Abstract
In this research work, we have studied the impact of Yttrium nanoparticles loaded with corrosion inhibitors dodecylamine (DOC) incorporated in polyolefin for the corrosion protection of steel. The surface analysis of pure polyolefin and polyolefin-Y2O3-DOC composite coatings by atomic force spectroscopy (AFM) shows that both of the coated samples’ roughness almost remain the same. Furthermore, the contact angle measurement shows an increase in the hydrophobicity of polyolefin-Y2O3 with inhibitor. The release behavior of the corrosion inhibitors DOC was also studied at different pH. The x-ray diffraction for the loaded product shows that no physical and structural changes occur during the loading of the corrosion inhibitor. The electrochemical impedance spectroscopy (EIS) analysis demonstrates that smart polyolefin-Y2O3-DOC coating has better anticorrosion properties than pure polyolefin coating due to the effective release of DOC. An increase in charge transfer and pore resistance confirms the better barrier properties of the polyolefin-Y2O3-DOC composite coating. The inhibition efficiency of the polyolefin modified by Y2O3 increased by 99% as compared to pure polyolefin coating. The carbon steel substrate became stable and the polymeric composite coating protectected the steel against corrosion in the oil and gas industry. In conclusion, the study shows that yttrium nanoparticles loaded with corrosion inhibitors incorporated in polyolefin have a significant impact on the corrosion protection of steel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbon steels are frequently used in the oil and gas industries, as well as engineering structures, due to their excellent mechanical properties and low cost. Nonetheless, their corrosion susceptibility can result in significant financial losses and environmental risks. Corrosion is widely regarded as the most damaging occurrence in the oil and gas industry, as it can cause significant economic losses, safety concerns, and even catastrophic incidents if not addressed promptly [1]. Many plants shut down due to corrosion to perform maintenance and fix any potential equipment and pipeline damage, which may be an expensive and time-consuming procedure.
For this reason, corrosion prevention has become a fundamental approach to minimizing the potential economic losses and environmental damage that corrosion may cause. One of the most popular and efficient methods of preventing corrosion on steel is the application of protective coatings [2,3,4]. Sol–gel coating [5], metallic coating [6], ceramic nano-coating [7], hybrid coating [8], and organic coatings [9] are some of the different coatings applied for corrosion protection. The advantages of using organic coatings are strong barrier capabilities, availability, processing ease, and flexibility to design formulas tailored for the application [10, 11].
Among the different types of organic coatings, organic polymer coating (OPC), temporary protective oil coating (TPOC), and volatile organic coating (VOC) are some of the commonly used organic coatings [12, 13]. VOC poses acute hazards to workers and a long-term environmental pollution hazard [14]. In polymer-based coating, polyolefin coating is used for coating due to its strong chemical resistance and robust adhesion to metal surfaces. Polyolefins have high electric resistance, causing cathodic protective shielding to carbon steel [15]. The coatings have the best mechanical properties of any polymeric coating [16, 17]. On the other hand, organic coatings can erode over time, so many significant techniques have been developed to address this issue [9, 18, 19]. Among these methods, the concept of “smart” and “self-healing” coatings has grown in popularity. Loading carriers with corrosion inhibitors or other healing species is one of the most efficient but challenging ways to produce “smart” organic coatings [20, 21]. Direct addition of corrosion inhibitors to formulations for coatings may result in high performance, but it typically results in the erosion of the coating matrix and lowers the barrier qualities of the coating [22, 23]. In the presence of environmental stimuli, such as pH changes, humidity, or moisture, the corrosion inhibitor embedded within the coating can diffuse into any defects in the coating and inhibit both anodic and cathodic reactions [24]. This problem has been overcome by using carrier-containing inhibitors which release inhibitors