qu, L. Zhou, Y. Ding, Appl. Catal. B Environ. 339, 123170 (2023). https://doi.org/10.1016/j.apcatb.2023.123170
J. Cai, Q. Zhao, W.Y. Hsu, C. Choi, Y. Liu, J.M.P. Martirez, C. Chen, J. Huang, E.A. Carter, Y. Huang, J. Am. Chem. Soc. 145(16), 9136–9143 (2023). https://doi.org/10.1021/jacs.3c00847
H. Pan, C.J. Barile, Energy Environ. Sci. 13(10), 3567–3578 (2020). https://doi.org/10.1039/d0ee02189j
B. Zhang, J. Zhang, M. Hua, Q. Wan, Z. Su, X. Tan, L. Liu, F. Zhang, G. Chen, D. Tan, X. Cheng, B. Han, L. Zheng, G. Mo, J. Am. Chem. Soc. 142(31), 13606–13613 (2020). https://doi.org/10.1021/jacs.0c06420
H. Yano, T. Tanaka, M. Nakayama, K. Ogura, J. Electroanal. Chem. 565(2), 287–293 (2004). https://doi.org/10.1016/j.jelechem.2003.10.021
H. Mistry, A.S. Varela, C.S. Bonifacio, I. Zegkinoglou, I. Sinev, Y.W. Choi, K. Kisslinger, E.A. Stach, J.C. Yang, P. Strasser, B.R. Cuenya, Nat. Commun. 7, 12123 (2016). https://doi.org/10.1038/ncomms12123
S. Zhao, S. Guo, C. Zhu, J. Gao, H. Li, H. Huang, Y. Liu, Z. Kang, RSC Adv. 7(3), 1376–1381 (2017). https://doi.org/10.1039/c6ra26868d
W. Zhang, Q. Qin, L. Dai, R. Qin, X. Zhao, X. Chen, D. Ou, J. Chen, T.T. Chuong, B. Wu, N. Zheng, Angew. Chem. 130(30), 9619–9623 (2018). https://doi.org/10.1002/ange.201804142
S. Mou, T. Wu, J. Xie, Y. Zhang, L. Ji, H. Huang, T. Wang, Y. Luo, X. Xiong, B. Tang, X. Sun, Adv. Mater. 31(36), 1903499 (2019). https://doi.org/10.1002/adma.201903499
J. Huang, Q. Hu, X. Guo, Q. Zeng, L. Wang, Green Chem. 20(13), 2967–2972 (2018). https://doi.org/10.1039/c7gc03744a
H. Xu, D. Rebollar, H. He, L. Chong, Y. Liu, C. Liu, C.J. Sun, T. Li, J.V. Muntean, R.E. Winans, D.J. Liu, T. Xu, Nat. Energy 5(8), 623–632 (2020). https://doi.org/10.1038/s41560-020-0666-x
Y. Song, R. Peng, D.K. Hensley, P.V. Bonnesen, L. Liang, Z. Wu, H.M. Meyer, M. Chi, C. Ma, B.G. Sumpter, A.J. Rondinone, ChemistrySelect 1(19), 6055–6061 (2016). https://doi.org/10.1002/slct.201601169
J. Du, S. Li, S. Liu, Y. Xin, B. Chen, H. Liu, B. Han, Chem. Sci. 11(19), 5098–5104 (2020). https://doi.org/10.1039/d0sc01133a
A.J. Martin, S. Mitchell, C. Mondelli, S. Jaydev, J. Perez-Ramirez, Nat. Catal. 5(10), 854–866 (2022). https://doi.org/10.1038/s41929-022-00842-y
A. Cao, R. Lu, G. Veser, Phys. Chem. Chem. Phys. 12(41), 13499–13510 (2010). https://doi.org/10.1039/c0cp00729c
H. Zhang, J. Pan, Q. Zhou, F. Xia, Small 17(7), 2005771 (2021). https://doi.org/10.1002/smll.202005771
B. Buesser, A.J. Grohn, S.E. Pratsinis, J. Phys. Chem. C 115(22), 11030–11035 (2011). https://doi.org/10.1021/jp2032302
J. Li, M. Zhu, Y.F. Han, ChemCatChem 13(2), 514–531 (2021). https://doi.org/10.1002/cctc.202001350
J. Qiao, Y. Liu, F. Hong, J. Zhang, Chem. Soc. Rev. 43(2), 631–675 (2014). https://doi.org/10.1039/c3cs60323g
A.G.M. Mostafa Hossain, T. Nagaoka and K. Ogura, Electrochim. Acta 41(17), 2773–2780 (1996).https://doi.org/10.1016/0013-4686(96)00136-3
