Abstract
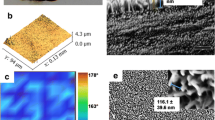
The wing architecture is an inspiration to fabricate novel materials with exquisite properties. The current study characterizes the structure and biological function of a termite’s wing. The topography of the surface of the wing was studied by electron microscopy, and surface profilometer. The physicochemical property of the surface was analyzed by Fourier transform infrared spectroscopy, X-ray diffraction spectroscopy, energy-dispersive X-ray spectroscopy, and gas chromatography–mass spectrometry analysis of the epicuticle content. Water Contact Angle measurement confirmed the hydrophobicity of the wing surface. When microorganisms come in contact with the surface of the wing, they adhere to the wing surface due to cell surface properties of their own and the surface chemistry of the wing. The current study reported the adhesion behavior of two bacterial species. The bactericidal activity of the wing was confirmed by counting the bacterial cell viability and examination under a confocal laser scanning microscope. Adhesion of bacteria was observed under the electron microscope. Bacterial oxidative stress, the topography of the wing, and the surface chemistry of the wing are the crucial factors that induce bactericidal activity. The nanostructure along with the chemical composition of the wing can be mimicked for the fabrication of novel material with antibacterial properties.







Similar content being viewed by others
References
Zhang, X. X., Wang, L., & Levanen, E. (2013). Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Advances, 3, 12003–12020.
Paikra, S. K., & Mishra, M. (2021). Role of physicochemical organization of Rhyothemis variegata wing in monitoring bactericidal activity. Surfaces and Interfaces, 27, 101576.
Zhao, D. Y., Tian, Q. Q., Wang, M. J., & Jin, Y. F. (2014). Study on the Hydrophobic Property of Shark-Skin-Inspired Micro-Riblets. Journal of Bionic Engineering, 11, 296–302.
Patil, D., Overland, M., Stoller, M., & Chatterjee, K. (2021). Bioinspired nanostructured bactericidal surfaces. Current Opinion in Chemical Engineering, 34, 100741.
Liu, D., Song, B. F., Yang, W. Q., Yang, X. J., Xue, D., & Lang, X. Y. (2021). A brief review on aerodynamic performance of wingtip slots and research prospect. Journal of Bionic Engineering, 18, 1255–1279.
Lee, Y., Yoo, Y., Kim, J., Widhiarini, S., Park, B., Park, H. C., Yoon, K. J., & Byun, D. (2009). Mimicking a superhydrophobic insect wing by argon and oxygen ion beam treatment on polytetrafluoroethylene film. Journal of Bionic Engineering, 6, 365–370.
Shahali, H., Hasan, J., Mathews, A., Wang, H., Yan, C., Tesfamichael, T., & Yarlagadda, P. (2019). Multi-biofunctional properties of three species of cicada wings and biomimetic fabrication of nanopatterned titanium pillars. Journal of Materials Chemistry B, 7, 1300–1310.
Hasan, J., Roy, A., Chatterjee, K., & Yarlagadda, P. K. (2019). Mimicking insect wings: the roadmap to bioinspiration. ACS Biomaterials Science & Engineering, 5, 3139–3160.
Bandara, C. D., Singh, S., Afara, I. O., Wolff, A., Tesfamichael, T., Ostrikov, K., & Oloyede, A. (2017). Bactericidal effects of natural nanotopography of dragonfly wing on Escherichia coli. ACS Applied Materials & Interfaces, 9, 6746–6760.
Nguyen, S. H., Webb, H. K., Mahon, P. J., Crawford, R. J., & Ivanova, E. P. (2014). Natural insect and plant micro-/nanostructsured surfaces: An excellent selection of valuable templates with superhydrophobic and self-cleaning properties. Molecules, 19, 13614–13630.
Ortiz, C., & Boyce, M. C. (2008). Bioinspired structural materials. Science, 319, 1053–1054.