in exchange for ions from the environment, which also help improve the coating’s barrier properties [25, 26]. The idea is to develop a protective self-healing smart polymeric coating that can enhance the corrosion-protection properties of polyolefin film applied on the carbon steel substrate. The use of shell-like capsules or porous matrices for corrosion inhibitor release has been successful; although, there are concerns regarding low mechanical stability and early inhibitor loss due to permeability through the shell material. The use of porous inorganic carriers as an alternative has been proposed to enable the reliable release of inhibitors when needed and for a longer life without the loss of mechanical stability [21, 27]. This method can overcome the problems associated with shell-like capsules and porous matrices, resulting in better performance and more dependable corrosion inhibition. The porous nature and high stability during coating formulation of rare-earth oxides including cerium oxide, yttrium oxide, and lanthanum oxide make them ideal candidates for loading corrosion inhibitors [28,29,30,31]. Mrad Mouna et al. [32] investigated the impact of cerium nitrate doping on the physicochemical and anti-corrosive properties of an electropolymerized-glycidoxypropyltriethoxysilane coating on an aluminum alloy. The results demonstrate that the coating’s ability to prevent corrosion is influenced by the amount of cerium present. More specifically, more effective corrosion protection is achieved by lowering the cerium content of the coating. Due to their stability, good compatibility with numerous polymeric matrices, and corrosion prevention capabilities, yttrium nanoparticles contribute desirable loading capacity and enhanced mechanical qualities when incorporated into polymeric matrices [17, 33, 34]. Nawaz et al. [35] developed polymeric nanocomposite coatings with yttrium nanoparticles containing corrosion inhibitors (imidazole) mixed into the epoxy formulation. The study shows that Y2O3 loaded with imidazole increases the corrosion resistance of epoxy-coated steel substrates. Figure 1 depicts a pictorial illustration of infusing Y2O3 with imidazole.
This study aims to investigate the morphology, structure analysis, and anticorrosion behavior of yttrium oxide (Y2O3) loaded with dodecylamine (DOC) when incorporated into the polyolefin matrix. The polymeric coating was deposited to the carbon steel substrate using the dip coating technique. The results suggest that Y2O3 loaded with DOC enhances the polymeric coating’s hydrophobicity, and an AFM analysis shows that the coating has been uniform and smooth. By using EIS analysis, the anticorrosion property of the polyolefin-Y2O3-DOC coating was compared with pure polyolefin coating. The smart coatings showed improved corrosion resistance, which can be attributed to the effective release of DOC from the enclosed Y2O3 incorporated into the polyolefin matrix.
2 Experimental
2.1 Material
Sigma-Aldrich supplied the yttrium nanoparticles (Y2O3), dodecylamine (DOC), sodium chloride (NaCl), and ethanol. The carbon steel substrate (35, 35, and 1.0 mm3) was provided by a local supplier. They were polished with emery papers of 80 and 120 grit size, respectively, properly washed with deionized water and acetone, and then dried. Plain carbon steel plates have a composition of Fe = 99.18%, C = 0.21%, Cu = 0.20%, Mn = 0.30%, P = 0.04%, and S = 0.04%.
2.2 Loading of corrosion inhibitor (DOC) into Y2O3
Encapsulation of yttrium nanoparticles, as a carrier for corrosion inhibitors, with the corrosion inhibitor dodecyl amine (DOC) was done. The corrosion inhibitor (DOC) was dissolved first in DI water using magnetic stirring, also 3 wt% of yttrium nanoparticles were added to the DOC inhibitor. Then, sonication was performed for 30 min to obtain a uniform distribution. After that, the solution is kept at 70 °C for overnight stirring. The prepared solution was kept for centrifuge with 5000 rpm for 20 min on the first cycle, and using DI water, we kept it again for a centrifuge for 10 min on the second cycle. Then, the obtained product was dried in the oven at 70 °C temperature for 24 h to remove the solvent. After that, the developed encapsulated 1 wt% of Y2O3-DOC was dispersed within the polyolefin matrix. We stored the polyolefin-Y2O3-DOC (1 wt%) solution for 24 h at room temperature in a vacuum chamber so that any bubbles will be removed from the prepared solution.
