Abstract
The African fungus Ceratocystis albifundus does not cause disease symptoms on its wide range of native woody hosts. However, on non-native Acacia mearnsii and orchard-grown Protea cynaroides, it represents an economically significant pathogen. Because previous studies exploring the biological fitness of C. albifundus were constrained by small sample sizes, we aimed to determine how commonly used measures of fitness (growth in culture, pathogenicity and sexual fertility status) vary across natural populations. For this purpose, a collection of 58 isolates originating from diverse hosts and geographic locations in South Africa were subjected to growth studies on synthetic culture medium, pathogenicity tests on A. mearnsii saplings, and sequence-based assays of fertility status. We found that these traits were generally not correlated with one another, although isolates from the Summer rainfall region and from native hosts induced significantly shorter lesions on A. mearnsii than isolates from the Winter rainfall region and from diseased A. mearnsii and orchard-grown P. cynaroides tissues. In other words, aggressiveness of C. albifundus to A. mearnsii was significantly influenced by the isolates’ geographic origin and host species, irrespective of their fertility status or growth rates. Additionally, the broad lack of correlation among growth, pathogenicity and fertility suggested that these fitness components are likely underpinned by distinct genetic and molecular mechanisms. Our study thus provides a robust foundation for further exploration of the fitness landscape in this important tree pathogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ceratocystis albifundus De Beer, Wingfield & Morris (Ascomycota) is an African fungus that resides in the family Ceratocystidaceae Locq. ex Réblová, W. Gams & Seifert, together with numerous other economically important plant pathogens (De Beer et al. 2014). In southern and eastern Africa, C. albifundus infects different woody plants (e.g., Protea gigantea L., Burkea africana Hook. and Combretum molle R.Br. ex G.Don) native to the region, as well as invasive non-native species like Acacia melanoxylon R.Br. (Roux et al. 2007; Mbenoun et al. 2013). In these cases, the fungus does not cause any obvious signs of disease and the plants remain healthy (Roux et al. 2007). However, on the non-native forestry species Acacia mearnsii and on commercially cultivated Protea cynaroides L., C. albifundus is an aggressive pathogen, causing canker and wilt disease that results in tree mortality and significant economic losses to the forestry and cut-flower industries (Roux et al. 1999; Lee et al. 2020).
Like other members of the Ceratocystidaceae, C. albifundus is closely associated with insects (De Beer et al. 2014). In these associations, the fungus serves as a source of nutrition for the insects, which in turn assist with dispersal of the fungus. Accordingly, fungi in this family are adapted for insect dispersal by producing ascospores in sticky drops borne at the apices of long-necked ascocarps (De Beer et al. 2014). In the case of C. albifundus, specifically, the fungus is often vectored by indigenous, sap-feeding nitidulid beetles (Coleoptera: Nitidulidae) (Heath et al. 2009). Under native field conditions, C. albifundus colonizes fresh wounds on its tree host, and the visiting nitidulid beetles (attracted by the fruity fungal aroma produced) come into contact with the sticky spore drops, thereby dispersing the fungus locally across landscapes (Roux et al. 2007; Heath et al. 2010).
Since its initial discovery in South Africa (Wingfield et al. 1996), a substantial body of information on the biology and evolution of C. albifundus has accumulated. In a series of recent Lee et al. (2015, 2016, 2018) demonstrated that the reproductive biology of C. albifundus represents an essential component of its overall fitness. In filamentous fungi, fitness typically refers to survival and reproductive success in a particular environment (Pringle and Taylor 2002). With the aid of mycelial growth assays and pathogenicity tests, Lee et al. (2015) showed that compared to self-sterile isolates, self-fertile isolates grow more rapidly and are more aggressive when inoculated onto non-native Acacia and orchard-grown P. cynaroides. Aggressiveness is the quantitative component of pathogenicity (Pariaud et al. 2009). Lee et al. (2015, 2018) also showed that self-fertile isolates are more prevalent in natural populations and in the progeny produced from sexual interactions. Because self-fertile isolates do not need compatible mating partners to complete the sexual cycle (Wilken et al. 2014; Wilson et al. 2015), they are regarded as superior in their ability to produce infective propagules and to rapidly spread or cause epidemics at host population scales (Pariaud et al. 2009; Schoustra et al. 2010). This is because self-fertility facilitates the production of ascocarps carrying sticky spore drops to mediate insect-vectored transmission, which in turn affords self-fertile isolates a competitive advantage, ultimately ensuring survival and efficient reproduction of C. albifundus.
