Abstract
Nanofiltration is one of the most widely used membrane processes for water purification with high practical value because of a large number of chemical species that are separated through this process. Usually, for nanofiltration, high energy–consuming operations are involved including the generation of enough pressure for the rejection of jumps and lower molecular weight chemicals at the surface of the membrane. Recent developments in the synthesis of nanocomposite membranes with graphene and graphene derivatives have led to an increase in energy requirements and the increase in membranes performances. In the present review, we have presented the recent advances in the field of graphene-based composite membranes for nanofiltration with applications for both types of based solvents—aqueous solutions and organic solvents. The presentation will be focused especially on the performances of membranes and applications of these materials for the rejection of salts (Na+, Mg2+), heavy metals (Li2+), and lower molecular weight organic compounds (methylene blue, Congo red, Direct Red, Methyl orange, Reactive green 13, etc.). Modern synthesis methods like interfacial polymerization for obtaining thin-film composite nanofiltration membranes are also presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
By definition, a membrane is a selective barrier to a particular species of ions, molecules, or particles. As a result, of all the functional materials known and used today, membranes can be associated with a particular property, namely selectivity [1]. In addition, from the point of view of the history of these materials, we are dealing with an interesting journey in this decade. The very first functional material that ever existed on Earth was a membrane—the membrane of the first single-celled organism, and most organs in the human body act as membranes [2]. It became a matter of time before man tried to imitate the properties of these materials, thus defending the first membranes of cellulose derivatives at the beginning of the twentieth century. The first large-scale industrial applications emerged after the Second World War when large amounts of nitrocellulose leftover from unused bombs were transformed into membranes for water filtration and purification to make it drinkable, under the guardianship of the Marshall Plan, thus giving birth to the company Millipore [3]. From the point of view of polymers used in the manufacturing of membranes, virtually any polymer that can dissolve in a solvent, for which the solvent exists a total non-solvent miscible with it, can lead to the preparation of polymer membranes by phase inversion technique [4,5,6]. The most commonly used polymers for membrane synthesis are cellulose derivatives, polysulfones and polyethersulfones, polyamide, etc. Initially, the membranes appeared as a natural necessity for a natural need—access to drinking water. Over time, the unique property it possesses—selectivity—has been exploited for other uses; some seem unthinkable even 20–30 years ago. From the use of membranes in processes that are strictly related to filtration and separation, it has become possible to obtain membranes for guided bone regeneration, uses in the field of biosensors, and medium support for genetic or microfluidic engineering. Depending on the size of the pores on the active surface of the membrane and implicitly, the size of particles that can be retained on the surface by dimensional discrimination mechanisms is known and classified at present by the main membranation processes—conventional filtration, microfiltration, ultrafiltration, and nanofiltration.
Conventional filtration is the process with the highest use on the laboratory scale and is used for the removal of particles with a size greater than 50 μm, the isolation of crystals obtained from synthesis reactions, etc. Microfiltration and ultrafiltration are the membranary processes with the widest industrial use—filters in the pharmaceutical, oil, food, and dye industries. Ultrafiltration is also the membrane process that allows the use of membranes in the biomedical field, initially being able to replace the function of the kidney using blood filtering through membranes—hemodialysis. All membranes for ultrafiltration are the basis of most complex systems for controlled release of drugs (many of them already commercial), and in bone regeneration [7]. Also, there are commercially available variants, especially for use in dentistry or membranes used in tissue engineering [8,9,10]. In addition to conventional filtration processes based on dimensional discrimination, some niche applications have appeared over time, for example, reactive retention (the species of separation chemically interact with the surface of the membrane, with applications in chiral separations) [11, 12], molecular impregnated membranes (which can separate a single compound, regardless of optical isomery, from a mixture however complex), or separations in the magnetic field. Certainly, the challenges we are currently facing, especially in the field of environmental repair, water purification, tissue engineering, and biomedical sciences, will make the field of polymer membranes a topical one for a long time to come with a high degree of applicability, to respond in a timely manner to these challenges [13]. Initially, in the 1950s and 1960s, polymeric membrane means “a porous sheet” obtained from a single polymer. However, with the evolvement in the structural composition of several materials, there has been a significant advancement in the functionalized or composite membranes (in the first step of two or many polymers, then with various particles that either actively participated in the process of separation, such as zeolites, or improved mechanical and thermal properties—organoclays). The synthesis of carbon nanotubes in the early 1990s led to an explosion of carbon nanotube composite membranes. The same thing was expected to happen with the appearance of graphene [14,15,16]. Unlike nanotubes that in rare cases can actively participate in the separation process, having, in particular, the role of reinforcement with a spectacular increase in the mechanical and thermal resistance of the membranes, graphene, due to the large surface and especially due to the possibility to generate defects in their structure and act as a molecular or even atomic sieve has been investigated both for the ability to improve mechanical and thermal properties, and for the ability to allow only certain species to “cross” the membrane. The literature available in recent years of research in the field of composite membranes with graphene is one among those with the highest applicative value being in the field of nanofiltration. Why nanofiltration from all processes? Defects that can be generated on the surface of the graphene (vacancy of a few carbon atoms) can generate some “holes” that will allow passage only for solvent and will retain those chemical species that must be removed by ultrafiltration. Even without defects on the surface, the “net” structure can allow the retention of ions with a small atomic radius—Na+, K+, Li+, etc.—without the need for the high pressures required by the rule of this process of separation (based on electrostatic interactions that can occur between electrons delocalized from the surface of the graphene and positive charge of cations) [17,18,19]. This review aims to present readers with recent advances in the field of graphene composite membranes for nanofiltration processes. The presentation will focus on two main directions—methods of synthesis of these composite membranes and the performances of these membranes in the field of nanofiltration.
Nanofiltration is the process that currently leads to the synthesis of the largest amounts of the membrane with practical application, for the simple reason that it is that process that allows us to obtain drinking water from seawater (or another source of saltwater) by desalination [20]. Large areas of the globe benefit from this technology, especially the countries of the Middle East. Between polymer membranes, nanofiltration uses membranes with the smallest pores and the filtration process implicitly requiring the highest energy consumption [21, 22].