2.3 Deposition of the polymeric film
The withdrawal speed of the dip coating machine was adjusted to 18 mm/min, and the immersion speed was optimized to 22 mm/min with a dwell time of 1 min. The dip-coating parameters are optimized to get an optimal thickness without cracking problems. Initially, the withdrawal speed was kept higher at 24 mm/min, but it leads to more accumulation of polyolefin on the steel substrate which eventually causes cracks formation. So, the dip coating condition was optimized to get the best coating. For 20 min, the coatings were placed in the oven at 160 °C to cure them. At 160 °C, the polyolefin melts, allowing for crosslinking-enabled curing, which causes the white suspension solution to turn totally clear and create a firm contact with the carbon steel substrate. Once the temperature reaches 160 °C, the oven is turned off and the coatings are left in the oven to cool down to avoid cracking. Figure 1 shows the graphical representation of the experimental procedure which is applied in fabricating the polymeric coating on the carbon steel substrate.
3 Results and discussion
3.1 Morphology of the polymeric film
The AFM analysis has a significant role to study the surface roughness and topography of the polymeric film. The AFM analysis also determines the impact of yttrium nanoparticles on the roughness of the coatings. Figure 2a and b shows the AFM image of the pure polyolefin film and polyolefin-Y2O3 nanocomposite, respectively. It has been observed that the roughness of the pure polyolefin film is around 19.04 nm, and the polyolefin-Y2O3-DOC (1 wt%) composite-film roughness is around 24.78 nm. This concluded that incorporating the Y2O3 nanoparticles into the polyolefin has no significant effect on the surface roughness of the coating; it is increased due to the addition of particles. The hydrophobicity of the polymeric coating indicates the surface properties. The hydrophobicity of the water drops on the surface of the polymeric film was studied by the sessile drop method. Figure 2c and d shows the contact angle of the pure polyolefin and polyolefin-Y2O3-Doc (1 wt%) nanocomposite film, respectively. The contact angle of pure polyolefin is around 99.8° compared to 110° when adding Y2O3-Doc (1 wt%) in polyolefin. The addition of Y2O3-Doc has improved the hydrophobicity of the coating. The hydrophobic nature of the coating isolates the steel substrate from water penetration through coatings, which eventually improves the barrier properties of the coating. A hydrophobic film can significantly increase the lifespan of the metal and give outstanding anticorrosion performance by limiting the interaction between the metal and the corrosive aqueous species, the hydrophobicity of water eventually keeps the Cl ions (present in the brine water) isolated from the steel substrate [36].
3.2 Structure analysis
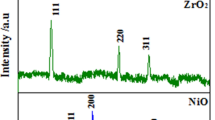
The UV–Vis spectroscopy analysis demonstrates the DOC’s self-release behavior. As a result, 3.5 wt% NaCl solutions with pH values of 2, 7, and 11 were prepared, and the loaded product (Y2O3-DOC) was dispersed in them. The UV–Vis spectra of Y2O3-DOC after 2 days of immersion are shown in Fig. 3a and b. The UV spectra profiles are similar at different pH levels, and a sharp peak at 204 nm can be attributed to the amine group found in DOC [37]. For all pH values, absorbance peaks can be seen (2, 7, and 11). An absorbance intensity of 0.25 AU was measured on the first day of immersion at pH 2 which slightly increases up to ~ 0.30 AU on the second day. Moreover, the absorbance intensity on the first day of immersion is 0.31 AU for pH 7 (Fig. 3b) and almost remains the same on the second day. The UV spectra taken at pH 11 show a higher intensity which indicates a more release of inhibitor as compared to pH 2 and 7. Figure 3c depicts the XRD pattern of Y2O3 and Y2O3-DOC, demonstrating the particle’s crystalline nature. Crystalline phases 111, 200, 220, and 311 may be responsible for the distinctive peaks at 29.11°, 33.81°, 48.45°, and 57.61°. The XRD patterns of Y2O3 and Y2O3-DOC do not differ significantly, and the absence of any additional peaks indicates that the presence of DOC has not caused any substantial structural or phase changes in the Y2O3.