In general, fungal fitness is dependent on various characters and/or combinations of characters that determine optimal life-history strategies (Schoustra et al. 2010). In addition to reproductive biology, other notable components of fitness may include growth rate in culture, sporulation, pathogenicity, substrate/host range, symbiotic relationships, and many other factors (Newton et al. 1997; McDonald and Linde 2002; Gilchrist et al. 2006; Cai et al. 2020). Because these traits are not typically correlated and vary substantially across populations (Merilä and Sheldon 1999; Bazzicalupo 2022), empirical data are needed in order to understand how individual fitness components are linked to the overall growth and survival of species in particular environments (de Visser et al. 1997; Pringle and Taylor 2002). Such studies have revealed that some characters also may be used as proxies for predicting the fitness and the long-term survival of certain fungi (de Visser et al. 1997; Brommer et al. 2004; Schoustra et al. 2010). However, in the case of C. albifundus, little is known regarding the variation that might be expected when growth and pathogenicity of isolates from different locations and hosts are compared, because previous studies addressing these questions were based on small numbers of isolates (Roux et al. 1999; Lee et al. 2015, 2016). This was also true for those studies reporting the influence of self-sterility on growth and pathogenicity of C. albifundus (Lee et al. 2015, 2016).
The aim of this study was to provide a population-wide framework for C. albifundus in South Africa regarding the variation that could be expected in growth, pathogenicity, and sexual fertility of the fungus. This was achieved by assembling a large collection of isolates from diverse hosts and locations, which were then used to investigate growth in culture, fertility status, and pathogenicity on A. mearnsii. We also investigated whether the growth and pathogenicity of isolates were influenced by their geographic origin and source or host. Finally, to identify possible proxies for the overall fitness of C. albifundus, we investigated whether the traits examined were correlated with one another. This study would provide valuable insights regarding the phenotypic diversity expected for populations of C. albifundus, thereby forming a sound foundation from which to explore the genetic and molecular mechanisms underpinning its fitness components.
Materials and methods
Fungal isolates and routine culturing
Fifty-eight C. albifundus isolates available in the Forestry and Agricultural Biotechnology Institute (FABI, University of Pretoria, South Africa) CMW culture collection were used in this study (Table S1). The set was compiled using viable isolates available from previous studies (Roux et al. 2001; Heath 2009; Mbenoun et al. 2014; Lee et al. 2016), with a small number obtained from unpublished work at FABI. Isolates originated from different plant hosts and beetles from across the fungus’s recorded range in the Summer and Winter rainfall regions of South Africa (Roffe et al. 2019). Following long-term storage in the CMW collection, isolates were revived by incubation on malt extract agar (MEA) containing 2% (w/v) malt extract (Biolab, Midrand, South Africa) and 2% (w/v) bacteriological agar (Biolab) under darkness at 25 °C for 7–14 days. These conditions were also used for to routinely grow the respective fungi.
Fertility status
The fertility status of all isolates was assayed using their unpublished genome sequences, which are available from the authors upon request. For this purpose, the isolates were scored based on the presence of the MAT1-2-1 gene. Because self-fertile isolates encode the full complement of genes expected for homothallic members of C. albifundus, the gene would be present in their genomes (Wilson et al. 2015). By contrast, it is absent from self-sterile isolates as they are the product of unidirectional mating type switching, which irreversibly deletes a region containing the MAT1-2-1 gene from the genome (Wilson et al. 2015). Accordingly, we searched the MAT1-2-1 protein sequence (GenBank accession number KF033902), against the translated sequences of each genome with the aid of local tBLASTn analyses in CLC Genomics Workbench v.10 (CLC bio, Aarhus, Denmark).
Colony growth assay
Radial colony growth on MEA was determined as previously described (Lee et al. 2015). For each isolate, a sterile 5 mm cork borer was used to take six mycelial plugs from the actively growing margin of a 2-week-old MEA culture, which were then transferred to the center 90 mm Petri plates containing MEA. The plates were sealed with Parafilm M® (Merck; Darmstadt, Germany), and incubated at 25 °C or at 30 °C, all in the dark. After 21 days, colony diameter was recorded by taking the means of two perpendicular measurements (mm) for each plate. The experiment was performed twice and three technical replicates were used for each condition.
Pathogenicity tests
Aggressiveness of isolates was assessed using 2-year-old A. mearnsii trees as described previously (Fourie et al. 2019). For each isolate, a sterile 5 mm cork borer was used to take a mycelial plug from the actively growing margin of a 3-week-old MEA culture and placing it on a wound on the plant stem. This wound was located 20 cm above ground level and made using a sterile 5 mm cork borer. Parafilm M® was used to hold the mycelial plug in position and to prevent desiccation and cross contamination. For each isolate, 10 plants were inoculated. For the control treatment, 10 plants were inoculated in the same way, except that agar plugs from sterile MEA medium were used. Plants were arranged according to complete randomization design in the same greenhouse, which were maintained at around 25 °C depending on the outside weather. After three weeks, the lengths of the lesions around the inoculation point were recorded. The experiment was conducted twice.