2 Graphene-based composite polymer membranes for nanofiltration
2.1 Synthesis methods for graphene-based composite nanofiltration membranes
The most common methods of synthesis for membranes refer to phase inversion or solvent evaporation. Phase reversal is the method of synthesis which starts from a concentrated polymer solution (deposited on a substrate) and can reach a membrane by immersion in a non-solvent (for polymer, but totally miscible with the polymer–solvent) or by evaporation of the initial solvent. Since nanofiltration requires pores with a very small diameter, the best method of synthesis between the two is solvent evaporation (the slower, the more favorable the formation of small pores). To obtain composite membranes with graphene, two strategies can be addressed: dispersion of graphene in an appropriate solvent, followed by strong mechanical shaking to homogenize the polymer and graphene solution or dispersion of graphene by ultrasonic directly in the polymer solution. Both methods have their advantages and shortcomings. In the first case, the integrity of the polymer is not affected, but the addition of the graphene solution decreases the concentration of the initial polymer solution, making it difficult to synthesize membranes of NF. In the second case, high-concentration polymer solutions can be obtained, with good dispersion of graphene, but the long ultrasonic time required for dispersion usually affects the polymer (breaking macromolecular chains with free radical formation and recombinations that cannot be controlled) leading to the synthesis of membranes with structural defects. However, the membranes obtained by this method do not present a sufficiently resistant active layer to the pressures required for NF processes. Requirements for increasing selectivity have led to the production of composite membranes with graphene for NF by unconventional methods, such as interfacial polymerization. Thus, using the support membrane of PES, a dispersion of GO was filtered through this membrane, followed by interfacial polymerization with obtaining a dense film of polyamide—which will actually represent the active layer of synthesized membranes (Fig. 1) [23]. The same principle was used for the synthesis of other membranation systems for NF [24,25,26]. Another innovative method is to obtain graphene films, dressed in the polymer. Thus, graphene films were nested in polyimide, the process followed by polymer reticulation to make the composite membrane stable to organic resistant solvent nanofiltration [27]. The presence of graphene improves both the membrane’s separation properties and its mechanical and thermal properties. In this case, the reticulation process is mandatory; otherwise, the polymeric film can be dissolved by the power solution during the filtration process.
Schematic process diagram of GO-PA composite membrane fabrication via spin-assisted IP technique. Reproduced with kind permission from Elsevier [23]
An easier way to obtain composite membranes is to filter graphene solutions through commercial membranes. Composite membranes of nitrated graphene doped with niobium were thus synthesized to improve the flow through the synthesized membranes [28]. After obtaining the laminated structure intermixed between the doped graphene and the polymer that makes up the membrane and after the reticulation of the membrane with 3-aminopropyl-triethoxysilane, flows were obtained six times greater than through the membrane without GO-Nb content. The use of functionalized graphene has generally led to better compatibility between graphene and polymer, which generally leads to both improved mechanical resistance and better performance infiltration processes [25]. Another modern synthesis technique refers to the coating of graphene films by electrospray. It is possible to obtain large membrane surfaces with homogeneous properties throughout the structure, and hydrodynamic resistance is much improved compared to classical methods of synthesis [29]. Not only the choice of polymer is important but also the derivation or functionalization of graphene. The synthesis of complex polyelectrolyte-based sodium carboxymethyl cellulose immobilized on GO can lead to a much-improved salt retention capacity in aqueous solutions [30]. By reticulation with glutaraldehyde, the system becomes stable enough to cope with high working pressures. In addition to membrane synthesis methods, strategies have been developed to improve the compatibility between graphene and polymer or to increase the stability of composite membranes through chemical interaction between the supporting polymer and the filling. Thus, the covalent interaction between the support polymer (polyimide) and graphene amino-functionalized (Fig. 2) has led to the production of membranes with high stability both in organic solvent (for the removal of dyes from the textile industry) or cations/heavy metals from aqueous solutions [31].
Schematic diagram of the aGQDs incorporated TFN SRNF preparation. Reproduced with kind permission from Elsevier [31]
2.2 Performances of graphene-based composite nanofiltration membranes
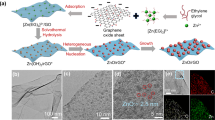
For an efficient separation of Mg2+ and Li2+ ions, membranes were synthesized with graphene oxide in the membrane structure of PES and the active layer of the nanofiltration membrane being made up of polyethylene (Fig. 3) [32]. After the synthesis and deposition of the PEI film, the membrane was immersed in trimesoyl chloride (TMC) for doping the surface of the graphene. At a content of 0.05% wt. GO, there was an increase in the flow of about 119%, and this TMC allowed interactions between the surface of the graphene and the groups O = S = O of PES, the membrane maintaining its hydrodynamic stability (constant flow and rejection—R(Mg2+) = 95.14%, R(Li+) = 20.93%), even after 7 days of continuous operation. The performance achieved by the reported system was superior to those obtained with other NF membranes that are forced to work at much higher pressures (16–20 atm) [33, 34] as opposed to 3 atm, the required operating pressure for the composite membrane with doped GO.