3.3 EIS analysis
The Bode plots of pure polyolefin and polyolefin-Y2O3-DOC (1 wt%) are presented in Fig. 4a. The impedance spectra of modified polyolefin coating after 24 h increased by 10 G.Ohm as compared to blank polyolefin which is slightly less than 1 M.Ohm. Which shows better corrosion protection due to the addition of DOC. The Bode plot shows that polyolefin-Y2O3 shows high charge transfer and pore resistance compared to pure polyolefin coating. Figure 4b shows the phase plot of the pure polyolefin and Polyolefin-Y2O3. The phase plot shows that polyolefin-Y2O3 shows capacitive behavior in a mid-frequency range which shows that polyolefin-Y2O3 is more protective against corrosion. Figure 4c shows the Bode plot of the polyolefin-Y2O3 with the inhibitor DOC after 24 h. Figure 4d shows the phase plot of the polyolefin-Y2O3 with the primary inhibitor DOC after 48 h. The impedance value remains stable and further slightly increased after 48 h of immersion.
a Shows the Bode plot of pure polyolefin and polyolefin-Y2O3. b Shows the phase plot of pure polyolefin and polyolefin-Y2O3. c Shows the Bode plot of pure polyolefin-Y2O3-DOC ( 1 wt%) after 24 and 48 h, respectively. d Shows the phase plot of pure polyolefin and polyolefin-Y2O3-DOC( 1 wt%) after 24 and 48 h, respectively
We have used two model to simulate the EIS fitting of pure polyolefin coating and polyolefin doping by Y2O3. Figure 5a shows the equivalent circuit used for the fitting of the EIS data of pure polylofin in a 3.5 wt% NaCl concentration. Figure 5b shows the model which is used to fit the EIS data of the polyolefin-Y2O3 with the inhibitor DOC.
The EIS parameters after fitting with an equivalent circuit is presented in Table 1. The pore resistance (RPO) also increased after the addition of particles that isolate the carbon steel substrate from the electrolyte and provide better barrier properties to the coating. The capacitance value of pure polyolefin coatings is higher compared to the modified due to conductive paths present in the coating. The charge transfer resistance (Rct) values show the interface between the carbon steel substrate, and the coating has better corrosion protection due to the inhibition effect of DOC. The inhibition layer formed due to the protective film formed on the steel surface. Higher resistance with a lower capacitance value shows the better corrosion property of the coating.
4 Conclusion
The development of novel eco-friendly polyolefin coating with the incorporation of nanoparticles for the protection of carbon steel from corrosion confirms promising results. The smart coatings were applied on the carbon steel substrate using the dip coating technique. The AFM analysis of pure polyolefin and polyolefin-Y2O3 with inhibitor DOC demonstrates a slight change in the roughness of the coating, which does not have any bad effect on the coating properties. The contact angle of modified polyolefin-Y2O3-DOC increases to 110°, and the surface becomes more hydrophobic which prevents the beginnings of corrosion activity at the surface of the polymeric film. The UV spectra taken at pH 11 show a higher intensity which indicates a more release of inhibitor as compared to pH 2 and 7. The EIS analysis shows that smart polyolefin-Y2O3-DOC coating demonstrates anticorrosion properties as compared to pure polyolefin coating due to an increase in charge-transfer resistance and pore resistance. The inhibition efficiency of the polyolefin modified by Y2O3 increased by 99% as compared to pure polyolefin coating. To investigate the potential for longer-term corrosion protection, the effects of incorporating higher concentrations of modified Y2O3 into polyolefin could be studied. The carbon steel substrate becomes stable and the polymeric composite coating protects the steel against corrosion in oil and gas industry.
Data availability
The data that support the findings of this study are available from the corresponding author, [Noora Al-Thani], upon reasonable request.