Statistical analyses
Data for growth in culture and aggressiveness were subjected to various analyses (see below). The mean colony diameter and mean lesion length measurements were visualized using histograms. Additionally, the growth and pathogenicity data were subjected to statistical analyses to determine if these traits were significantly linked to isolate origin or fertility status. For this purpose, the data were arranged into groups according to host, by lumping isolates into three groups, i.e., the P. cynaroides group (n = 29) containing isolates from orchard-grown P. cynaroides, the Acacia group (n = 7) containing isolates from A. mearnsii and A. melanoxylon, and the Native hosts group (n = 22; including the beetle isolates) containing the rest of the isolates. Isolates were also grouped based on fertility status (see below), or whether they came from hosts with diseases symptoms (Symptomatic group, n = 33) or from hosts that were asymptomatic (Asymptomatic group, n = 21). Finally, the isolates were grouped based on their geographic origin, with those originating from locations in the Western Cape Province that predominantly receives rain during April to September (Roffe et al. 2019) representing the Winter rainfall group (n = 35). Isolates originating from locations across the interior and eastern parts of South Africa, where rain predominantly fall during October to March (Roffe et al. 2019), represented the Summer rainfall group (n = 23). Furthermore, to exclude the possible impact that fertility status might have on the patterns observed, all analyses were repeated on datasets from which isolates scored as self-sterile were removed.
Normality in the growth and pathogenicity data were explored using the online Kolmogorov-Smirnov test available at https://www.socscistatistics.com. Online tools from this platform were also used for comparison of means, by subjecting the data to one-way ANOVA (analysis of variance) or two-tailed Student’s t-tests using a confidence level of 95%. Here the null hypothesis assumed no differences between the means for the different groups. For the comparison of three or more means, significant differences were determined using Tukey’s Honestly Significant Difference (HSD) tests. All ANOVAs and Tukey’s HSD tests were performed using the online tools available at https://acetabulum.dk/anova.html. Additionally, Chi squared (X2) tests were used to determine whether the observed ratio of self-fertility to sterility departed significantly (α = 0.05) from data presented previously (Lee et al. 2018) with the online tool at https://www.socscistatistics.com.
The growth and pathogenicity data were also subjected to Pearson’s product-moment correlation analyses. Correlations between these continuous data and categorical data were based on the point-biserial correlation coefficient (rpbc). The latter test is a derivation of Pearson’s product-moment correlation analysis where one of the variables is continuous and the other dichotomous. Categorical data included fertility status (self-fertile or self-sterile), and the plant host’s disease status (symptoms or no symptoms). To explore a possible correlation between different sets of categorical data, we used the Phi (Φ) coefficient, which is also analogous to Pearson’s product-moment correlation coefficient (rPearson) but that estimates the degree of association between two binary variables.
Multivariate correlations were explored using Principal Component Analysis (PCA) with SRplot (https://www.bioinformatics.com.cn/en). The latter software was also used to generate biplots in order to graphically display Principal Component (PC) scores and to project variable vectors onto the PCs. These analyses were performed to determine if there are associations among the various data points recorded for the fungi. These included lesion lengths induced on A. mearnsii samplings, colony sizes following growth at 25 and 30 °C, whether strains originated from diseased or healthy plant hosts, whether they were self-fertile or self-sterile, and whether they were collected in the Summer or Winter rainfall regions.
Results
Fertility status
Of the 58 isolates examined based on the presence/absence of the MAT1-2-1 gene, 45 were scored as self-fertile and 13 were scored as self-sterile (Fig. 1). The ratio of ca. 4:1 self-fertility to self-sterility observed for the entire isolate collection differed significantly (X2 = 8.6568; p < 0.05) from the 1:1 ratio that would be expected following Mendelian segregation. However, it did not differ significantly (X2 = 2.1913; p = 0.1388) from the average ratio (ca. 7:1) reported previously for instances where self-fertility dominated among isolates collected from native hosts or P. cynaroides orchards (Lee et al. 2018). Additionally, there was no obvious pattern regarding the distribution of self-sterile and self-fertile isolates according to host. For example, the self-sterile isolates originated from P. cynaroides (n = 7) and Acacia species (n = 2), as well as different native tree species (n = 4). Similarly, no clear patterns were observed in terms of geographic origin, with self-sterile and self-fertile isolates recorded from both the Summer and Winter rainfall regions.