Schematic representation of membrane synthesis process. Reproduced with kind permission from Elsevier [32]
In the case of membranes with an active layer deposited over a support, the choice of suitable support is very important that presents chemical compatibility and roughness that does not affect the structure of the synthesized composite [35]. Too much roughness will deform the coating by implicitly affecting the separative properties by increasing the contact surface with favoring surface clogging, while an incompatibility between the two used systems can lead to hydrodynamic instability during the filtration process. For example, for a better compatibility between the support layer (in this case polycarbonate) and the active layer (containing GO), the polycarbonate surface was treated with argon ion beam, thus “leveling” the surface of the support layer (Fig. 4) [36]. By processing the surface support membranes, the flow of water through the composite membrane increased 6.4 times.
a–g Schematics illustrating the procedure used to prepare an ultrathin graphene oxide (GO) membrane on a wrinkled polycarbonate (PC) support. a Ar ion beam treatment of the PC support. b Wrinkle formation. c Fabrication of GO membrane on the wrinkled PC support. d Photographs of a GO membrane with 20 nm thickness on flat PC and wrinkled PC supports. e–g Top-view and cross-sectional view SEM images of GO on flat PC (upper set) and GO on wrinkled PC (lower set) supports. Reproduced with kind permission from Elsevier [36]
A new series of thin film composite membranes (TFC) has been synthesized based on graphene quantum dots (GQDs), modified with amino and sulfonic acid groupings on the surface, using GQDs as nanochannels for high-performance nanofiltration [37]. The membranes were able to remove Na+, Ca2+, and Mg2+ in percentages between 98 and 45%. The stability of the composites obtained was explained both by the gQDs structure and by the chemical interactions that occur in the amino groups of GQDs and the O = S = O groups in the structure of the polyethersulfone (supporting membrane), the whole assembly being covered by a thin layer of polyethylene. Also, GQDs were the basis of the synthesis of supermolecular architectures based on cyclodextrin obtaining membranes for NF used to filter and remove Congo red and Eriochrome black T (Fig. 5) [38]. In addition to the remarkable filtration capacity, due to the cavitates in cyclodextrins, the membranes also showed a remarkable resistance to chlorides due to the covalent interactions that formed the basis of the formation of architectures with separation properties. Similar performance was achieved for other composite membranes with graphene quantum dots [39, 40].
Schematic diagram of the structure of β-CD/GQDs NF membranes. Reproduced with permission from Elsevier [38]
Separation from small or medium-mass organic molecules is also a challenge in the field of nanofiltration. Using polyacrylonitrile membranes as support, GO solutions were filtered, ultrased at different times, followed by coating membranes supporting polyethyleneimine through interfacial polymerization and surface reticulation [41]. The membranes were used for the filtration of methylene blue from aqueous solutions as the function of the time allotted to the dispersion of graphene, the retention ranging from 56 to 98% (for dispersion in the ultrasonic field for 120′). The results were found to be superior to other systems previously reported in the literature, this being explained by the large contact area of the exfoliated graphene in the composite membrane structure [42, 43]. Laminated reduced GO treated in plasma was intercalated among cellulose nanofibers with the formation of nanostructured membranes, used in the retention of Fuchsin Acid, Rose Bengal, and Brilliant Blue [44]. The advantage of plasma treatment lies in maintaining the stability of the lamellar structures, including during the process of synthesis of the composite membrane with the preservation of properties (large contact surface in the membrane structure with increased efficiency of the separation process, without affecting the flow properties). The separation of different dyes has been studied using a complex membrane system obtained from GO starch functionalized filtered in a commercial membrane of PES (to match graphene with polymer and to increase the flow of water through the membrane) (Fig. 6). GO–starch composites were integrated in the polyamide (PA) top layer by esterification. The entire system was deposited on polyester and was studied for the retention of five organic compounds—Safranin O, Acid orange 2, Sucrose, Reactive violet 1, and Reactive green 13 (feed solutions in water, 200 ppm)—the retention ranging between 92.4 and 99.6% [45]. The membranes being negatively charged, having a thinner top layer also exhibited high rejection of Na2SO4 (96%) at the highest pure water flow of 10 L/m2hbar and a molecular weight cut-off of 330 Yes.
Schematic of synthesis of GO–starch nanocomposite membranes. Reproduced with kind permission from Elsevier [45]
The retention of methylene blue and Congo red was studied on composites GO covalent organic framework (COF), obtained from 1,3,5-triformylbenzene and para-phenylenediamine, using a commercial nylon membrane as support [46]. Outstanding performance, especially in dye retention and high solvent flow, was attributed to the hierarchically organized GB/COF composite. In addition, Nylon-based membranes were synthesized for the retention of dyes by an innovative process. After filtering a graphene solution through the Nylon membrane, the membrane was stabilized by electrospinning and electrospray using all Nylon6 (depositing 6–12 such successive layers) [47]. The synthesized membranes were proven to have remarkable hydrodynamic stability due to the multiple layers applied and good performance at the retention of methylene blue and methyl orange. Similar systems have been synthesized for the separation of sodium sulfate, rhodamine B, and acid from aqueous solutions [48]. Functionalization of the surface of graphene with choline chloride and ethylene glycol–based novel deep eutectic solvent led to a better interaction between graphene and dye (Congo Red, Methyl Blue, Evan Blue, Direct Red) using a polyethersulfone membrane as support [49]. Anionic colorants, Direct black 38, Xylenol orange, and Pumiceau S, were removed using phosphorylated chitosan–modified graphene oxide nanosheets incorporated as selective layer (using polyacrylonitriles as support membrane) with good retention results—58.8%, 76.3%, and 75.1% [50]. The use of an optimal amount of GO can improve both the properties of the active layer (hydrophilicity and negative loading of) as well as the porosity of the underlying layer. Ionic interactions that occur between composite membrane components as well as between separate species have led both to an increase in filtration efficiency and improvement in the membrane’s antifouling properties.
The use of graphene decorated with TiO2 took the process of nanofiltration of dyes a step further—their removal and photo-oxidative degradation during the same process [51]. The presence of TiO2 nanoparticles at GO decoration (microwave field synthesis) led to membranes being obtained in the polyamide/polyacrylonitrile system with high stability when removing heavy metals from organic solvents (hexane and heptane) [52]. In addition to the aforementioned advantages, the presence of TiO2 on the surface of the graphene significantly improved the flow through the membrane and also antifouling resistance, thus increasing the life and use of membranes [53]. The increase in flows and antifouling resistance through the composite membranes with graphene decorated with TiO2 has also been observed in the case of systems synthesized by other methods, such as hydrothermal [54]. Decorating GO with other metal nanoparticles (e.g., niobium) leads both to improved performance by increasing water flow and by being able to combine the separation process with a catalytic one (either photocatalysis or oxidative degradation) [55].