References
S. Ameri, P.J. Szary, Identifying Research, Development, and Training Needs for Oil and Gas Pipeline Safety and Security (Division of Research and Technology and U.S. Department of Transportation Federal Highway Administration the State of New Jersey, 2005)
M. Zheludkevich, J. Tedim, M. Ferreira, “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 82, 314–323 (2012)
M. Ates, A review on conducting polymer coatings for corrosion protection. J. Adhes. Sci. Technol. 30(14), 1510–1536 (2016)
G.P. Bierwagen, Reflections on corrosion control by organic coatings. Prog. Org. Coat. 28(1), 43–48 (1996)
D. Snihirova et al., Hydroxyapatite microparticles as feedback-active reservoirs of corrosion inhibitors. ACS Appl. Mater. Interfaces. 2(11), 3011–3022 (2010)
F. Presuel-Moreno, M. Jakab, N. Tailleart, M. Goldman, J. Scully, Corrosion-resistant metallic coatings. Mater. Today 11(10), 14–23 (2008)
A.A. Farag, Applications of nanomaterials in corrosion protection coatings and inhibitors. Corros. Rev. 38(1), 67–86 (2020)
Y. Zhou et al., Robust superhydrophobic surface based on multiple hybrid coatings for application in corrosion protection. ACS Appl. Mater. Interfaces. 11(6), 6512–6526 (2019)
A.A. Olajire, Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 269, 572–606 (2018)
D. Thomas, E. Philip, R. Sindhu, S. B. Ulaeto, A. Pugazhendhi, and M. K. Awasthi “Developments in smart organic coatings for anticorrosion applications: a review,” (Biomass Conversion and Biorefinery 2022), p. 1:17
S. García et al., Self-healing anticorrosive organic coating based on an encapsulated water reactive silyl ester: Synthesis and proof of concept. Prog. Org. Coat. 70(2–3), 142–149 (2011)
F.N. Jones, M.E. Nichols, S.P. Pappas, Organic Coatings: Science and Technology, 4th edn. (John Wiley & Sons, 2017), 512 p.
M. de Meijer, Review on the durability of exterior wood coatings with reduced VOC-content. Prog. Org. Coat. 43(4), 217–225 (2001)
T. Lomonaco et al., Release of harmful volatile organic compounds (VOCs) from photo-degraded plastic debris: a neglected source of environmental pollution. J. Hazard. Mater. 394, 122596 (2020)
M. Zamanzadeh, G. T. Bayer, and A. K. Chikkam, “Cathodic protection, coatings that shield cathodic protection, stress corrosion cracking and corrosion assessment in aging coated pipe lines and buried utility structures, ” (in NACE International Corrosion Conference Proceedings, 2018), NACE International, pp. 1-19
R. Raj, M. Taryba, Y. Morozov, R. Kahraman, R. Shakoor, M. Montemor, On the synergistic corrosion inhibition and polymer healing effects of polyolefin coatings modified with Ce-loaded hydroxyapatite particles applied on steel. Electrochim. Acta 388, 138648 (2021)
M. Nawaz et al., Improved properties of polyolefin nanocomposite coatings modified with ceria nanoparticles loaded with 2-mercaptobenzothiazole. Prog. Org. Coat. 171, 107046 (2022)
C. Bressy, C. Hugues, A. Margaillan, Characterization of chemically active antifouling paints using electrochemical impedance spectrometry and erosion tests. Prog. Org. Coat. 64(1), 89–97 (2009)
C.R. Hegedus, S.J. Spadafora, A.T. Eng, Organic coating technology for the protection of aircraft against corrosion, Corrosion Detection and Management of Advanced Airframe Materials, Agard Conference Proceedings 565, 17 (1995)
M. Attaei, M.G. Taryba, R.A. Shakoor, R. Kahraman, A.C. Marques, M.F. Montemor, Highly protective polyolefin coating modified with ceria nano particles treated with N, N, N’, N’-Tetrakis (2-hydroxyethyl) ethylenediamine for corrosion protection of carbon steel. Corros. Sci. 198, 110162 (2022)
E. Shchukina, H. Wang, D.G. Shchukin, Nanocontainer-based self-healing coatings: current progress and future perspectives. Chem. Commun. 55(27), 3859–3867 (2019)