Information of host, origin and fertility status of the Ceratocystis albifundus isolates examined, as well as bar graphs with standard deviation showing the size of lesions induced on 2-year-old Acacia mearnsii trees and colony diameters after 7 days of growth at 25 and 30 °C on malt extract agar medium. In the pathogenicity panel, isolates that did not differ significantly from the control treatment are indicated in grey
Colony growth assay
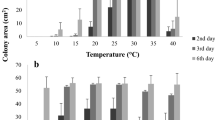
The mean colony diameter following three weeks of growth at 25 and 30 °C varied widely across the 58 isolates examined (Fig. 1). Measurements of 14–54 mm at 25 °C and 21–55 mm at 30 °C were recorded. Most isolates grew faster at the higher temperature, but there were some exceptions. Similar results were observed from the repeated experiment. Both datasets were normally distributed with Kolmogorov-Smirnov D statistic values of 0.08581 (p = 0.74529) for the 25 °C growth data and 0.10411 (p = 0.51145) for the 30 °C data. Comparisons of the various defined groups of isolates using ANOVA and Student’s t-tests generally mostly did not significant differences in growth (Supplementary Table S2 and S3). However, when the 58 isolates were grouped in terms of their fertility status, geographic origin, host species and the disease status of their original host, isolates from the respective Winter rainfall and Symptomatic groups all yielded significantly (p < 0.05) shorter mean colony diameters at 25 °C (Fig. 2A).
Mean colony diameter following 21 days of incubation at 25 °C (yellow) and 30 °C (green) for all 58 isolates examined (A), and only for the 45 self-fertile isolates examined (B). Bars represent standard deviation, while different letters within isolate groups indicate significant (p < 0.05) differences between/among means (see Tables S2 and S3 for details)
For the subset of 45 isolates that included only the self-fertile individuals, the growth data were also normally distributed at both 25 °C (D = 0.1173; p = 0.7430) and 30 °C (D = 0.1236; p = 0.4350). Again, no significant differences were detected among or between most of the defined isolate groups (Supplementary Fig. S1, Tables S2 and S3). The only exceptions were the Acacia group and the Native hosts group that grew significantly (p < 0.05) more rapidly at 25 °C than the P. cynaroides group. However, the Winter rainfall group (mainly obtained from P. cynaroides and A. melanoxylon) grew significantly (p < 0.05) more slowly at 25 °C than Summer rainfall group (mainly isolates obtained from native hosts and from A. mearnsii) (Fig. 2B; Supplementary Tables S2 and S3). The same was also true for the Symptomatic groups (mainly isolates obtained from P. cynaroides and A. mearnsii) that grew significantly (p < 0.05) more slowly at 25 °C than the Asymptomatic group (mainly isolates obtained from native hosts and from A. melanoxylon).
Pathogenicity tests
When C. albifundus isolates were inoculated on A. mearnsii saplings, the mean lesion lengths ranged from 16 mm (CMW42450) to 140 mm (CMW42438) (Fig. 1), with the data being normally distributed (D = 0.0740, p = 0.8851). ANOVA indicated that the recorded measurements for 13 isolates (indicated in grey in the “pathogenicity” panel of Fig. 2) did not differ significantly from the control (Supplementary Table S2). These 13 apparently non-pathogenic isolates originated from diseased P. cynaroides (n = 10) and Acacia species (n = 3), and all but one was scored as self-fertile.
ANOVA and Student’s t-tests revealed significant (p < 0.05) differences among or between the defined groups of isolates, when their original hosts were considered (Supplementary Tables S2 and S3). Isolates obtained from Acacia and orchard-grown Protea (i.e., the P. cynaroides and Acacia groups) produced significantly longer lesions than isolates originating from native hosts lacking signs of disease (i.e., the Native hosts group) (Fig. 3). Similarly, isolates from the Symptomatic group produced significantly (p < 0.05) longer legions than the Asymptomatic group. When isolates were grouped based on geographic origin, those in the Winter rainfall group formed significantly (p < 0.05) longer lesions than those in the Summer rainfall group. Additionally, the isolates from the Self-fertile group produced significantly (p < 0.05) longer lesions than those form the Self-sterile group (Fig. 3; Supplementary Table S3).
The dataset containing mean lesion lengths for only the 45 self-fertile isolates were normally distributed (D = 0.0695; p = 0.9650), with ANOVA and Student’s t-tests also showing significant (p < 0.05) differences among and between various groups of means (Fig. 3; Supplementary Tables S2 and S3). As was the case for the larger dataset, isolates included in the Native hosts group again produced the shortest lesions (p < 0.05), while the Symptomatic group produced significantly longer lesions (p < 0.05) than the Asymptomatic group. Isolates in the Winter rainfall group produced significantly (p < 0.05) longer lesions than those in the Summer rainfall group.