Better compatibility between the support membrane and the active layer with graphene content can also be achieved by the use of functionalized polymers. Thus, good results were obtained by the use of aminated polyvinyl chloride (APVC) membranes as support for the GO/polyamide system [56]. The chemical interaction between the two structures and polymers ensures a structural stability which translates into a longer use of the NF membrane with the preservation of separative and flow performance. Other systems investigated used, in addition to graphene, additions of hydrogels (sodium alginate–polyvinyl alcohol) to increase water flow [57], functionalization with organic species [58], crosslinked polyimide [27], pH-sensitive oligomers with variable geometry [59], interspersing self-assembled porphyrin molecules [60], polyvinylidene fluoride (PVDF)/styrene-maleic anhydride (SMA) copolymer [61], hollow fiber interspersed polymers [62], or forms of advanced organization of graphene film in the structure of the active layer [63, 64]. Some of the performances of the membranes studied are presented in Table 1.
A special class of composite membranes with graphene for nanofiltration processes is represented by the use of graphene with their porosity; in that case, graphene actively participates, as a molecular sieve, with a much more important role in the nanofiltration process [65]. Main properties that make graphene suitable for these applications (like spall pore size that enhances salt rejection by size exclusion, formation of nanochannels that enable nanofiltration, hydrophilic pores and edges that enhance water flux or ionization of functional groups at the pore’s perimeter that affects water transport and salt leakage) are presented in Fig. 7.
Important properties of graphene and graphene oxide that enhance the specific properties of the nanofiltration membrane for improving desalination. Reproduced with kind permission from Elsevier [65]
The principle of action for these membranes is based on the use of graphene with its porosity [66]. Graphene as such is completely impermeable for water [67]. However, this permeability can be obtained by generating atomic vacations and defects in the structure of the graphene, which will transform it from “sheet” to “sieve” [68, 69]. Such nanoporous graphene will peer the exclusive passage of water molecules, acting as a filter at the molecular or even atomic level (for atoms with large dimensions—for example, Co, Cu). This porosity can be obtained in the structure of graphene by bombarding with heavy ions, oxidative etching, or laser ablation [70,71,72,73,74]. The filtration capacity of composite membranes will depend on the size of the nanopores generated, the ion and electrostatic loading of the separate species, the presence of other functional groups on the surface of the graphene, and the interactions it establishes with the supporting polymer [75]. Too large atom holes (more than 3–5 carbon atoms) will also allow the passage of ions or organic molecules with low molecular mass. Too few separate ions (from highly diluted solutions) could also easily pass through the synthesized membranes. The presence of other functional groups and the interactions between graphene and the polymer of the supporting membrane can affect the free volume of pores and implicitly the ability to flow through the synthesized membranes, leading to very small flows that would make the obtained membrane materials unusable in practical applications. Also, a very important aspect in the case of these materials is related to the orientation of graphene in the membrane structure. They should have a parallel oriented lamellar structure so that they can perform the filter function. This problem can be solved by functionalizing the surface of the graphene with polymer, followed by the polymerization of the obtained adducts. For example, a thin-film nanofiltration membrane was obtained from system GO/TiO2/piperazine and trimesoyl chloride [54]. Such membranes are much more suitable for the retention of metals than for the retention of small ions (which can pass through nanopores). Thus, membranes were developed for the retention of Mg2+, Pb2+, Ni2+, Cd2+, and Zn2+ based on polyethyleneimine [76], Pb2+, Ni2+, Cd2+, Co2+, and Zn2+ with membrane capacity keeping its performance unchanged up to 10 successive filtration cycles [77] or even Nd3+, Gd3+, and Y3+ [78, 79].
3 Conclusions and future perspectives
Nanofiltration is an innovative process that currently gives us one of the most important components of life—water. The expansion of human activities has made it necessary to develop more and more advanced and selective methods to remove salts from water or organic micropollutants [80,81,82,83,84]. Large areas of the planet currently depend on desalination processes to have drinking water, and large industries to purify water or residual solvents—the pharmaceutical or dye industry being among the largest consumers of nanofiltration membranes. The use of graphene [85,86,87] and graphene derivatives when obtaining composite membranes has opened the possibility of achieving improved performance in nanofiltration processes, with the decrease of energy consumption and, implicitly, costs as well as in many other applications including as photocatalysts. Graphene as filler in nanofiltration membranes will assure the possibility of retaining any amount of heavy metals or cations from salts by adsorption at the surface of graphene. Also, small organic molecules, especially dyes, are rejected at the surface of graphene which acts as a molecular sieve. This has also a major impact in terms of the porosity of the active layer of the membrane and also in terms of energy consumption; at the laboratory scale, the composite nanofiltration membranes with graphene show high rejection yields at lower pressures than classical nanofiltration membranes. However, large parts of the synthesis processes remain at a high-cost price, which makes it impossible for large-scale use at this moment.
Obtaining molecular sieves based on graphene with defects in the structure will pave the way for highly selective separations with the gradual passage of these materials into large-scale use. However, it remains challenging to obtain aligned and parallel structures, so that the principle on which these composite membranes work retains its value. In addition, it is necessary to develop easier synthesis processes to be able to obtain large quantities of membranes that make possible applicability on an industrial scale. The cost price of graphene is still high; if we refer to the large quantities of material needed to obtain composite membranes, this remains an impediment.
By decorating graphene with metal oxides or other metal nanoparticles, it will open up the possibility of performing two processes simultaneously—the separation of chemical species and their degradation. By using, for example, TiO2, photooxidative degradation will be possible, while using zinc nanoparticles, niobium, and tin membrane reactors will be able to obtain catalytic degradation of separate species. This will have a huge impact on environmental contamination processes, especially in terms of dye molecules, pesticides, hormones, drugs, as well as in terms of reuse of the materials from which the membranes were obtained. The advances offered by these processes will influence not only the “philosophy of separation” but also the principles of the circular economy, by decreasing the consumption of raw materials and energy consumption.