Rajabi, H., Moghadami, M., & Darvizeh, A. (2011). Investigation of microstructure, natural frequencies and vibration modes of dragonfly Wing. Journal of Bionic Engineering, 8, 165–173.
Wedmore, I., Mcmanus, J. G., Pusateri, A. E., & Holcomb, J. B. (2006). A special report on the chitosan-based hemostatic dressing: experience in current combat operations. Journal of Trauma and Acute Care Surgery, 60, 655–658.
Meyers, M. A., Chen, P.-Y., Lin, A.Y.-M., & Seki, Y. (2008). Biological materials: structure and mechanical properties. Progress in Materials Science, 53, 1–206.
Kumari, N., Sood, N., & Krishnan, V. (2020). Beetle wing inspired fabrication of nanojunction based biomimetic SERS substrates for sensitive detection of analytes. Materials Technology. https://doi.org/10.1080/10667857.10662020.11816382
Kumari, N., Kumar, A., & Krishnan, V. (2021). Ultrathin Au–Ag heterojunctions on nanoarchitectonics based biomimetic substrates for dip catalysis. Journal of Inorganic and Organometallic Polymers and Materials, 31, 1954–1966.
Sharma, V., Kumar, S., Reddy, K. L., Bahuguna, A., & Krishnan, V. (2016). Bioinspired functional surfaces for technological applications. Journal of Molecular and Engineering Materials, 4, 1640006.
Gao, X. Y., & Guo, Z. G. (2017). Biomimetic superhydrophobic surfaces with transition metals and their oxides: a review. Journal of Bionic Engineering, 14, 401–439.
Cosme, L., Jr., Turchen, L. M., & Guedes, R. N. C. (2020). Chemical constituents of tropical woods and resistance to the invasive drywood termite Cryptotermes brevis. Journal of Applied Entomology, 144, 270–277.
Kaya, M., Sofi, K., Sargin, I., & Mujtaba, M. (2016). Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydrate Polymers, 145, 64–70.
Wang, K. J., Zhang, J. Q., Fang, Y. Q., Chen, D. B., Liu, L. P., Han, Z. W., & Ren, L. Q. (2019). Micro/nano-scale characterization and fatigue fracture resistance of mechanoreceptor with crack-shaped slit arrays in scorpion. Journal of Bionic Engineering, 16, 410–422.
Vargas, M., Albors, A., Chiralt, A., & González-Martínez, C. (2009). Characterization of chitosan–oleic acid composite films. Food Hydrocolloids, 23, 536–547.
Du, Y.-Z., Wang, L., Yuan, H., Wei, X.-H., & Hu, F.-Q. (2009). Preparation and characteristics of linoleic acid-grafted chitosan oligosaccharide micelles as a carrier for doxorubicin. Colloids and Surfaces B: Biointerfaces, 69, 257–263.
Atarian, M., Rajaei, A., Tabatabaei, M., Mohsenifar, A., & Bodaghi, H. (2019). Formulation of Pickering sunflower oil-in-water emulsion stabilized by chitosan-stearic acid nanogel and studying its oxidative stability. Carbohydrate Polymers, 210, 47–55.
Fernandez, J. G., & Ingber, D. E. (2012). Unexpected strength and toughness in chitosan-fibroin laminates inspired by insect cuticle. Advanced Materials, 24, 480–484.
Cai, Y., Bing, W., Xu, X., Zhang, Y., Chen, Z., & Gu, Z. (2021). Topographical nanostructures for physical sterilization. Drug Delivery and Translational Research, 11, 1376–1389.
Mcmahon, A., Lu, H., & Butovich, I. A. (2013). The spectrophotometric sulfo-phospho-vanillin assessment of total lipids in human meibomian gland secretions. Lipids, 48, 513–525.
Ivanova, E. P., Nguyen, S. H., Webb, H. K., Hasan, J., Truong, V. K., Lamb, R. N., Duan, X., Tobin, M. J., Mahon, P. J., & Crawford, R. J. (2013). Molecular organization of the nanoscale surface structures of the dragonfly Hemianax papuensis wing epicuticle. PLoS ONE, 8, 67893.