Correlation analyses
For exploring possible correlations between the characters studied, bivariate analyses were used to determine the degree to which the different sets of data were linearly related (Supplementary Table S4). For the larger dataset containing both self-fertile and self-sterile isolates, a moderate positive correlation (rPearson = 0.6291, p < 0.00001) between colony growth at 25 and 30 °C for the 58 isolates was obtained, while growth at 25 °C was weakly correlated with, respectively, fertility status (r(pbc) = 0.2952, p = 0.0272) and the disease status of the isolates’ original host (r(pbc) = −0.3634, p = 0.0042). Pathogenicity to A. meansii showed a weak to moderately positive correlation with the isolates’ fertility status (r(pbc) = 0.4674, p = 0.00028), disease status of their host species (r(pbc) = 0.2524, p = 0.0497), and their geographic origin (r(pbc) 0.46272, p = 0.0003). None of the other analyses yielded any significant correlations. Furthermore, the results for the various correlation analyses with only the data for the 45 self-fertile isolates generally yielded patterns like those obtained when analyzing all the isolates (see Supplementary Table S4). In other words, weak to moderate correlations were detected when we compared their growth at the two temperatures, as well as for the comparisons involving pathogenicity against A. meansii to the isolates’ geographic origin and to the disease status of their hosts. Also, in this smaller isolate collection, mycelial growth at 25 °C had a weak, but significant correlation with the disease status of the plants from which the fungi were obtained. Note, however, that the similar trends observed (using both datasets) for comparisons involving the isolates’ geographic origin and diseased status of their hosts could likely be ascribed to the fact that most members of the Winter rainfall group originated from diseased P. cynaroides, while most of the Summer rainfall group originated from asymptomatic native hosts. Due to the small number of isolates originating from insects, they were not included in any of the correlation analyses.
For exploring multivariate relations among the variables examined, we used a PCA biplot to visualize the data for the first two PCs (Fig. 4). When both self-fertile and self-sterile isolates were included, the two major PCs accounted for 60.2% of the total variability in the data. This clustering pattern of the isolates along PC1 was mainly influenced by disease status of the isolates’ host species, whether the isolates came from the Winter or Summer rainfall region, and the isolates’ apparent pathogenicity to A. mearnsii, as well as their mycelial growth rate at both of the temperatures tested (see vectors labeled “Lesion”, “Region”, “Host”, “Growth25” and “Growth30” in Fig. 4A). However, as suggested by the results of the bivariate analyses (Supplementary Table S4), the directions of the “Host”, “Growth25” and “Growth30” vectors were mostly opposite to those of the “Lesion” and “Region” vectors. Additionally, whether the isolates were self-fertile of self-sterile had no affect the clustering pattern along PC1 (see the vector labeled “Fertility” in Fig. 4A). Indeed, when fertility was excluded as a variable and the PCA was conducted on the self-fertile isolates only, the first two PCs accounted for 68.5% of the total variability in the data, with the directions of the “Host”, “Growth25” and “Growth30” vectors again being mostly opposite to those of the “Lesion” and “Region” vectors (Fig. 4B).
Biplots of principal component (PC) analyses performed on the pathogenicity, growth, geographic origin, and host data for the C. albifundus isolates used in this study, by either including (A) or excluding (B) fertility status as a variable. Individual points represent PC scores for the observations of each isolate relative to the first two PCs. The arrows indicate loadings associated with the isolates’ geographic origin (“Region”), host of origin (“Host”), as well as the lesion length obtained in the pathogenicity test (“Lesion”), and colony growth at 25 and 30 °C (“Growth25” and “Growth30”)
Discussion
This study provides the first comprehensive view of how pathogenicity and growth of C. albifundus might be expected to vary across populations in South Africa. All previous studies (Roux 1996; Roux et al. 2007; Lee et al. 2015) exploring these traits utilized only a small number of C. albifundus isolates. By making use of a collection of 58 isolates (originating from different hosts and geographic regions, and associated with disease or only occurring on wounds in the absence of disease) we showed that C. albifundus varied widely in its aggressiveness on A. mearnsii and its mycelial growth rate in culture at 25 and 30 °C. These patterns also indicated that growth and pathogenicity cannot serve as proxies for one another, although self-fertile isolates can generally be expected to be more aggressive than self-sterile isolates. Nevertheless, the isolates’ fertility status did not seem to have a substantial impact on the overall patterns observed. For example, exclusion of the self-sterile isolates from the pathogenicity data showed that the differences observed regarding the isolates’ host species and disease status, as well as their geographic origin, were similar to those seen when using the entire collection containing both self-fertile and self-sterile isolates. Exploration of these pathogenicity and growth patterns might thus hold valuable clues as to how the individual traits contribute to the overall fitness of C. albifundus (Dutta et al. 2021; Bazzicalupo 2022).