Another direction of the future will be the synthesis of composite membranes with super carbonic architectures (graphene sheet parallel interconnected with the help of open carbon nanotubes, in this case, nanotubes acting as active pores). Such structures could change the paradigm of separations—instead of removing impurities from water, for example, we separate water from impurities, the diameter of nanotubes allowing only the passage of water molecules. The idea is not new, but there is a current limitation that could not be solved—water would have very high speed through such pores due to the absence of friction, which would lead to an increase of several thousand times in the flow in nanofiltration processes at the same time as a dramatic decrease in energy consumption. Nevertheless, it is precisely this very large increase in flow that raises a practical problem still unresolved—pumps and pipes that can bear the transport of a very large amount of water in a very short time. In addition, now, the costs of obtaining such super carbonic structures are extremely high and need both easier methods of preparation and the possibility to integrate these architectures into supporting polymer membranes.
References
M. Ulbricht, Advanced functional polymer membranes. Polymer 47(7), 2217–2262 (2006)
J.M. Lehn, Supramolecular chemistry: concepts and perspectives. Wiley ISBN 3–527- 2931, 1–6 (1995)
X. Kang, Y. Cheng, Y. Wen, J. Qi, X. Li, Bio-inspired co-deposited preparation of GO composite loose nanofiltration membrane for dye contaminated wastewater sustainable treatment. J. Hazard. Mater. 400, 123121 (2020)
A. Palla-Papavlu, S.I. Voicu, M. Dinescu, Sensitive materials and coating technologies for surface acoustic wave sensors. Chemosensors 9(5), 105 (2021)
I. Chiulan, E.B. Heggset, S.I. Voicu, G. Chinga-Carrasco, Photopolymerization of biobased polymers in a biomedical engineering perspective. Biomacromolecules 22(5), 1795–1814 (2021)
A. Muhulet, C. Tuncel, F. Miculescu, A.M. Pandele, C. Bobirica, C. Orbeci, L. Bobirica, A. Palla-Papavlu, S.I. Voicu, Synthesis and characterization of polysulfone-TiO2 doped MWCNT composite membranes by sonochemical method. Appl. Phys. A 126(3), 233 (2020)
A.M. Pandele, A. Constantinescu, I.C. Radu, F. Miculescu, S.I. Voicu, L.T. Ciocan, Synthesis and characterization of PLA–microstructured hydroxyapatite composite films. Materials 13(2), 274 (2020)
M. Oprea, S.I. Voicu, Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 247, 116683 (2020)
M. Oprea, S.I. Voicu, Cellulose composites with graphene for tissue engineering applications. Materials 13(23), 5347 (2020)
M. Oprea, S.I. Voicu, Recent advances in applications of cellulose derivatives-based composite membranes with hydroxyapatite. Materials 13, 2481 (2020)
A.M. Pandele, H. Iovu, C. Orbeci, C. Tuncel, A. Nicolescu, C. Deleanu, F. Miculescu, S.I. Voicu, Surface modified cellulose acetate membranes for the reactive retention of tetracycline. Sep. Purif. Technol. 249, 117145 (2020)
M.D. Raicopol, C. Andronescu, S.I. Voicu, E. Vasile, A.M. Pandele, Cellulose acetate/layered double hydroxide adsorptive membranes for efficient removal of pharmaceutical environmental contaminants. Carbohydr. Polym. 214, 204–212 (2019)
S.I. Voicu, V.K. Thakur, Aminopropyltriethoxysilane as a linker for cellulose-based functional materials: new horizons and future challenges. Curr. Opin. Green. Sust. Chem. 30, 100480 (2021)
M. Ionita, L.E. Crica, S.I. Voicu, A.M. Pandele, H. Iovu, Fabrication of cellulose triacetate/graphene oxide porous membrane. Polym. Adv. Technol. 27(3), 350–357 (2016)
S.I. Voicu, A.M. Pandele, E. Vasile, R. Rughinis, L.E. Crica, L. Pilan, M. Ionita, The impact of sonication time through polysulfonegraphene oxide composite films properties. Dig. J. Nanomater. Biostruct. 8(4), 1389–1394 (2013)
A.M. Pandele, O.S. Serbanescu, S.I. Voicu, Polysulfone composite membranes with carbonaceous structure. Synth. Appl. Coat. 10(7), 609 (2020)
V. Satulu, B. Mitu, A.M. Pandele, S.I. Voicu, L. Kravets, G. Dinescu, Composite polyethylene terephthalate track membranes with thin teflon-like layers: preparation and surface properties. Appl. Surf. Sci. 476, 452–459 (2019)
O.S. Serbanescu, S.I. Voicu, V.K. Thakur, Polysulfone functionalized membranes: properties and challenges. Mater. Today Chem. 17, 100302 (2020)
O.S. Serbanescu, A.M. Pandele, F. Miculescu, S.I. Voicu, Synthesis and characterization of cellulose acetate membranes with self-indicating properties by changing the membrane surface color for separation of Gd (III). Coatings 10(5), 468 (2020)
S.I. Voicu, A. Dobrica, S. Sava, A. Ivan, L. Naftanaila, Cationic surfactants-controlled geometry and dimensions of polymeric membrane pores. J. Optoelectron. Adv. Mater. 14(11–12), 923–928 (2012)