Gołębiowski, M., Boguś, M. I., Paszkiewicz, M., & Stepnowski, P. (2010). The composition of the free fatty acids from Dendrolimus pini exuviae. Journal of Insect Physiology, 56, 391–397.
Van Dooremalen, C., & Ellers, J. (2010). A moderate change in temperature induces changes in fatty acid composition of storage and membrane lipids in a soil arthropod. Journal of Insect Physiology, 56, 178–184.
Gołębiowski, M., Boguś, M. I., Paszkiewicz, M., & Stepnowski, P. (2011). Cuticular lipids of insects as potential biofungicides: methods of lipid composition analysis. Analytical and Bioanalytical Chemistry, 399, 3177–3191.
Ivanova, E. P., Hasan, J., Webb, H. K., Truong, V. K., Watson, G. S., Watson, J. A., Baulin, V. A., Pogodin, S., Wang, J. Y., & Tobin, M. J. (2012). Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small (Weinheim an der Bergstrasse, Germany), 8, 2489–2494.
Sahoo, J. K., Paikra, S. K., Mishra, M., & Sahoo, H. (2019). Amine functionalized magnetic iron oxide nanoparticles: synthesis, antibacterial activity and rapid removal of Congo red dye. Journal of Molecular Liquids, 282, 428–440.
Watson, G. S., Cribb, B. W., & Watson, J. A. (2010). How micro/nanoarchitecture facilitates anti-wetting: an elegant hierarchical design on the termite wing. ACS Nano, 4, 129–136.
Kreuz, P., Arnold, W., & Kesel, A. (2001). Acoustic microscopic analysis of the biological structure of insect wing membranes with emphasis on their waxy surface. Annals of Biomedical Engineering, 29, 1054–1058.
Kaya, M., Bitim, B., Mujtaba, M., & Koyuncu, T. (2015). Surface morphology of chitin highly related with the isolated body part of butterfly (Argynnis pandora). International Journal of Biological Macromolecules, 81, 443–449.
Zeier, J., & Schreiber, L. (1999). Fourier transform infrared-spectroscopic characterisation of isolated endodermal cell walls from plant roots: chemical nature in relation to anatomical development. Planta, 209, 537–542.
Padmavathi, A. R., Abinaya, B., & Pandian, S. K. (2014). Phenol, 2, 4-bis (1, 1-dimethylethyl) of marine bacterial origin inhibits quorum sensing mediated biofilm formation in the uropathogen Serratia marcescens. Biofouling, 30, 1111–1122.
Lian, J., Pan, G., Xu, J., Zhu, Z., & Ni, J. (2019). Wenzel-cassie wetting transition of droplet on rough metal substrates. Science of Advanced Materials, 11, 533–539.
Kaya, M., Mujtaba, M., Ehrlich, H., Salaberria, A. M., Baran, T., Amemiya, C. T., Galli, R., Akyuz, L., Sargin, I., & Labidi, J. (2017). On chemistry of γ-chitin. Carbohydrate Polymers, 176, 177–186.
Hossin, M. A., Al Shaqsi, N. H. K., Al Touby, S. S. J., & Al Sibani, M. A. (2021). A review of polymeric chitin extraction, characterization, and applications. Arabian Journal of Geosciences, 14, 1870.
Kelleher, S. M., Habimana, O., Lawler, J., O’reilly, B., Daniels, S., Casey, E., & Cowley, A. (2015). Cicada wing surface topography: an investigation into the bactericidal properties of nanostructural features. ACS Applied Materials & Interfaces, 8, 14966–14974.
Rzhepishevska, O., Limanska, N., Galkin, M., Lacoma, A., Lundquist, M., Sokol, D., Hakobyan, S., Sjostedt, A., Prat, C., & Ramstedt, M. (2018). Characterization of clinically relevant model bacterial strains of Pseudomonas aeruginosa for anti-biofilm testing of materials. Acta Biomaterialia, 76, 99–107.