The phenotypic separation of C. albifundus isolates from the Winter and Summer rainfall regions of South Africa is consistent with what has been reported from recent microsatellite-based population genetic studies (Lee et al. 2016). We found that isolates from the Summer rainfall region or from native hosts induced significantly shorter lesions on 2-year-old A. mearnsii saplings than those from the Winter rainfall region. In part, this was not unexpected as geographic differentiation in microbial populations is often linked to climatic conditions that determine the distribution of both microbes and their plant hosts (Roberts and Wiedmann 2003; Bazzicalupo 2022). The fact that C. albifundus appears not to be pathogenic to the plants with which it shares a native range probably also reflects its co-evolutionary relationship with these species (Roux et al. 2001; Bazzicalupo 2022). Indeed, Lee et al. (2016) argued that the occurrence of C. albifundus in the Winter rainfall region is likely due to recent introduction(s) into that area, combined with host range expansions and/or host shifts (Roy 2001; Woolhouse et al. 2002). This scenario is congruent with the notion that fungal strains from “new encounter” hosts (in this case P. cynaroides and A. melanoxylon) are typically more virulent or aggressive than those in their native range (Desprez-Loustau et al. 2007; Keane 1997; Parker and Gilbert 2004). It would be interesting to see whether the analysis of more extensive collections of isolates from particularly the Summer rainfall region in South Africa would yield similar results. Also, future work could explore the hypothesis that aggressiveness of C. albifundus to A. mearnsii and the cultivar of P. cynaroides grown in orchards is a consequence of these plants not possessing suitable mechanisms for fending off the fungus.
The ratio of self-fertile to self-sterile isolates in the collection of C. albifundus isolates utilized in this study was strongly skewed towards self-fertility, which is consistent with previous reports (Lee et al. 2015, 2018). This situation might arise when fewer ascospores bearing the self-sterile trait originate in an ascus and/or when ascospores bearing the trait fail to germinate (Lee et al. 2018). Our findings were also consistent with previous suggestions that self-sterile isolates are less aggressive than their self-fertile counterparts (Lee et al. 2015, 2018). However, with a few exceptions, the self-sterile isolates mostly did not grow more slowly than the self-fertile isolates (see Supplementary Fig. S1). Nevertheless, given their limited aggressiveness, it is unclear why self-sterility is retained in C. albifundus populations, although the presence of self-sterile isolates potentially promotes outcrossing to fully exploit the benefits of sexual recombination (Lin and Heitman 2007; Heitman 2010; Billiard et al. 2011). The presence of self-sterile isolates in populations could thus mediate re-assortment of advantageous alleles, or lead to the introduction of new alleles, while self-fertile isolates ensure through selfing the multiplication and transmission of advantageous allelic combinations that are locally adapted (McDonald and Linde 2002).
Our results suggest that fertility, growth, and pathogenicity each represent distinct components of fitness that contribute separately towards optimizing the life-history strategy of C. albifundus. The distribution patterns associated with these traits were all different from one another and none of them were correlated, which are similar to reports from other fungi (Schoustra et al. 2010). Also, given the complexities of the C. albifundus life cycle (i.e., vegetative and sexual reproduction involving both selfing and outcrossing) (Lee et al. 2020), different types of interactions among these and other traits likely determine the organism’s overall fitness (de Visser et al. 1997; Gilchrist et al. 2006). For example, in the case of saprophytic filamentous fungi, rapid mycelial growth and high levels of spore production are often inversely correlated, thus representing fitness trade-offs for ensuring either exploration of nearby nutritional resources or dispersal to new sites (Pringle and Taylor 2002; Gilchrist et al. 2006; Cai et al. 2020). Therefore, the individual contributions of growth, fertility, and pathogenicity to the fitness of C. albifundus requires further investigation, especially how these traits might be influenced by different biotic and abiotic factors. This is particularly true given the impact of plant host and geography on the patterns observed here, as well as the potential involvement of insects and other fungi in the general ecology of C. albifundus (Heath et al. 2009; Mbenoun et al. 2014).
Taken together, our findings provide a valuable resource for future investigations into the molecular basis and mechanisms underlying growth in culture and pathogenicity in C. albifundus. Colony diameter and aggressiveness on A. mearnsii saplings both displayed continuous distributions, irrespective of the isolates’ fertility status, geographic origin, or their hosts species. This suggests that these traits are quantitative and governed by multiple loci and/or genes (Croll and McDonald 2017). Similar patterns have been reported in other fungi for growth in culture (Olson 2006; Schoustra and Punzalan 2012), and pathogenicity (Idnurm and Howlett 2001; Li et al. 2012). Furthermore, the general lack of correlation between the traits suggest that different sets of loci likely determine the patterns observed (Woolhouse et al. 2002; Croll and McDonald 2017). These data, combined with the unpublished genome sequences used in the current study, would thus allow for comprehensive and detailed analyses of the molecular nature of the traits examined and subsequent inference of the contribution of each to the overall fitness of C. albifundus. Such information might also reveal avenues to manage the pathogen’s pathways of movement and disease on commercially cultivated Acacia and Protea.