M. Zhou, J. Chen, W. Zhou, J. Sun, H. Tang, Developing composite nanofiltration membranes with highly stable antifouling property based on hydrophilic roughness. Sep. Purif. Technol. 256, 117799 (2021)
Y. Zhang, T.-S. Chung, Graphene oxide membranes for nanofiltration. Curr. Opin. Chem. Eng. 16, 9–15 (2017)
X. Kang, X. Liu, J. Liu, Y. Wen, J. Qi, X. Li, Spin-assisted interfacial polymerization strategy for graphene oxide-polyamide composite nanofiltration membrane with high performance. Appl. Surf. Sci. 508, 145198 (2020)
R.R. Hu, Y.J. He, M.R. Huang, G.K. Zhao, H.W. Zhu, Strong adhesion of graphene oxide coating on polymer separation membranes. Langmuir 34, 10569–10579 (2018)
Q.L. Xie, S.S. Zhang, Z. Hong, H.J. Ma, B.R. Zeng, X. Gong, W.Y. Shao, Q.Q. Wang, A novel double-modified strategy to enhance the performance of thin-film nanocomposite nanofiltration membranes: incorporating functionalized graphene into supporting and selective layers. Chem. Eng. J. 368, 186–201 (2019)
S.X. Zheng, Q.S. Tu, J.J. Urban, S.F. Li, B.X. Mi, Swelling of graphene oxide membranes in aqueous solution: characterization of interlayer spacing and insight into water transport mechanisms. ACS Nano 11, 6440–6450 (2017)
M.-L. Liu, J. Wang, J.-L. Guo, T.-D. Lu, X.-L. Cao, S.-P. Sun, Graphene oxide/cross-linked polyimide (GO/CLPI) composite membranes for organic solvent nanofiltration. Chem. Eng. Res. Des. 146, 182–189 (2019)
M. Kunimatsu, K. Nakagawa, T. Yoshioka, T. Shintani, T. Yasui, E. Kamio, S.C. Edman Tsang, J. Li, H. Matsuyama, Design of niobate nanosheet- graphene oxide composite nanofiltration membranes with improved permeability. J. Membr. Sci. 595, 117598 (2020)
L. Chen, J.-H. Moon, X. Ma, L. Zhang, Q. Chen, L. Chen, R. Peng, P. Si, J. Feng, Y. Li, J. Lou, L. Ci, High performance graphene oxide nanofiltration membrane prepared by electrospraying for wastewater purification. Carbon 130, 487–494 (2018)
Y.-C. Wang, S.R. Kumar, C.-M. Shih, W.-S. Hung, Q.-F. An, H.-C. Hsu, S.-H. Huang, S.J. Lue, High permeance nanofiltration thin film composites with a polyelectrolyte complex top layer containing graphene oxide nanosheets. J. Membr. Sci. 540, 391–400 (2017)
S. Li, C. Li, B. Su, M.Z. Hu, X. Gao, C. Gao, Amino-functionalized graphene quantum dots (aGQDs)-embedded thin film nanocomposites for solvent resistant nanofiltration (SRNF) membranes based on covalence interactions. J. Membr. Sci. 588, 117212 (2019)
P. Xu, J. Hong, X. Qian, Z. Xu, H. Xia, Q.-Q. Ni, “Bridge” graphene oxide modified positive charged nanofiltration thin membrane with high efficiency for Mg2+/Li+ separation. Desalination 488, 114522 (2020)
S.-Y. Sun, L.-J. Cai, X.-Y. Nie, X. Song, J.-G. Yu, Separation of magnesium and lithium from brine using a Desal nanofiltration membrane. J. Water Process Eng. 7, 210–217 (2015)
G. Yang, H. Shi, W. Liu, W. Xingn, A. Xu, Investigation of Mg2+/Li+ separation by nanofiltration. Chin. J. Chem. Eng. 19, 586–591 (2011)
M. Zhang, J. Sun, Y. Mao, G. Liu, W. Jin, Effect of substrate on formation and nanofiltration performance of graphene oxide membranes. J. Membr. Sci. 574, 196–204 (2019)
Y.T. Nam, S.J. Kim, K.M. Kang, W.-B. Jung, D.W. Kim, H.-T. Jung, Enhanced nanofiltration performance of graphene-based membranes on wrinkled polymer supports. Carbon 148, 370–377 (2019)
Y. Lin, Q. Shen, Y. Kawabata, J. Segawa, X. Cao, K. Guan, T. Istirokhatun, T. Yoshioka, H. Matsuyama, Graphene quantum dots (GQDs)-assembled membranes with intrinsic functionalized nanochannels for high-performance nanofiltration. Chem. Eng. J. 127602(2020). https://doi.org/10.1016/j.cej.2020.127602
J. Xue, J. Shen, R. Zhang, F. Wang, S. Liang, X. You, Q. Yu, Y. Hao, Y. Su, Z. Jiang, High-flux nanofiltration membranes prepared with β-cyclodextrin and graphene quantum dots. J. Membr. Sci. 612, 118465 (2020)
Y. Liang, C. Li, S. Li, B. Su, M.Z. Hu, X. Gao, C. Gao, Graphene quantum dots (GQDs)-polyethyleneimine as interlayer for the fabrication of high performance organic solvent nanofiltration (OSN) membranes. Chem. Eng. J. 380, 122462 (2020)
R. Bi, R. Zhang, J. Shen, Y. Liu, M. He, X. You, Y. Su, Z. Jiang, Graphene quantum dots engineered nanofiltration membrane for ultrafast molecular separation. J. Membr. Sci. 572, 504–511 (2019)
V. Kandjou, Z. Gonzalez, B. Acevedo, J.M. Munuera, J.I. Paredes, S. Melendi-Espina, Influence of graphene oxide’s characteristics on the fabrication and performance of crosslinked nanofiltration membranes. J. Taiwan Inst. Chem. Eng. 119, 158–165 (2021)
J. Feng, J. Zhu, L. Wei, J. Li, W. Yan, Effect of hydroxyl group of carboxylic acids on the adsorption of acid red G and methylene blue on TiO2. Chem. Eng. J. 269, 316–322 (2015)
L. Nie, K. Goh, Y. Wang, J. Lee, Y. Huang, H.E. Enis Karahan, K. Zhou, M.D. Guiver, T.H. Bae, Realizing small-flake graphene oxide membranes for ultrafast size-dependent organic solvent nanofiltration. Sci. Adv. 6, 1–13 (2020)