Das, M. C., Sandhu, P., Gupta, P., Rudrapaul, P., De, U. C., Tribedi, P., Akhter, Y., & Bhattacharjee, S. (2016). Attenuation of Pseudomonas aeruginosa biofilm formation by Vitexin: a combinatorial study with azithromycin and gentamicin. Scientific Reports, 6, 23347.
Truong, V. K., Lapovok, R., Estrin, Y. S., Rundell, S., Wang, J. Y., Fluke, C. J., Crawford, R. J., & Ivanova, E. P. (2010). The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials, 31, 3674–3683.
Benhabiles, S., Salah, R., Lounici, H., Drouiche, N., Goosen, M. F. A., & Mameri, N. (2012). Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocolloids, 29, 48–56.
Da Silva Lucas, A. J., Oreste, E. Q., Costa, H. L. G., López, H. M., Saad, C. D. M., & Prentice, C. (2021). Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chemistry, 343, 128550.
Kravanja, G., Primozic, M., Knez, Z., & Leitgeb, M. (2019). Chitosan-based (Nano)materials for novel biomedical applications. Molecules, 24, 1960.
Ahmed, S., & Ikram, S. (2016). Chitosan based scaffolds and their applications in wound healing. Achievements in the Life Sciences, 10, 27–37.
Karthikeyan, C., Varaprasad, K., Venugopal, S. K., Shakila, S., Venkatraman, B., & Sadiku, R. (2021). Biocidal (bacterial and cancer cells) activities of chitosan/CuO nanomaterial, synthesized via a green process. Carbohydrate Polymers, 259, 117762.
Roy, S., Mondal, A., Yadav, V., Sarkar, A., Banerjee, R., Sanpui, P., & Jaiswal, A. (2019). Mechanistic insight into the antibacterial activity of chitosan exfoliated MoS2 nanosheets: membrane damage, metabolic inactivation, and oxidative stress. ACS Applied Bio Materials, 2, 2738–2755.
Leonida, M. D., Belbekhouche, S., Benzecry, A., Peddineni, M., Suria, A., & Carbonnier, B. (2018). Antibacterial hop extracts encapsulated in nanochitosan matrices. International Journal of Biological Macromolecules, 120, 1335–1343.
Chang, A. K. T., Frias, R. R., Jr., Alvarez, L. V., Bigol, U. G., & Guzman, J. P. M. D. (2019). Comparative antibacterial activity of commercial chitosan and chitosan extracted from Auricularia sp. Biocatalysis and Agricultural Biotechnology, 17, 189–195.
Jenkins, J., Mantell, J., Neal, C., Gholinia, A., Verkade, P., Nobbs, A. H., & Su, B. (2020). Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nature Communications, 11, 1626.
Acknowledgements
SKP and SM are thankful to MHRD for fellowship assistance. NN is thankful to the Department of science and technology, DST-Inspire, India for financial assistance, and JB is thankful to DBT, India for fellowship assistance. The authors would like to express sincere gratitude to Dr. Elena P. Ivanova for providing valuable suggestions regarding the surface property of the wing to improve the quality of work. We are also grateful to the central instrument facility of NIT, Rourkela, Odisha, India for providing the instruments. Our lab is also supported by the Department of Biotechnology, Government of India, Grant No. BT/PR21857/NNT/28/1238/2017, Science and engineering research board (SERB) EMR/2017/003054, Odisha DBT (Department of biotechnology) 3325/ST(BIO)-02/2017. Anonymous reviewers are thankfully acknowledged for their valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paikra, S.K., Mukherjee, S., Nayak, N. et al. Impact of Hierarchical Architecture of Cryptotermes brevis Wing on the Modulation of Bacterial Adhesion. J Bionic Eng 19, 516–529 (2022). https://doi.org/10.1007/s42235-021-00148-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-021-00148-y