Data availability
Genome sequences are available from the authors upon request.
References
Bazzicalupo A (2022) Local adaptation in fungi. FEMS Microbiol Rev 46:fuac026
Billiard S, Lopez-Villavicencio M, Devier B, Hood ME, Fairhead C, Giraud T (2011) Having sex, yes, but with whom? Inferences from fungi on the evolution of anisogamy and mating types. Biol Rev Cambridge Philos Soc 86:421–442
Brommer JE, Gustafsson L, Pietiäinen H, Merilä J (2004) Single-generation estimates of individual fitness as proxies for long-term genetic contribution. Am Nat 163:505–517
Cai F, Gao R, Zhao Z, Ding M, Jiang S, Yagtu C, Zhu H, Zhang J, Ebner T, Mayrhofer-Reinhartshuber M, Kainz P (2020) Evolutionary compromises in fungal fitness: hydrophobins can hinder the adverse dispersal of conidiospores and challenge their survival. ISME J 14:2610–2624
Croll D, McDonald BA (2017) The genetic basis of local adaptation for pathogenic fungi in agricultural ecosystems. Mol Ecol 26:2027–2040
De Beer ZW, Duong TA, Barnes I, Wingfield BD, Wingfield MJ (2014) Redefining Ceratocystis and allied genera. Stud Mycol 79:187–219
de Visser JAGM, Hoekstra RF, Van den Ende H (1997) Test of interaction between genetic markers that affect fitness in Aspergillus niger. Evolution 51:1499–1505
Desprez-Loustau ML, Robin C, Buée M, Courtecuisse R, Garbaye J, Suffert F, Sache I, Rizzo DM (2007) The fungal dimension of biological invasions. Trends Ecol Evol 22:472–480
Dutta A, Hartmann FE, Francisco CS, McDonald BA, Croll D (2021) Mapping the adaptive landscape of a major agricultural pathogen reveals evolutionary constraints across heterogeneous environments. ISME J 15:1402–1419
Fourie A, van der Nest MA, de Vos L, Wingfield MJ, Wingfield BD, Barnes I (2019) QTL mapping of mycelial growth and aggressiveness to distinct hosts in Ceratocystis pathogens. Fungal Genet Biol 131:103242
Gilchrist MA, Sulsky DL, Pringle A (2006) Identifying fitness and optimal life-history strategies for an asexual filamentous fungus. Evolution 60:970–979
Heath RN (2009) Studies to consider the possible origins of three canker pathogens of Eucalyptus in South Africa. PhD Thesis, University of Pretoria, Pretoria, South Africa.
Heath R, Linde M, Groeneveld H, Wingfield B, Wingfield M, Roux J (2010) Factors influencing infection of Acacia mearnsii by the wilt pathogen Ceratocystis albifundus in South Africa. Forest Pathol 40(6):500–509
Heath RN, Wingfield MJ, Van Wyk M, Roux J (2009) Insect associates of Ceratocystis albifundus and patterns of association in a native savanna ecosystem in South Africa. Environ Entomol 38:356–364
Heitman J (2010) Evolution of eukaryotic microbial pathogens via covert sexual reproduction. Cell Host Microbe 8:86–99
Idnurm A, Howlett BJ (2001) Pathogenicity genes of phytopathogenic fungi. Mol Plant Pathol 2:241–255
Keane P (1997) Diseases in natural plant communities. In: Brown JF, Ogle HJ (eds) Plant pathogens and plant diseases. Rockvale Publications, Armidale, Australia, pp 518–532
Lee DH, Roux J, Wingfield BD, Barnes I, Mostert L, Wingfield MJ (2016) The genetic landscape of Ceratocystis albifundus populations in South Africa reveals a recent fungal introduction event. Fungal Biol 120:690–700
Lee DH, Roux J, Wingfield BD, Wingfield MJ (2018) Non-mendelian segregation influences the infection biology and genetic structure of the African tree pathogen Ceratocystis albifundus. Fungal Biol 122:222–230
Lee DH, Wingfield BD, Roux J, Wingfield MJ (2020) Quantification of outcrossing events in haploid fungi using microsatellite markers. J Fungi 6:48
Lee DH, Roux J, Wingfield BD, Wingfield MJ (2015) Variation in growth rates and aggressiveness of naturally occurring self-fertile and self-sterile isolates of the wilt pathogen Ceratocystis albifundus. Plant Pathol 64:1103–1109
Li G, Zhou X, Xu J-R (2012) Genetic control of infection-related development in Magnaporthe oryzae. Curr Opin Microbiol 15:678–684
Lin X, Heitman J (2007) Mechanisms of homothallism in fungi and transitions between heterothallism and homothallism. In: Heitman J, Kronstad JW, Taylor JW, Casselton LA (eds) Sex in fungi: molecular determination and evolutionary implications. ASM Press, Washington, DC, pp 35–57