S. Mohammed, H.M. Hegab, R. Ou, S. Liu, H. Ma, X. Chen, T. Sridhar, H. Wang, Effect of oxygen plasma treatment on the nanofiltration performance of reduced graphene oxide/cellulose nanofiber composite membranes. Green Chem. Eng. 2(1), 122–131 (2021)
J.P. Ambre, K.B. Dhopte, R.P. Nemade, D.H. Vishwanath, High flux hyperbranched starch–graphene oxide piperazinamide composite nanofiltration membrane. J. Environ. Chem. Eng. 7, 103300 (2019)
L. Chen, W. Wang, Q. Fang, K. Zuo, G. Hou, Q. Ai, Q. Li, L. Ci, J. Lou, High performance hierarchically nanostructured graphene oxide/covalent organic framework hybrid membranes for stable organic solvent nanofiltration. Appl. Mater. Today 20, 100791 (2020)
L. Chen, Y. Li, L. Chen, N. Li, C. Dong, Q. Chen, B. Liu, Q. Ai, P. Si, J. Feng, L. Zhang, J. Suhr, J. Lou, L. Ci, A large-area free standing graphene oxide multilayer membrane with high stability for nanofiltration applications. Chem. Eng. J. 345, 536–544 (2018)
B. Zhao, Z. Guo, H. Wang, L. Wang, Y. Qian, X. Long, C. Ma, Z. Zhang, J. Li, H. Zhang, Enhanced water permeance of a polyamide thin-film composite nanofiltration membrane with a metal-organic framework interlayer. J. Membr. Sci. 625, 119154 (2021)
N. Mehrabi, H. Lin, N. Aich, Deep eutectic solvent functionalized graphene oxide nanofiltration membranes with superior water permeance and dye desalination performance. Chem. Eng. J. 412, 128577 (2021)
Y. Song, Y. Sun, M. Chen, P. Huang, T. Li, X. Zhang, K. Jiang, Efficient removal and fouling-resistant of anionic dyes by nanofiltration membrane with phosphorylated chitosan modified graphene oxide nanosheets incorporated selective layer. J. Water Process Eng. 34, 101086 (2020)
Y. Liu, Z. Yu, Y. Peng, L. Shao, X. Li, H. Zeng, A novel photocatalytic self-cleaning TiO2 nanorods inserted graphene oxide based nanofiltration membrane. Chem. Phys. Lett. 749, 137424 (2020)
H. Abadikhah, E.N. Kalali, S. Behzadi, S.A. Khan, X. Xu, M.E. Shabestari, S. Agathopoulos, High flux thin film nanocomposite membrane incorporated with functionalized TiO2@reduced graphene oxide nanohybrids for organic solvent nanofiltration. Chem. Eng. Sci. 204, 99–109 (2019)
J. Wang, Y. Wang, J. Zhu, Y. Zhang, J. Liu, B. Van der Bruggen, Construction of TiO2@graphene oxide incorporated antifouling nanofiltration membrane with elevated filtration performance. J. Membr. Sci. 533, 279–288 (2017)
M. Safarpour, V. Vatanpour, A. Khataee, M. Esmaeili, Development of a novel high flux and fouling-resistant thin film composite nanofiltration membrane by embedding reduced graphene oxide/TiO2. Sep. Purif. Technol. 154, 96–107 (2015)
M. Kunimatsu, K. Nakagawa, T. Yoshioka, T. Shintani, T. Yasui, E. Kamio, S.C. Edman Tsang, J. Li, H. Matsuy, Design of niobate nanosheet-graphene oxide composite nanofiltration membranes with improved permeability. J. Membr. Sci. 595, 117598 (2020)
Y. Qin, H. Liu, Y. Liu, M. Chen, K. Chen, Y. Huang, C. Xiao, Design of a novel interfacial enhanced GO-PA/APVC nanofiltration membrane with stripe-like structure. J. Membr. Sci. 604, 118064 (2020)
S. Amiri, A. Asghari, V. Vatanpour, M. Ra, Fabrication and characterization of a novel polyvinyl alcohol-graphene oxide-sodium alginate nanocomposite hydrogel blended PES nanofiltration membrane for improved water purification. Sep. Purif. Technol. 250, 117216 (2020)
J. Hou, Y. Chen, W. Shi, C. Bao, X. Hu, Graphene oxide/methylene blue composite membrane for dyes separation: formation mechanism and separation performance. Appl. Surf. Sci. 505, 144145 (2020)
W. Shao, C. Liu, H. Ma, Z. Hong, Q. Xie, Y. Lu, Fabrication of pH-sensitive thin-film nanocomposite nanofiltration membranes with enhanced performance by incorporating amine functionalized graphene oxide. Appl. Surf. Sci. 487, 1209–1221 (2019)
T. Gao, L. Huang, C. Li, G. Xu, G. Shi, Graphene membranes with tuneable nanochannels by intercalating self-assembled porphyrin molecules for organic solvent nanofiltration. Carbon 124, 263–270 (2017)
D. Kang, H. Shao, G. Chen, X. Dong, S. Qin, Fabrication of highly permeable PVDF loose nanofiltration composite membranes for the effective separation of dye/salt mixtures. J. Membr. Sci. 621, 118951 (2021)
L. Tian, Y. Jiang, S. Li, L. Han, B. Su, Graphene oxide interlayered thin-film nanocomposite hollow fiber nanofiltration membranes with enhanced aqueous electrolyte separation performance. Sep. Purif. Technol. 248, 117153 (2020)
Y. Zhong, S. Mahmud, Z. He, Y. Yang, Z. Zhang, F. Guo, Z. Chen, Z. Xiong, Y. Zhao, Graphene oxide modified membrane for highly efficient wastewater treatment by dynamic combination of nanofiltration and catalysis. J. Hazard. Mater. 397, 122774 (2020)