Y. Hori, H. Konishi, T. Futamura, A. Murata, O. Koga, H. Sakurai, K. Oguma, Electrochim. Acta, 50(27), 5354–5369 (2005). https://doi.org/10.1016/j.electacta.2005.03.015
R. Senthil Kumar, S. Senthil Kumar, M. Anbu Kulandainathan, Electrochem. Commun. 25(1), 70–73 (2012). https://doi.org/10.1016/j.elecom.2012.09.018
Y. Hori, R. Takahashi, Y. Yoshinami, A. Murata, J. Phys. Chem. B. 101, 7075–7081 (1997).https://doi.org/10.1021/jp970284i
M. Zabilskiy, V.L. Sushkevich, D. Palagin, M.A. Newton, F. Krumeich, J.A. Van Bokhoven, Nat. Commun. 11(1), 2409 (2020). https://doi.org/10.1038/s41467-020-16342-1
M. Zabilskiy, V.L. Sushkevich, M.A. Newton, J.A. Van Bokhoven, ACS Catal. 10(23), 14240–14244 (2020). https://doi.org/10.1021/acscatal.0c03661
I.M. Badawy, A.M. Ismail, G.E. Khedr, M.M. Taha, N.K. Allam, Sci. Rep. 12(1), 13456 (2022). https://doi.org/10.1038/s41598-022-17317-6
J. Zeng, T. Rino, K. Bejtka, M. Castellino, A. Sacco, M.A. Farkhondehfal, A. Chiodoni, F. Drago, C.F. Pirri, Chemsuschem 13(16), 4128–4139 (2020). https://doi.org/10.1002/cssc.202000971
F.C.F. Marcos, R.S. Alvim, L. Lin, L.E. Betancourt, D.D. Petrolini, S.D. Senanayake, R.M.B. Alves, J.M. Assaf, J.A. Rodriguez, R. Giudici, E.M. Assaf, Chem. Eng. J. 452, 139519 (2023).https://doi.org/10.1016/j.cej.2022.139519
A. Strijevskaya, A. Yamaguchi, S. Shoji, S. Ueda, A. Hashimoto, Y. Wen, A.C. Wardhana, J.E. Lee, M. Liu, H. Abe, M. Miyauchi, A.C.S. Appl, Mater. Interfaces 15(19), 23299–23305 (2023). https://doi.org/10.1021/acsami.3c02874
T. Yan, N. Li, L. Wang, W. Ran, P.N. Duchesne, L. Wan, N.T. Nguyen, L. Wang, M. Xia, G.A. Ozin, Nat. Commun. 11(1), 6095 (2020). https://doi.org/10.1038/s41467-020-19997-y
X. Cao, B. Wulan, Y. Wang, J. Ma, S. Hou, J. Zhang, Sci. Bull. 68(10), 1008–1016 (2023). https://doi.org/10.1016/j.scib.2023.04.026
P. Gao, F. Li, L. Zhang, N. Zhao, F. Xiao, W. Wei, L. Zhong, Y. Sun, J. CO2 Util. 2, 16–23 (2013). https://doi.org/10.1016/j.jcou.2013.06.003
Acknowledgements
The authors of this paper would like to acknowledge Qatar University for their financial support (Grant Number: QUPD—CENG—23/24—510). The authors are also thankful to the department of chemical engineering, Qatar University for their support to research.
Funding
Open Access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Contributions
The conceptualization and design of the study were aided by Anand Kumar and V.S.K Yadav. V.S.K Yadav wrote the first draft of the manuscript, and all other authors provided feedback on the drafts. The final manuscript was read and approved by all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yadav, V.S.K., Al-Marri, M.J., Saad, M.A.H.S. et al. Understanding the progress and challenges in the fields of thermo-catalysis and electro-catalysis for the CO2 conversion to fuels. emergent mater. 7, 1–16 (2024). https://doi.org/10.1007/s42247-023-00606-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-023-00606-9