Mbenoun M, Wingfield M, Boyogueno A, Wingfield B, Roux J (2013) Molecular phylogenetic analyses reveal three new Ceratocystis species and provide evidence for geographic differentiation of the genus in Africa. Mycol Progress 13:219–240
Mbenoun M, Wingfield MJ, Begoude Boyogueno AD, Wingfield BD, Roux J (2014) Molecular phylogenetic analyses reveal three new Ceratocystis species and provide evidence for geographic differentiation of the genus in Africa. Mycol Prog 13:219–240
McDonald BA, Linde C (2002) Pathogen population genetics, evolutionary potential, and durable resistance. Annu Rev Phytopathol 40:349–379
Merilä J, Sheldon BC (1999) Genetic architecture of fitness and nonfitness traits: empirical patterns and development of ideas. Heredity 83:103–109
Newton MR, Kinkel LL, Leonard KJ (1997) Competition and density-dependent fitness in a plant parasitic fungus. Ecology 78:1774–1784
Olson Å (2006) Genetic linkage between growth rate and the intersterility genes S and P in the basidiomycete Heterobasidion annosum s.lat. Mycol Res 110:979–984
Pariaud B, Ravigné V, Halkett F, Goyeau H, Carlier J, Lannou C (2009) Aggressiveness and its role in the adaptation of plant pathogens. Plant Pathol 58:409–424
Parker IM, Gilbert GS (2004) The evolutionary ecology of novel plant-pathogen interactions. Annu Rev Ecol Evol Syst 35:675–700
Pringle A, Taylor JW (2002) The fitness of filamentous fungi. Trends Microbiol 10:474–481
Roberts AJ, Wiedmann M (2003) Pathogen, host and environmental factors contributing to the pathogenesis of listeriosis. Cell Mol Life Sci 60:904–918
Roffe SJ, Fitchett JM, Curtis CJ (2019) Classifying and mapping rainfall seasonality in South Africa: a review. S Afr Geogr J 101:158–174
Roux J (1996) A preliminary study of the diseases of Acacia mearnsii in South Africa. MSc Dissertation, University of the Orange Free State, Bloemfontein, South Africa, 1996
Roux J, Dunlop R, Wingfield MJ (1999) Susceptibility of elite Acacia mearnsii families to Ceratocystis wilt in South Africa. J For Res 4:187–190
Roux J, Harrington T, Steimel J, Wingfield M (2001) Genetic variation in the wattle wilt pathogen Ceratocystis albifundus. Mycoscience 42:327–332
Roux J, Heath RN, Labuschagne L, Nkuekam GK, Wingfield MJ (2007) Occurrence of the wattle wilt pathogen, Ceratocystis albifundus on native South African trees. For Pathol 37:292–302
Roy BA (2001) Patterns of association between crucifers and their flower-mimic pathogens: host jumps are more common than coevolution or cospeciation. Evolution 55:41–53
Schoustra S, Rundle HD, Dali R, Kassen R (2010) Fitness-associated sexual reproduction in a filamentous fungus. Curr Biol 20:1350–1355
Schoustra S, Punzalan D (2012) Correlation of mycelial growth rate with other phenotypic characters in evolved genotypes of Aspergillus nidulans. Fungal Biol 116:630–636
Wilken PM, Steenkamp ET, Wingfield MJ, De Beer ZW, Wingfield BD (2014) DNA loss at the Ceratocystis fimbriata mating locus results in self-sterility. PLoS One 9:e92180
Wilson AM, Wilken PM, Van der Nest MA, Steenkamp ET, Wingfield MJ, Wingfield BD (2015) Homothallism: an umbrella term for describing diverse sexual behaviours. IMA Fungus 6:207–214
Wingfield MJ, De Beer C, Visser C, Wingfield BD (1996) A new Ceratocystis species defined using morphological and ribosomal DNA sequence comparisons. Syst Appl Microbiol 19:191–202
Woolhouse MEJ, Webster JP, Domingo E, Charlesworth B, Levin BR (2002) Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat Gen 32:569–577
Funding
Open access funding provided by University of Pretoria. We thank the Department of Science and Innovation (DSI)—National Research Foundation (NRF) Centre of Excellence in Tree Health Biotechnology and SARChI Chair in Fungal Genomics for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Danki, V., Steenkamp, E., de Vos, L. et al. Growth, pathogenicity and sexual fertility of the African tree pathogen Ceratocystis albifundus. J Plant Pathol (2024). https://doi.org/10.1007/s42161-024-01634-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-024-01634-y