R. Hu, R. Zhang, Y. He, G. Zhao, H. Zhu, Graphene oxide-in-polymer nanofiltration membranes with enhanced permeability by interfacial polymerization. J. Membr. Sci. 564, 813–819 (2018)
A. Anand, B. Unnikrishnan, J.-Y. Mao, H.-J. Lin, C.-C. Huang, Graphene-based nanofiltration membranes for improving salt rejection, water flux and antifouling—a review. Desalination 429, 119–133 (2018)
A.K. Geim, Graphene: status and prospects. Science 324, 1530–1535 (2009)
V. Berry, Impermeability of graphene and its applications. Carbon 62, 1–10 (2013)
A. Nicolaı, B.G. Sumpter, V. Meunier, Tunable water desalination across graphene oxide framework membranes. Phys. Chem. Chem. Phys. 16, 8646–8654 (2014)
J. Lawler, Incorporation of graphene-related carbon nanosheets in membrane fabrication for water treatment: a review. Membranes 6, 57 (2016)
H. Vázqueza, E.H. Åhlgrena, O. Ochedowski, A.A. Leino, R. Mirzayev, R. Kozubek, H. Lebius, M. Karlušic, M. Jakšic, A.V. Krasheninnikov, J. Kotakoski, M. Schleberger, K. Nordluna, F. Djurabekova, Creating nanoporous graphene with swift heavy ions. Carbon 114, 511–518 (2017)
M.D. Fischbein, M. Drndić, Electron beam nanosculpting of suspended graphene sheets. Appl. Phys. Lett. 93, 113107 (2008)
C. Yu, B. Zhang, F. Yana, J. Zhao, J. Li, L. Li, J. Li, Engineering nano-porous graphene oxide by hydroxyl radicals. Carbon 105, 291–296 (2016)
D.S. Fox, P. Maguire, Y. Zhou, C. Rodenburg, A. O’Neill, J.N. Coleman, H. Zhang, Sub-5nm graphene nanopore fabrication by nitrogen ion etching induced by a low energy electron beam. Nanotechnology 27, 195302 (2016)
S.C. O’Hern, M.S.H. Boutilier, J.-C. Idrobo, Y. Song, J. Kong, T. Laoui, M. Atieh, R. Karnik, Selective ionic transport through tunable subnanometer pores in single layer graphene membranes. Nano Lett. 14, 1234–1241 (2014)
D. An, L. Yang, T.-J. Wang, B. Liu, Separation performance of graphene oxide membrane in aqueous solution. Ind. Eng. Chem. Res. 55, 4803–4810 (2016)
Y. Zhang, S. Zhang, T.-S. Chung, Nanometric graphene oxide framework membranes with enhanced heavy metal removal via nanofiltration. Environ. Sci. Technol. 49, 10235–10242 (2015)
R. Sitko, M. Musielak, B. Zawisza, E. Talik, A. Gagor, Graphene oxide/cellulose membranes in adsorption of divalent metal ions. RSC Adv. 6, 96595–96605 (2016)
R.M. Ashour, H.N. Abdelhamid, A.F. Abdel-Magied, A.A. Abdel-Khalek, M.M. Ali, A. Uheida, M. Muhammed, X. Zou, J. Dutta, Rare earth ions adsorption onto graphene oxide nanosheets. Solvent Extr. Ion Exch. 35, 91–103 (2017)
A.Y. Romanchuk, A.S. Slesarev, S.N. Kalmykov, D.V. Kosynkin, J.M. Tour, Graphene oxide for effective radionuclide removal. Phys. Chem. Chem. Phys. 15, 2321–2327 (2013)
B. Ates, S. Koytepe, A. Ulu, C. Gurses, V.K. Thakur, Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 120, 9304–9362 (2020)
R. Kumar, P. Raizada, N. Verma, A. Hosseini-Bandegharaei, V.K. Thakur, Q.V. Le, V.-H. Nguyen, R. Selvasembian, P. Singh, Recent advances on water disinfection using bismuth based modified photocatalysts: strategies and challenges. J. Clean. Prod. 297, 126617 (2021)
B. Sharma, S. Thakur, G. Mamba, R.K. Prateek, V.K. Gupta, V.K. Thakur. Gupta, Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. J Environ. Chem. Eng. 9, 104608 (2020)
A. Verma, S. Thakur, G. Mamba, R.K. Prateek, P. Gupta, V.K. Thakur. Thakur, Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 148, 1130–1139 (2020)
A.K. Rana, Y.K. Mishra, V.K. Gupta, V.K. Thakur, Sustainable materials in the removal of pesticides from contaminated water: perspective on macro to nanoscale cellulose. Sci. Total Environ. 797, 149129 (2021)
D. Trache, V.K. Thakur, R. Boukherroub, Cellulose nanocrystals/graphene hybrids—a promising new class of materials for advanced applications. Nanomaterials 10, 1523 (2020)
M. Miculescu, V.K. Thakur, F. Miculescu, S.I. Voicu, Graphene-based polymer nanocomposite membranes: a review. Polym. Adv. Technol. 27, 844–859 (2016)
N. Chandel, K. Sharma, A. Sudhaik, P. Raizada, A. Hosseini-Bandegharaei, V.K. Thakur, P. Singh, Magnetically separable ZnO/ZnFe2O4 and ZnO/CoFe2O4 photocatalysts supported onto nitrogen-doped graphene for photocatalytic degradation of toxic dyes. Arab. J. Chem. 13, 4324–4340 (2020)
Acknowledgements
This work was supported by a grant from the Ministry of Research, Innovation and Digitization, CNCS/CCCDI–UEFISCDI, project number PN-III-P4-ID-PCE-2020-1154, Hemodialysis combined with stimuli-responsive drug delivery – a new generation of polymeric membranes for advanced biomedical applications within PNCDI III.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Voicu, S.I., Thakur, V.K. Graphene-based composite membranes for nanofiltration: performances and future perspectives. emergent mater. 5, 1429–1441 (2022). https://doi.org/10.1007/s42247-021-00291-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-021-00291-6