K. Yasakau, M. Zheludkevich, O. Karavai, M. Ferreira, Influence of inhibitor addition on the corrosion protection performance of sol–gel coatings on AA2024. Prog. Org. Coat. 63(3), 352–361 (2008)
C.D. Dieleman, P.J. Denissen, S.J. Garcia, Long-term active corrosion protection of damaged Coated-AA2024-T3 by embedded electrospun inhibiting nanonetworks. Adv. Mater. Interfaces 5(12), 1800176 (2018)
G. Zhang et al., Corrosion protection properties of different inhibitors containing PEO/LDHs composite coating on magnesium alloy AZ31. Sci. Rep. 11(1), 2774 (2021)
R. Raj et al., Calcium carbonate particles loaded with triethanolamine and polyethylenimine for enhanced corrosion protection of epoxy coated steel. Corros. Sci. 167, 108548 (2020)
Y. Zhang, M. Yu, C. Chen, S. Li, J. Liu, Self-healing coatings based on stimuli-responsive release of corrosion inhibitors: a review. Frontiers in Materials 8, 613 (2022)
F. Ubaid et al., Multifunctional self-healing polymeric nanocomposite coatings for corrosion inhibition of steel. Surf. Coat. Technol. 372, 121–133 (2019)
J. De Damborenea, A. Conde, and M. Arenas, Corrosion inhibition with rare earth metal compounds in aqueous solutions, (in Rare Earth-Based Corrosion Inhibitors: Elsevier, 2014), pp. 84-116
W. Yao, Y. Chen, L. Wu, B. Jiang, F. Pan, Preparation of slippery liquid-infused porous surface based on MgAlLa-layered double hydroxide for effective corrosion protection on AZ31 Mg alloy. J. Taiwan Inst. Chem. Eng. 131, 104176 (2022)
D.G. Shchukin, H. Möhwald, Self-repairing coatings containing active nanoreservoirs. Small 3(6), 926–943 (2007)
W. Sassi et al., A challenge to succeed the electroplating of nanocomposite Ni–Cr alloy onto porous substrate under ultrasonic waves and from a continuous flow titanium nanofluids. J. Alloy. Compd. 828, 154437 (2020)
M. Mrad, W. Sassi, J.-Y. Hihn, and F. Montemor, Designing of anti-corrosive poly (γ-glycidoxypropyltriethoxysilane)-coated aluminum alloy via electro-polymerization: effect of cerium nitrate concentration, (Polymer Bulletin, 2023) pp. 1–23
R. Augustine et al., Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 156, 153–170 (2020)
H. Jiu, Y. Fu, L. Zhang, Y. Sun, Y. Wang, T. Han, Preparation and luminescent properties of hollow Y2O3: Tb3+ microspheres. Micro & Nano Letters 7(9), 947–950 (2012)
M. Nawaz, N. Naeem, R. Kahraman, M. Montemor, W. Haider, R. Shakoor, Effectiveness of epoxy coating modified with yttrium oxide loaded with imidazole on the corrosion protection of steel. Nanomaterials 11(9), 2291 (2021)
A. Thakur, A. Kumar, S. Kaya, R. Marzouki, F. Zhang, L. Guo, Recent advancements in surface modification, characterization and functionalization for enhancing the biocompatibility and corrosion resistance of biomedical implants. Coatings 12(10), 1459 (2022)
M. Nawaz, S. Habib, A. Khan, R. Shakoor, R. Kahraman, Cellulose microfibers (CMFs) as a smart carrier for autonomous self-healing in epoxy coatings. New J. Chem. 44(15), 5702–5710 (2020)
Acknowledgements
The findings made herein are solely the responsibility of the authors. The authors are very grateful to the Centre for Advanced Materials (CAM), Qatar University for the inclusive support.
Funding
Open Access funding provided by the Qatar National Library. This study was made possible by NPRP grants NPRP12S0203-190038 from the Qatar National Research Fund (a member of Qatar Foundation).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mallick, S., Bhadra, J., Shakoor, R.A. et al. Influence of polyolefin modified by Y2O3 as protective coatings against carbon steel corrosion into 3.5% NaCl media. emergent mater. 6, 2019–2026 (2023). https://doi.org/10.1007/s42247-023-00555-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-023-00555-3